Abstract
Anemia is a hallmark of chronic kidney disease (CKD), worsens with disease progression, and profoundly affects a patient's well-being. Major pathogenic factors are inadequate kidney erythropoietin (EPO) production and absolute and functional iron deficiency. The 2 mainstays of current anemia treatment are a) replacement therapy with recombinant EPO or 1 of its glycosylated derivatives, administered subcutaneously or intravenously, and b) intravenous (IV) iron injections. Over the past 5 years, hypoxia-inducible factor (HIF)-prolyl hydroxylase inhibitors (HIF-PHIs) have been approved in many countries for the management of anemia in both nondialysis and dialysis-dependent patients with CKD. Due to cardiovascular safety concerns, only 2 HIF-PHIs, daprodustat and vadadustat, have been approved for marketing in the United States, and only for patients on maintenance dialysis. HIF-PHIs are oral agents that are effective at improving and maintaining hemoglobin levels by activating HIF signaling in anemic patients with CKD. They stimulate the production of endogenous EPO, increase total iron-binding capacity through their direct effects on transferrin gene transcription, lower plasma hepcidin indirectly, and have beneficial effects on red blood cell parameters. Here, we discuss the mechanisms of action and pharmacologic properties of different HIF-PHIs. We discuss unwanted on-target and off-target effects, review cardiovascular and other safety concerns, and provide a benefit/risk-based perspective on how this new class of oral drugs might impact current anemia management in CKD. A clinical case is presented that highlights the clinical complexities and therapeutic challenges in managing anemia in CKD.
Learning Objectives
Understand the mechanisms of action and safety profiles of HIF-PHIs
Understand both the clinical complexities and therapeutic challenges in managing anemia associated with CKD
CLINICAL CASE
Patient H, a 74-year-old man on maintenance hemodialysis (HD), has type 2 diabetes mellitus, hypertension, coronary artery disease, atherosclerotic disease of the carotid arteries, and benign prostatic hyperplasia. His end-stage kidney disease (ESKD) is secondary to hypertensive nephrosclerosis and diabetes mellitus. His outpatient HD regimen, thrice weekly through a tunneled dialysis catheter, has been stable with good dialysis adequacy and volume control. On admission to the hospital for workup of intermittent macroscopic hematuria, his laboratory results are as follows: hemoglobin (Hb) level, 6.4 g/dL (baseline, 9.0 to 9.5 g/dL); mean corpuscular volume, 85.3 fL; serum iron, 41 µg/dL; total iron-binding capacity (TIBC), 245 µg/dL; transferrin saturation (TSAT), 17%; and ferritin level, 1130 ng/mL (100.9 ng/mL 3 months prior to admission). C-reactive protein (CRP) of 162 mg/L and intact parathyroid hormone of 621.3 pg/mL are elevated. Prior to admission, he was receiving epoetin alfa via IV during dialysis totaling 18 000 IU/wk and reports taking oral iron. The patient's hospital course is complicated by a transient ischemic attack felt to be due to a significant common carotid artery stenosis and endarterectomy is recommended.
