STUDY TITLE: A Phase III, Randomized Study of Nivolumab Plus AVD or Brentuximab Vedotin Plus AVD in Patients (Age ≥ 12 years) with Newly Diagnosed Advanced Stage Classical Hodgkin Lymphoma (SWOG Intergroup S1826)
CLINICALTRIALS.GOV IDENTIFIER: NCT03907488
SPONSOR: SWOG, National Cancer Institute (grant awards U10CA180888, U10CA180819)
ACCRUAL GOAL: 987 participants
PARTICIPATING CENTERS: All SWOG sites (lead organization) and Cancer Trials Support Unit sites (including the Children’s Oncology Group, Alliance, and ECOG-ACRIN), and the Canadian Cancer Trials Group
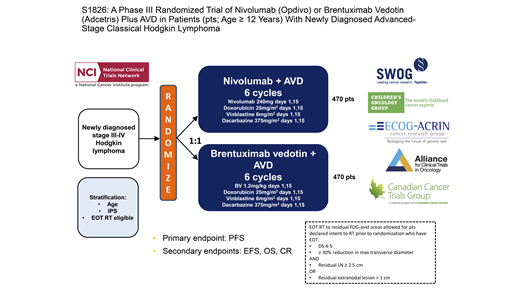
STUDY DESIGN: This trial is enrolling patients 12 years and older (no upper age limit) with untreated newly diagnosed stage III or IV classical Hodgkin lymphoma (HL). Patients are randomized 1:1 to receive six cycles of treatment with either nivolumab (N) plus doxorubicin, vinblastine, and dacarbazine (AVD) chemotherapy or brentuximab vedotin (BV) plus AVD. Patients randomized to receive BV-AVD are required to receive granulocyte-colony stimulating factor primary prophylaxis for neutropenia. Patients are eligible to receive radiation therapy (RT) to residually FDG-avid areas on the end-of-treatment positron emission tomography (PET) scan at the discretion of the treating investigator. Patients will be stratified during randomization based on age, international prognostic score, and whether the patient is intended for RT.
The primary endpoint of the study will compare the progression-free survival (PFS) in patients with newly diagnosed advanced-stage classical HL randomized to receive N-AVD versus BV-AVD. Secondary endpoints include overall survival, event-free survival, the end-of-treatment complete metabolic response rate, and the safety and tolerability of N-AVD as compared to BV-AVD. Another key secondary endpoint will compare patient-reported symptoms and health-related quality of life between the study arms.
RATIONALE: Although a majority of patients with advanced stage HL will be cured with initial therapy, about 20 to 25 percent of patients will have relapsed or refractory disease.1 Standard initial therapy for adults with advanced-stage HL for more than 20 years has been ABVD (doxorubicin, bleomycin, vincristine, dacarbazine), which is associated with pulmonary toxicity due to bleomycin. Recent studies have demonstrated that in patients with a negative interim PET scan, the omission of bleomycin after two cycles is noninferior with regards to survival as compared to a full course of ABVD. However, a significant proportion of patients have a positive interim PET scan and require escalation to BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) — an effective but toxic regimen associated with secondary malignancies and infertility.2 More recently, the addition of BV to AVD demonstrated improved modified and traditionally defined PFS compared to ABVD. However, the BV-AVD regimen was associated with higher rates of neutropenia, sepsis, and peripheral neuropathy compared to ABVD, and there still was an approximately 20 percent failure rate.3 There remains room to improve outcomes in patients with newly diagnosed advanced-stage HL.
In adolescents and young adults in North America, there is a similar approximately 20 percent failure rate with ABVE-PC (adriamycin, bleomycin, vincristine, etoposide phosphate-prednisone, cyclophosphamide) chemotherapy combined with RT use in a majority of patients. Hence, adolescent and young adult patients with advanced-stage HL could benefit from novel therapies and efforts to decrease radiation use while maintaining or potentially improving efficacy.
The PD-1 pathway plays a critical role in the pathogenesis of HL and the disease is exquisitely sensitive to PD-1 blockade, as demonstrated in phase I and II studies of nivolumab and pembrolizumab in patients with heavily treated relapsed/refractory HL.4,5 A phase II trial evaluating N-AVD demonstrated promising safety and efficacy in patients with newly diagnosed HL.6 The incorporation of PD-1 blockade into front-line therapy represents a clear opportunity to attempt to improve the outcomes and tolerability of initial therapy for patients with newly diagnosed advanced-stage HL.
COMMENT: This study represents an unprecedented effort in HL research across all the North American clinical trial cooperative groups to improve the cure rate in advanced stage HL and to harmonize treatment approaches in HL across pediatric and adult patients. The study evaluates the use of nivolumab, a drug that is highly effective against the disease and well-tolerated, as part of front-line therapy for advanced-stage HL. The control arm in the study is BV-AVD, which was demonstrated to be superior to non-PET–adapted ABVD in the ECHELON-1 study. Aside from BEACOPP, which is a toxic regimen rarely used in North America, BV-AVD is the regimen that yields the best PFS in advanced-stage HL. Historically, the approach to treatment of advanced-stage HL in pediatric patients has incorporated RT in a high proportion of patients. The goal is to reserve RT for the patients who are most likely to benefit and minimize RT use in the youngest patients at highest risk for secondary complications.
References
Competing Interests
Dr. Herrera has consulted for and receives research funding from Bristol-Myers Squibb and Seattle Genetics. Dr. Kahl indicated no relevant conflicts of interest.