A diagnosis of Burkitt lymphoma (BL) typically causes a fair bit of anxiety for affected patients and their providers. But with appropriate therapy, the proportion of patients cured is quite high; in fact, higher than other aggressive B-cell lymphomas. The problem is getting there. Historically, the regimens considered acceptable for BL included R-hyper-CVAD (rituximab with hyper cyclophosphamide, vincristine, adriamycin, and dexamethasone) with alternating R-cytarabine/methotrexate; CODOX-M (cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate) with alternating IVAC (ifosfamide, etoposide, high-dose cytarabine), or “Magrath”; and the “modified” Magrath regimen. The modified Magrath regimen is a spin-off of the original, with some key dose modifications to improve tolerability in adults. Even with these modifications, the regimen is a significant challenge to administer to patients older than 40 years, as is hyper-CVAD, which is really a childhood acute lymphoblastic leukemia regimen. With this in mind, investigators from the National Cancer Institute tested the dose-adjusted (DA) EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) regimen in adult patients with BL and reported highly encouraging results.1 As with any single-institution study, however, patient selection issues may bias results. With that in mind, these investigators conducted a multicenter trial (22 sites) testing the DA-EPOCH-R regimen in adults with BL.
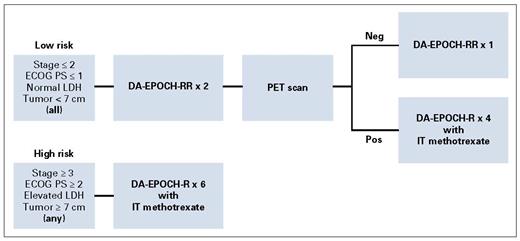
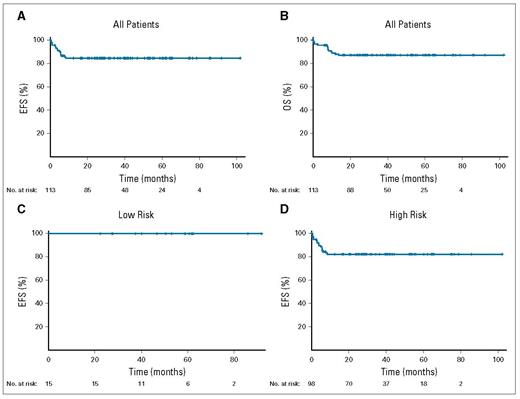
One hundred thirteen patients were enrolled, with a median age of 49 years (range, 18-86 years). The treatment plan was tailored to the patient’s risk category (Figure 1). Patients with low-risk disease could receive as little as three cycles of therapy with no central nervous system (CNS) –directed therapy. With a median follow up of 59 months, the event-free survival for the whole population is 85 percent. When analyzing by risk category, 100 percent of the low-risk patients (n=15) and 82 percent of the high-risk patients were cured (n=98; Figure 2). The toxicities were typical for the EPOCH-R regimen, and 4 percent of patients died during therapy due to treatment toxicity. Patients with either CNS involvement or bone marrow involvement (n=29) did decidedly less well, with cure rates arounds 60 percent.
Risk-stratified treatment based on pretreatment characteristics. Reprinted with permission. © 2020 American Society of Clinical Oncology. All rights reserved. Roschewski M et al: J Clin Oncol Vol. 38, 2020: 2519-2529.
Risk-stratified treatment based on pretreatment characteristics. Reprinted with permission. © 2020 American Society of Clinical Oncology. All rights reserved. Roschewski M et al: J Clin Oncol Vol. 38, 2020: 2519-2529.
Kaplan-Meier estimates of the event-free survival (EFS) and overall survival (OS) of patients enrolled with Burkitt lymphoma. Reprinted with permission. © 2020 American Society of Clinical Oncology. All rights reserved. Roschewski M et al: J Clin Oncol Vol. 38, 2020: 2519-2529.
Kaplan-Meier estimates of the event-free survival (EFS) and overall survival (OS) of patients enrolled with Burkitt lymphoma. Reprinted with permission. © 2020 American Society of Clinical Oncology. All rights reserved. Roschewski M et al: J Clin Oncol Vol. 38, 2020: 2519-2529.
In Brief
This is an important study that convincingly demonstrates that the DA-EPOCH-R regimen is highly efficacious in a multicenter prospective clinical trial in BL. There is no question that this regimen is easier to deliver than traditional BL regimens, and this is particularly true for older BL patients. It would be reasonable to consider this “the standard” for most patients, with the more traditional intensive regimens reserved for younger patients with bone marrow and/or CNS involvement at diagnosis. Because there is some patient selection inherent in all clinical trials, it will be important to see how DA-EPOCH-R performs in general practice. There are ongoing efforts to collect “real-world” data in BL and these reports should provide additional important data which could influence practice.
References
Competing Interests
Dr. Kahl reports consulting fees and research funding from Genentech and Roche.