Abstract
While targeted therapies such as Bruton's tyrosine kinase and BCL2 inhibitors have fundamentally changed the treatment of mantle cell lymphoma (MCL), not all patients respond to these therapies, and responses are finite and can be fleeting, especially with high-risk MCL. As patients progress through successive therapies, the clinical course is characterized by shortening response times,1 frequent disease acceleration, and limited survival outcomes. Recently, the sensitivity of MCL to novel immune-based therapies is being realized with favorable results, as chimeric antigen receptor–modified T cells and bispecific T-cell–engaging antibodies are being investigated and implemented into practice for patients. However, critical issues remain to understand the role of these agents in routine practice. In this review, we discuss the current landscape regarding these agents, examine our approach to incorporating them into practice, and consider unanswered questions that we must ultimately address to improve outcomes for patients.
Learning Objectives
Evaluate current evidence for CD19-directed CAR-T therapy in treating R/R MCL
Compare the implementation of CAR-T therapies with BsAb therapies in the landscape of treating R/R MCL
CLINICAL CASE
A 56-year-old man with mantle cell lymphoma (MCL) self-referred for evaluation and management. He had been diagnosed with stage III blastoid MCL roughly 1 year prior to our evaluation and received rituximab, cyclophosphamide, doxorubicin, etoposide, vincristine, and prednisolone for 6 cycles, attaining an end-of-treatment partial response (PR). Rituximab maintenance was pursued, but disease progression was noted within 2 months, and the treatment was changed to acalabrutinib. Within 6 months, further disease progression occurred, prompting self-referral to our center. At this point the patient's disease was progressing rapidly. What is the optimal management of multiple refractory blastoid MCL in this setting?
Introduction
Although significant advances have been made in treating relapsed/refractory (R/R) MCL with the advent of molecularly targeted therapies, it remains a challenging clinical entity characterized by the progressive shortening of response duration with subsequent lines of therapy and short survival times among patients whose disease is progressing.1,2 The promise of immune-based therapies in treating MCL has been recognized for many years; recent advances have accelerated the use of these therapies, with approved or investigational agents showing tangible benefits in this difficult-to-treat clinical setting. The advent of anti-CD20 monoclonal antibodies (MAbs) established the role of immunotherapy in treating MCL.3 The final predecessor to the current era of immunotherapy is lenalidomide, which remains an accepted up-front and relapse therapy (typically combined with an anti-CD20 MAb).4,5 Lenalidomide is thought to act directly on tumor cells but also through modulating the tumor immune microenvironment for therapeutic effect.6
CD19-directed chimeric antigen receptor–modified T-cell (CAR T) therapy has fundamentally altered the treatment landscape for diffuse large B-cell lymphoma (DLBCL) and is now the standard of care for patients with primary refractory/early relapsing (<12 months from frontline immunochemotherapy) disease based on data from multiple trials. This class of drugs (axicabtagene autoleucel, tisagenlecleucel, and lisocabtagene maraleucel [liso-cel]) is also approved by the US Food and Drug Administration (FDA) for treating follicular lymphoma and third-line–+ DLBCL. CD19-directed CAR-T therapies treating R/R MCL are also highly effective with 2 agents: brexucabtagene autoleucel (brexu-cel, FDA approved in 2020) and liso-cel (FDA approved in June 2024).
Bispecific T-cell–engaging antibodies (BsAbs) are therapies that simultaneously engage CD20 on normal and malignant B cells and CD3 on host T cells to direct immune activation toward tumor eradication. These agents have been a major advancement in treating R/R DLBCL and follicular lymphoma, and very early data are showing promise in treating R/R MCL. Both classes of agents carry a risk for cytokine release syndrome (CRS) and neurotoxicity that warrants attention and is the subject of ongoing investigations for mitigation strategies.