Introduction to hypoxia-inducible factor activators
Anemia is a common complication of advanced chronic kidney disease (CKD) and profoundly affects a patient's well-being. Major etiological factors are the inability of the diseased kidney to adequately increase erythropoietin (EPO) production in response to tissue hypoxia, as well as absolute and functional iron deficiency.1 Current mainstays of anemia therapy in CKD are IV or subcutaneous injections of erythropoiesis-stimulating agents (ESAs), which are recombinant human EPO or one of its glycosylated derivatives, and oral or IV iron supplementation.2 EPO therapy has reduced the need for blood transfusions in patients with severe anemia, with some studies showing improved overall quality of life.3
Anemia in CKD is associated with a substantial Hb-dependent increase in cardiovascular risk, putting forward the notion that raising Hb may improve cardiovascular outcome in CKD.3 However, several randomized controlled studies in which patients were treated with ESAs with the goal to normalize Hb demonstrated increased risk for cardiac and cerebrovascular events, vascular access thrombosis, progression to ESKD, or all-cause mortality for patients in the high Hb target cohorts (13-14 g/dL).3 These studies, which included patients on dialysis and not on dialysis, led to the currently recommended Hb target ranges for patients with CKD, which are 10 to 11 g/dL in the United States and 10 to 12 g/dL outside the United States.2 The degree to which increased cardiovascular risk is directly linked to ESAs has been debated. However, high ESA doses have been associated with worse cardiovascular outcome in clinical studies and have raised safety concerns, prompting the US Food and Drug Administration to issue a black box warning for ESAs.3 These safety concerns provided the rationale for developing alternative strategies for treating CKD anemia that more comprehensively address the underlying pathophysiology.4-6
The new class of oral hypoxia-inducible factor-prolyl hydroxylase inhibitors (HIF-PHIs), which was first introduced into clinical practice in China and Japan with the approval of roxadustat in late 2018 and 2019, is now marketed for the treatment of anemia of CKD in many countries (Table 1).4 HIF-PHIs treat anemia of CKD effectively and raise Hb levels in a dose-dependent manner.4,7 In the United States, daprodustat and vadadustat have been recently approved for the treatment of anemia in CKD, but only for patients undergoing maintenance dialysis.8,9 This limitation is due to cardiovascular safety concerns associated with HIF-PHI use in patients with CKD who are not on dialysis or are transitioning to dialysis. The US Food and Drug Administration's cardiovascular safety evaluations that led to these restrictions were based on the global phase 3 ASCEND program for daprodustat (Anemia Studies in Chronic Kidney Disease: Erythropoiesis via a novel prolyl hydroxylase inhibitor daprodustat) and the PRO2TECT and INNO2VATE trials for vadadustat.10-15 Roxadustat, the third HIF-PHI studied in global cardiovascular safety trials, was not approved in the United States but is approved in the European Union and other countries for both patients on dialysis and not on dialysis (for an overview of all relevant roxadustat trials, see Ku et al6).
HIF-PHIs approved for marketing
| HIF-PHI . | Recommended starting dose . | Maximal dose . | Dosing frequency . | Countries with approval for marketing . |
|---|---|---|---|---|
| Daprodustat | ND-CKD: 2 - 4 mg (ESA-naive), 4 mg (switch from ESA) DD-CKD: [Japan] 4 mg, [United States] 1 - 4 mg (ESA-naive), 4-12 mg (switch from ESA) | 24 mg | QD | United States (DD-CKD only), Japan |
| Desidustat | ND-CKD: 100 mg (ESA-naive), 100,125 or 150 mg (switch from ESA) DD-CKD: 100 mg | 150 mg | TIW | India |
| Enarodustat | ND-CKD and DD-CKD-PD: 2 mg DD-CKD: 4 mg | 8 mg | QD | China, Japan, Korea |
| Molidustat | ND-CKD: 25 mg (ESA-naive), 25 - 50 mg (switch from ESA) DD-CKD: 75 mg | 200 mg | QD | Japan |