Critical unanswered questions remain for each of these classes of medicines. First, optimizing patient selection is important toward identifying patients who are likely or unlikely to benefit. Second, defining the optimal sequencing of these therapies and weighing them against the treatment landscape in each individual patient is critical. Third, partner therapies that may synergize with CAR T or BsAbs are currently undefined. Fourth, the curative potential of these therapies is unknown and require longer follow-up. In R/R DLBCL, approximately 30% to 40% of patients experience durable remissions with CAR-T therapy, possibly indicating a cure.7 Fifth, whether modifications to the components of the construct or the administration of these agents can make them more tolerable and accessible is being investigated. CRS, neurotoxicity, infections, and prolonged cytopenias remain major challenges. Finally, correlative studies defining molecular mechanisms of resistance are nascent in MCL and may elucidate new avenues for treatment.
In this review we outline published data regarding the CAR-T and BsAb therapy used in R/R MCL, offer our perspective on their use in current practice, and state questions for future inquiry that will ultimately require collaborative multicenter efforts to effectively answer.
CAR-T therapy for R/R MCL
Brexucabtagene autoleucel
Supportive data for the use of brexu-cel, a CD28-costimulated CAR-T agent, are derived from the registrational ZUMA-2 trial and subsequent studies of its implementation in real-world nontrial settings.8-10 Wang and colleagues published a 3-year follow-up on 68 patients treated with brexu-cel in the ZUMA-2 study in 2023 with a median follow-up of 35.6 months.
Many patients' disease had adverse-risk features, including 31% with blastoid, 82% with Ki67 greater than or equal to 30%, 62% Bruton's tyrosine kinase inhibitor (BTKi) refractory, and 40% refractory to previous therapy. The overall response rate (ORR) was 91%, including a 68% complete response (CR) rate and a 24% PR; 25 patients had stable disease or a PR later converted to a CR, and the median duration of response (mDOR) was 28.2 months. The median progression-free survival (mPFS) and overall survival (OS) were 25.8 and 46.6 months, respectively. Across patient/tumor characteristics, response-based efficacy was generally maintained, including according to Ki67 percentage, tumor TP53 mutation, and POD24 status. Patients with blastoid MCL appeared to have inferior 30-month OS, whereas other subgroups derived comparable benefit. Finally, survival analyses suggest continual relapses over time without a clear plateau of long-term responders.
Regarding toxicity, CRS of any grade occurred in 62 (91%) patients; grade 2 CRS occurred in 47% of patients and grade 3 or higher in 15% of patients. Neurologic events of any grade occurred in 63% of patients, including 31% with a grade higher than 2. Notably, infections of higher than grade 2 occurred in 44% of patients in the updated 3-year analysis. Cytopenias were also common: cytopenias of grade 3 and higher occurred in 94% of patients overall. Some cytopenias were prolonged, with neutropenia of grade 3 and higher and thrombocytopenia occurring more than 90 days from infusion in 16% of patients and anemia in 12% of patients.
The United States CAR-T Consortium published nontrial data regarding brexu-cel in 2023. Most (79%) patients included in their analysis would have been ineligible for ZUMA-2.9 Among 168 patients who received brexu-cel, the ORR was 90%, including a CR of 82%. With a median follow-up of 14.3 months, the median PFS was 16.4 months and the median OS not reached. TP53 alterations and a history of POD24 were associated with a lower ORR. Additionally, PFS differed according to the Mantle Cell Lymphoma International Prognostic Index score, Ki67, TP53 status, blastoid histology, and complex karyotype. As with the 3-year ZUMA-2 experience of brexu-cel, a pattern of continuous relapses is apparent. In these analyses, 90% of patients experienced CRS, including 8% with grade 3 or higher and 1 death; 61% of patients experienced neurologic toxicity, including 32% of grade 3 or higher. Nonrelapse mortality, primarily driven by infections, was 9.1% at 1 year, further corroborating the risk for infections post CAR T. The FDA granted an accelerated regulatory approval to brexu-cel on 24 July 2020 for treating R/R MCL.
Lisocabtagene maraleucel
The MCL cohort of the TRANSCEND study of liso-cel was published in 2023 and detailed safety and efficacy data among patients with R/R MCL who underwent leukapheresis (N = 104) and liso-cel infusion (N = 92; median follow-up, 16.1 months).11-13 Nine percent of patients were high risk according to the Simplified Mantle Cell Lymphoma International Prognostic Index, 75% had tumors with Ki67 equal to or greater than 30%, 31% were blastoid, and 23% harbored mutated TP53. Nearly all (94%) patients had received prior BTKi therapy. The ORR and CR rates among infused patients were comparable to those observed with brexu-cel: 83.1% and 72.3%, respectively. The mDOR was 15.7 months. Regarding survival, the median PFS and OS were 15.3 and 18.2 months, respectively. With limited follow-up, relapses continue to occur. An updated subgroup analysis by adverse characteristics demonstrated that response rates, PFS, and OS across subgroups were consistent with the overall population. Patients with a TP53 mutation had a numerically lower mDOR than the overall population, likely due to more responders achieving a PR than a CR. However, of 11 patients with TP53- mutated MCL who achieved CR, 6 were in ongoing response at the time of analysis.13 Among patients with secondary central nervous system lymphoma, the ORR was 86%, the CR rate was 71%, and 3 of 5 patients with a CR were in ongoing response, suggesting a role for CAR T in treating MCL with central nervous system involvement.
The toxicity profile was favorable compared to brexu-cel (Table 1), including lower rates of CRS and immune effector cell–associated neurotoxicity syndrome (both overall and grades >2). Specifically, the overall rates and rates of grade 3 and higher of CRS and immune effector cell–associated neurotoxicity syndrome were 61% and 1%, and 31% and 9%, respectively. Infections of grade 3 and higher were less frequent as well, occurring in 15% of patients, albeit with shorter follow-up. FDA approval for liso-cel was granted in June 2024 based on these encouraging data.
Comparative efficacy and toxicity of brexucabtagene and lisocabtagene
| Agent . | ORR . | CRR . | Median follow-up (mo) . | mDOR (mo) . | mPFS (mo) . | mOS (mo) . | CRS. overall . | CRS > grade 2 . | Neurotoxicity, overall . | Neurotoxicity > grade 2 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Brexu-cel (N = 68)10 | 91% | 68% | 35.6 | 28.2 | 25.8 | 46.6 | 91% | 15% | 63% | 31% |
| Liso-cel (N = 83)12 | 83% | 72% | 16.1 | 15.7 | 15.3 | 18.2 | 61% | 1% | 31% | 9% |
| Agent . | ORR . | CRR . | Median follow-up (mo) . | mDOR (mo) . | mPFS (mo) . | mOS (mo) . | CRS. overall . | CRS > grade 2 . | Neurotoxicity, overall . | Neurotoxicity > grade 2 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Brexu-cel (N = 68)10 | 91% | 68% | 35.6 | 28.2 | 25.8 | 46.6 | 91% | 15% | 63% | 31% |
| Liso-cel (N = 83)12 | 83% | 72% | 16.1 | 15.7 | 15.3 | 18.2 | 61% | 1% | 31% | 9% |
CRR, complete response rate.
Our perspective and future directions/questions
We consider CAR-T therapy for all patients presenting with R/R MCL at our center and weigh its potential benefits against expected toxicities and other standard-of-care or investigational therapies available while evaluating the patient's fitness and goals/preferences for therapy. In practice, we particularly consider CAR T for patients progressing on a BTKi or other second-line therapy or who were primary refractory or had a short remission duration following frontline therapy. However, the optimal sequencing of CAR T with other novel agents in the R/R setting is not firmly established and is highly individualized. We frequently refer older adult patients for a comprehensive geriatric assessment to optimize modifiable factors and further refine the risks of therapy. In our experience, most patients with MCL require bridging therapy, and although sensitivity to bridging is not required to proceed to infusion, we attempt to debulk the disease as much as feasible, anticipating that doing so reduces the risk of toxicity. With the caveats of cross-trial comparisons, we are encouraged by the favorable tolerability of liso-cel compared to brexu-cel, which may enable additional patients to receive CAR-T therapy now that liso-cel is FDA approved.