| Roxadustat | [European Union] 70 mg for BW <100 kg, 100 mg for BW ≥100 kg [Japan] 50 mg (ESA-naive), 70 - 100 mg (switch from ESA) | 3.0 mg/kg BW | TIW | China, Chile, Egypt, European Union, Iceland, Japan, Kuwait, Lichtenstein, Mexico, Norway, Russia, Saudi Arabia, South Africa, South, Korea, Turkey, United Arab Emirates, United Kingdom |
| Vadadustat | 300 mg | 600 mg | QD | For DD-CKD only: United States, Australia, European Union, Korea, Taiwan For both DD- and ND-CKD: Japan |
| HIF-PHI . | Recommended starting dose . | Maximal dose . | Dosing frequency . | Countries with approval for marketing . |
|---|---|---|---|---|
| Daprodustat | ND-CKD: 2 - 4 mg (ESA-naive), 4 mg (switch from ESA) DD-CKD: [Japan] 4 mg, [United States] 1 - 4 mg (ESA-naive), 4-12 mg (switch from ESA) | 24 mg | QD | United States (DD-CKD only), Japan |
| Desidustat | ND-CKD: 100 mg (ESA-naive), 100,125 or 150 mg (switch from ESA) DD-CKD: 100 mg | 150 mg | TIW | India |
| Enarodustat | ND-CKD and DD-CKD-PD: 2 mg DD-CKD: 4 mg | 8 mg | QD | China, Japan, Korea |
| Molidustat | ND-CKD: 25 mg (ESA-naive), 25 - 50 mg (switch from ESA) DD-CKD: 75 mg | 200 mg | QD | Japan |
| Roxadustat | [European Union] 70 mg for BW <100 kg, 100 mg for BW ≥100 kg [Japan] 50 mg (ESA-naive), 70 - 100 mg (switch from ESA) | 3.0 mg/kg BW | TIW | China, Chile, Egypt, European Union, Iceland, Japan, Kuwait, Lichtenstein, Mexico, Norway, Russia, Saudi Arabia, South Africa, South, Korea, Turkey, United Arab Emirates, United Kingdom |
| Vadadustat | 300 mg | 600 mg | QD | For DD-CKD only: United States, Australia, European Union, Korea, Taiwan For both DD- and ND-CKD: Japan |
ESA-naive is defined as no previous use of ESA.
BW, body weight; DD, dialysis-dependent (maintenance); ND, non–dialysis-dependent; QD, once daily; TIW, three times weekly.
HIFs are evolutionarily conserved, ubiquitously expressed, heterodimeric transcription factors essential for cellular survival under hypoxic conditions. HIF-PHIs reversibly inhibit prolyl hydroxylase domain (PHD) dioxygenases, the cellular oxygen sensors that control HIF transcription factor activity by initiating proteasomal degradation of the HIF-α subunit. HIF1 and HIF2, the most extensively studied HIFs, regulate many genes that control various biological processes and cellular pathways, including EPO and iron metabolism genes such as transferrin, divalent metal transporter 1, and duodenal cytochrome B (Figure 1).4
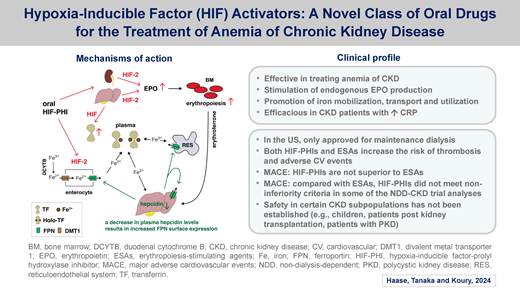
Mechanisms of action of HIF-PHIs. Schematic overview of HIF activity regulation by PHD dioxygenases. The oxygen-sensitive HIF-α subunit is constitutively synthesized and rapidly degraded under normoxic conditions. Proteasomal degradation of HIF-α is initiated by prolyl-hydroxylation and mediated by the von Hippel-Lindau (VHL)-E3-ubiquitin ligase complex. PHD1, PHD2, and PHD3 utilize molecular oxygen and 2-oxoglutarate (2OG) for HIF-α hydroxylation. PHD2 is the main regulator of HIF activity in most cells. Hypoxia or exposure to HIF-PHIs reduces PHD catalytic activity, which results in intracellular accumulation of HIF-α and its nuclear translocation. In the nucleus, HIF-α heterodimerizes with constitutively expressed HIF-β, forming the HIF transcription factor, which increases the expression of HIF target genes such as EPO, ceruloplasmin (CP), divalent metal transporter 1 (DMT1), duodenal cytochrome b (DCYTB), ferroportin (FPN), transferrin (TF), lactate dehydrogenase (LDH), VEGF, and others. Also shown are examples of HIF-regulated biological processes. HIF-2 induces renal and hepatic EPO synthesis in response to hypoxia or HIF-PHI administration. Chemical structures of HIF-PHIs daprodustat (2-[(1,3-dicyclohexyl-2,4,6-trioxo-1,3-diazinane-5-carbonyl)amino]acetic acid) and vadadustat (2-[[5-(3-chlorophenyl)-3-hydroxypyridine-2-carbonyl]amino]acetic acid) are shown at the top. A common feature of HIF-PHIs is the presence of a carbonylglycine side chain, which is structurally analogous to 2-OG.