Whether CAR-T therapy has a curative potential in treating R/R MCL is undetermined. Current evidence from both brexu-cel and liso-cel suggests continued relapses such that long-term disease eradication may be uncommon compared to the 30% to 40% of patients with R/R DLBCL who are cured with CAR T. A longer follow-up might ultimately provide an answer to the question of curability. Given the toxicity associated with CAR T and the promise of BsAb antibodies, the possible lack of curative potential of CAR T is a critical question.
Therapeutic partners that may augment CAR-T function or abrogate toxicity are under active investigation. We are encouraged by data for the IL-1r antagonist anakinra in mitigating the immune side effects (CRS/neurotoxicity) of CAR-T agents without compromising efficacy.14 The TARMAC study (N = 20) investigated time-limited ibrutinib before and after CAR-T infusion in R/R MCL, hypothesizing that overlapping therapy might improve efficacy and safety.15 Its data, with limited follow-up, demonstrate promising results and validate the feasibility of implementing such trials in this clinical setting. Whether other MCL therapies, overlapping with or in a “maintenance” fashion post-CAR T, may increase the response rate or prolong response duration is unproven but warrants further investigation. Additionally, novel targets or dual antigen targeting may be promising avenues in the future.16 Finally, for MCL patients with high-risk disease features, the incorporation of CAR T-cell therapy in their up-front regimen might modify the current unfavorable trajectory of their disease course and is worthy of investigation.
Disparities in access to CAR-T therapy persist in the United States and require further study to understand their patterns and causes.17 With this knowledge the field may ultimately design and implement strategies to mitigate these inequalities and ensure adequate access across ethnically and geographically diverse populations.
BsAb antibody therapy for MCL
Epcoritamab
Epcoritamab is an immunoglobulin G1 antibody derived from a mouse antihuman CD3 antibody and a human anti-CD20 antibody. In a phase 1/2 study involving patients with all B-cell non-Hodgkin lymphoma (NHL), 4 patients with R/R MCL were treated. All had received at least 2 prior lines of therapy. Some responses were observed: 1 CR, 1 PR, and 1 stable disease.18 Notably, only 1 of these 4 patients received epcoritamab at what was selected to be the recommended phase 2 dose, and both responses occurred in patients with poor-risk histology.
Glofitamab
Another CD3xCD20 BsAb antibody studied in R/R MCL is glofitamab. The phase 1 study, published in 2021, enrolled across B-cell lymphoma histologies and included 6 patients with MCL, 3 of whom received the recommended phase 2 dose.19,20 Response evaluations are detailed for 5 of the 6 patients, of whom 4 attained a CR.
A dedicated study of glofitamab in treating R/R MCL was reported in abstract form in 2021 and updated in 2024 with 60 patients. The study population was pretreated with a median of 2 prior lines of therapy, 52% with a prior BTKi and 73% refractory to previous therapy. The ORR was 85%, including a 78% CR. The response rate differed according to BTKi exposure: the ORR was 96.6% for BTKi naive vs 74.2% for BTKi-exposed. The mDOR was 16.2 months and the median duration of CR (mDOCR), 15.4 months.
CRS was the most common adverse event, occurring in 70% of patients. Grade 1 CRS occurred in 36.7% of patients, grade 2 in 21.7% of patients, and grades of 3 or higher in 11.6% of patients. Neurotoxicity occurred in 5 patients, all in grades 1 to 2.
The ongoing international GLOBRYTE study (NCT06084936) is comparing glofitamab vs investigator's choice therapy in R/R MCL (Table 3) and results are awaited.
Mosunetuzumab
Mosunetuzumab is another full-length humanized BsAb antibody investigated in a phase 1/2 study across B-cell NHL.23 Fifteen patients with MCL enrolled, but details concerning responses for MCL are unavailable.