Mechanisms of action of HIF-PHIs. Schematic overview of HIF activity regulation by PHD dioxygenases. The oxygen-sensitive HIF-α subunit is constitutively synthesized and rapidly degraded under normoxic conditions. Proteasomal degradation of HIF-α is initiated by prolyl-hydroxylation and mediated by the von Hippel-Lindau (VHL)-E3-ubiquitin ligase complex. PHD1, PHD2, and PHD3 utilize molecular oxygen and 2-oxoglutarate (2OG) for HIF-α hydroxylation. PHD2 is the main regulator of HIF activity in most cells. Hypoxia or exposure to HIF-PHIs reduces PHD catalytic activity, which results in intracellular accumulation of HIF-α and its nuclear translocation. In the nucleus, HIF-α heterodimerizes with constitutively expressed HIF-β, forming the HIF transcription factor, which increases the expression of HIF target genes such as EPO, ceruloplasmin (CP), divalent metal transporter 1 (DMT1), duodenal cytochrome b (DCYTB), ferroportin (FPN), transferrin (TF), lactate dehydrogenase (LDH), VEGF, and others. Also shown are examples of HIF-regulated biological processes. HIF-2 induces renal and hepatic EPO synthesis in response to hypoxia or HIF-PHI administration. Chemical structures of HIF-PHIs daprodustat (2-[(1,3-dicyclohexyl-2,4,6-trioxo-1,3-diazinane-5-carbonyl)amino]acetic acid) and vadadustat (2-[[5-(3-chlorophenyl)-3-hydroxypyridine-2-carbonyl]amino]acetic acid) are shown at the top. A common feature of HIF-PHIs is the presence of a carbonylglycine side chain, which is structurally analogous to 2-OG.
As anemia drugs, HIF-PHIs promote erythropoiesis primarily through increased production of endogenous EPO (HIF2 dependent) and by facilitating iron uptake, transport, and mobilization for Hb synthesis (Figure 1).4 HIF-PHIs stimulate the endogenous production of EPO in both the kidneys and liver.1,16,17 However, the contribution of liver-derived EPO to serum EPO levels in patients treated with HIF-PHIs has not been studied in clinical trials but has been investigated in experimental models of chronic kidney injury.17,18 Animal studies suggest that the availability of HIF-PHIs to induce EPO production in the kidney depends on the severity of fibrosis and degree of myofibroblast transdifferentiation of kidney interstitial cells that have the capacity to produce EPO—ie, much less kidney EPO production is expected from more severely fibrosed kidneys.17
Because HIF-PHIs are oral drugs, their bioavailability may be affected by the coadministration of other drugs, such as iron and non–iron-containing phosphate binders,9 or by medical conditions that result in gastrointestinal malabsorption. Additionally, due to the high burden of medications required to treat the many comorbidities affecting patients with CKD, HIF-PHIs should be carefully screened for potential drug interactions.4 Therefore, medical conditions affecting bioavailability and drug interactions must be considered when prescribing HIF-PHIs.
HIF-PHI dosing and non-erythropoietic actions
Because HIF transcription factors control multiple biological processes, systemic PHD inhibition can potentially produce adverse, HIF-dependent, on-target effects. HIF-mediated effects on cellular differentiation and growth, vascular homeostasis and hemodynamics, and inflammation and cellular metabolism are well documented in animal studies and might affect clinical outcomes of patients with CKD. Moreover, PHDs belong to a larger group of 2-oxoglutarate-dependent dioxygenases, which hydroxylate targets involved in collagen synthesis, gene regulation, metabolism, and other biological processes. Therefore, HIF-PHIs, which are competitive structural 2-oxoglutarate analogues, may inhibit other dioxygenases with clinically relevant effects that are HIF independent.19-21 The generation of undesirable on-target and/or off-target effects in patients is likely to depend on the dosing and pharmacokinetic properties of an individual HIF-PHI.