Subsequent MCL-specific data were presented combining mosunetuzumab with the anti-CD79b antibody-drug conjugate polatuzumab-vedotin.24 Patients (N = 20) had received a median of 3 prior lines of therapy: all had received a BTKi, 35% had received CAR T, and 85% were refractory to previous therapy. Many patients' disease harbored adverse-risk features: 65% had tumors with Ki67 greater than or equal to 50%, 50% had a blastoid/pleomorphic histology, and 20% had TP53-mutated MCL. Treatment was administered as a fixed duration for 17 three-week cycles. The overall and CR rates were 75% and 70%, respectively, and the mDOCR was not reached. CRS occurred in 50% of patients, but all events were grades 1 and 2. Neurotoxicity occurred in 3 patients (15%), all grades 1 and 2.
Odronextamab
The final CD3xCD20 BsAb antibody with data in MCL is odronextamab.25 This agent was administered to 12 patients with R/R MCL in a study across B-cell NHL. Among these patients, 6 (50%) responded, including 4 CRs and 2 PRs. The estimated mDOR was 10.9 months. CRS occurred in 61% of patients, including 54% with grades 1 and 2, 6% with grade 3, and 1 grade 4 event. Eighteen (12%) patients experienced neurotoxicity, including 4 instances of grade 3 or higher.
Our perspective
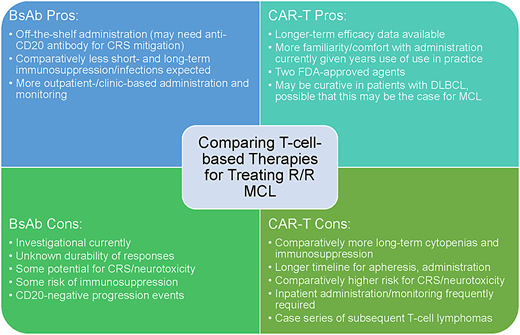
The promising results of BsAb antibodies in treating R/R DLBCL and follicular lymphoma give us hope that similar results will be realized for MCL, likely as combination therapies. The current evidence supporting these agents, specifically in MCL, is sparse (Table 2), but several trials are ongoing (Table 3); the results are eagerly awaited to inform practice. As with other B-cell NHL histologies, key questions for BsAb antibodies are the durability of response, the frequency and severity of toxicity, and patient selection/treatment sequencing given other active agents. Whether prior therapies, especially bendamustine, may induce T-cell exhaustion is also a consideration that warrants evaluation. Finally, weighing BsAb antibodies vs CAR-T therapies in R/R MCL may be challenging in the future for certain patients (Figure 1).
Data for BsAb in treating R/R MCL
| Agent’ . | N . | Activity . |
|---|---|---|
| Epcoritamab18 | 4 | One patient with CR, 1 PR, and 1 stable disease. Both responses occurred in patients with blastoid or pleomorphic MCL. |
| Glofitamab21,22 | 29 | ORR 85%, CR 78.3%; mDOR 16.2 mo, mDOCR 15.4 mo |
| Mosunetuzumab + polatuzumab-vedotin23,24 | 20 | ORR 75%, CRR 70%; mDOCR NR |
| Odronextamab25 | 12 | ORR 50%, CRR 33%; mDOR 10.9 mo |
| Agent’ . | N . | Activity . |
|---|---|---|
| Epcoritamab18 | 4 | One patient with CR, 1 PR, and 1 stable disease. Both responses occurred in patients with blastoid or pleomorphic MCL. |
| Glofitamab21,22 | 29 | ORR 85%, CR 78.3%; mDOR 16.2 mo, mDOCR 15.4 mo |
| Mosunetuzumab + polatuzumab-vedotin23,24 | 20 | ORR 75%, CRR 70%; mDOCR NR |
| Odronextamab25 | 12 | ORR 50%, CRR 33%; mDOR 10.9 mo |
CRR, complete response rate; NR, not reached.
Ongoing BsAb trials in MCL (including for previously untreated patients)
| Setting . | Agent(s) . | NCT . | Geography . | Notes . |
|---|---|---|---|---|
| R/R | Epcoritamab, ibrutinib | NCT05283720 | Global | |
| R/R | Epcoritamab, ibrutinib, venetoclax | |||
| Treatment naïve | Epcoritamab, ibrutinib, venetoclax | |||
| R/R | Glofitamab, lenalidomide | NCT06192888 | United States | Prior BTKi mandatory; prior lenalidomide acceptable if >12 months interval since treatment and previously sensitive |
| R/R | Glofitamab, pirtobrutinib | NCT05833763 | Australia | Prior BTKi mandatory |
| NCT06252675 | United States | Must have responded to prior BTKi therapy | ||
| Treatment naïve | Glofitamab, ibrutinib | NCT06357676 | United States | Either high-risk disease or ≥65 years old |
| R/R | Acalabrutinib, obinutuzumab, and glofitamab | NCT06054776 | United States | Incorporates measurable residual disease testing |
| Treatment naïve | Glofitamab, obinutuzumab, venetoclax, and lenalidomide | NCT05861050 | United States | At least one high-risk disease feature must be present |
| R/R | Glofitamab vs investigator's choice (rituximab-lenalidomide or rituximab-bendamustine) | NCT06084936 | Global | Prior BTKi mandatory |
| Mosunetuzumab | NCT05207670 | United States | Prior BTKi mandatory | |
| Mosunetuzumab – polatuzumab-vedotin | NCT03671018 | United States, Europe | ≥2 prior lines of systemic therapy including BTKi, anti-CD20 MAb, and chemotherapy |
| Setting . | Agent(s) . | NCT . | Geography . | Notes . |
|---|---|---|---|---|
| R/R | Epcoritamab, ibrutinib | NCT05283720 | Global | |
| R/R | Epcoritamab, ibrutinib, venetoclax | |||
| Treatment naïve | Epcoritamab, ibrutinib, venetoclax | |||
| R/R | Glofitamab, lenalidomide | NCT06192888 | United States | Prior BTKi mandatory; prior lenalidomide acceptable if >12 months interval since treatment and previously sensitive |
| R/R | Glofitamab, pirtobrutinib | NCT05833763 | Australia | Prior BTKi mandatory |
| NCT06252675 | United States | Must have responded to prior BTKi therapy | ||
| Treatment naïve | Glofitamab, ibrutinib | NCT06357676 | United States | Either high-risk disease or ≥65 years old |
| R/R | Acalabrutinib, obinutuzumab, and glofitamab | NCT06054776 | United States | Incorporates measurable residual disease testing |
| Treatment naïve | Glofitamab, obinutuzumab, venetoclax, and lenalidomide | NCT05861050 | United States | At least one high-risk disease feature must be present |
| R/R | Glofitamab vs investigator's choice (rituximab-lenalidomide or rituximab-bendamustine) | NCT06084936 | Global | Prior BTKi mandatory |
| Mosunetuzumab | NCT05207670 | United States | Prior BTKi mandatory | |
| Mosunetuzumab – polatuzumab-vedotin | NCT03671018 | United States, Europe | ≥2 prior lines of systemic therapy including BTKi, anti-CD20 MAb, and chemotherapy |
CLINICAL CASE (continued)
The patient underwent apheresis for brexu-cel, received R-DHAOx with clinical and radiographic response, underwent brexu-cel infusion, and is in an ongoing CR at 9 months post infusion. His course was complicated by grade 3 neurotoxicity requiring a brief admission to the intensive care unit, but no CRS occurred.
Acknowledgment
The authors thank Dr. Anita Kumar for assistance.
Conflict-of-interest disclosure
Zachary D. Epstein-Peterson: advisory board: Genmab; research funding: Kymera, Viracta.
M. Lia Palomba: advisory board: Synthekine, Cellectar, Mustang- Bio, Bristol Myers Squibb, Novartis.
Off-label drug use
Zachary D. Epstein-Peterson: All bispecific antibodies are investigational at time of manuscript preparation.
M. Lia Palomba: All bispecific antibodies are investigational at time of manuscript preparation.