HIF-regulated genes are not equally sensitive to hypoxia and/or HIF activation. For example, the degree of hypoxia needed to induce EPO transcription in rat kidneys is much less than that required to induce vascular endothelial growth factor (VEGF) in kidneys or other organs.22 Higher doses of HIF-PHIs may therefore activate HIF-regulated processes that otherwise would not have been activated with lower doses. In clinical studies, for example, daprodustat, in doses above the recommended maximum, statistically significantly elevated serum VEGF concentrations.23,24 Additionally, non-erythropoietic actions have consistently been reported in clinical studies with roxadustat and daprodustat, such as reductions in total serum cholesterol, low- and high-density lipoprotein cholesterol, and triglycerides.4,6 Thus, when initiating HIF-PHI treatment, the lowest dose possible should be used to avoid the risk of undesirable effects.
HIF-PHIs and iron metabolism
Iron is required for the efficient production of red blood cells (RBCs), and regular monitoring of iron status is critically important for anemic patients with CKD who are treated with ESAs or HIF-PHIs, as these agents increase erythropoietic activity and therefore the need for iron. In dialysis patients, iron status is monitored, often monthly, by measuring serum TIBC, TSAT, and ferritin to guide iron-replacement therapy. The current definitions of iron deficiency in CKD patients are related to the high prevalence of inflammation and the routine administration of iron supplements to those patients with more advanced disease who are receiving dialysis.5 For patients not receiving dialysis, absolute iron deficiency is defined as a TSAT level lower than 20% and ferritin below 100 µg/L and functional iron deficiency as a TSAT level lower than 20% and ferritin higher than 100 µg/L. For patients receiving dialysis, absolute iron deficiency is defined as a TSAT level lower than 20% and ferritin below 200 µg/L and functional iron deficiency as a TSAT level lower than 20% and ferritin above 200 µg/L. Importantly, iron deficiency has been associated with increased risk of ischemic stroke in a large epidemiological study and in a standard animal model.25,26 Laboratory studies and clinical presentation indicate that patient H has absolute iron deficiency complicated by functional iron deficiency due to iron sequestration induced by severe inflammation, which further increases his preexisting risk for major cardiovascular events due to ESKD and underlying atherosclerotic vascular disease.
Do HIF-PHIs reduce the need for IV iron?
HIF activation increases duodenal iron absorption and iron mobilization from hepatic and reticuloendothelial stores through the transcriptional upregulation of iron metabolism genes (Figure 1). Increased TIBC and variably decreased TSAT are therefore expected with HIF-PHI treatment and have been consistently observed.6 The reduced serum levels of iron-regulatory peptide hepcidin reported in clinical trials occurred indirectly due to increased erythropoietic activity and the production of erythroferrone.4 However, greater and more persistent hepcidin suppression with HIF-PHI therapy than with ESA therapy likely results from the salutary effects of HIF on TIBC and TSAT.27,28 This improved iron availability to the erythroid marrow resulted in increased mean corpuscular volume and mean corpuscular Hb of RBCs in patients on HIF-PHI therapy compared with those on ESA therapy.29 Thus, HIF-PHIs can potentially enhance iron absorption and utilization, thereby reducing IV iron supplementation as suggested by several studies.4,6 However, in the design of most phase 3 clinical trials HIF-PHI effects on iron metabolism were not primary end points, so the degree of HIF-HPI reduction in IV iron administration requires examination in postmarketing analyses.4,6 However, completely avoiding IV iron supplementation in HIF-HPI-treated patients with ESKD is very unlikely.
Cardiovascular and other safety concerns
Despite predictions from preliminary analyses of phase 3 trials that some HIF-PHIs might positively impact the cardiovascular risk of patients with CKD, the cardiovascular superiority of HIF-PHIs over ESAs was not established in any large global safety trial. Non-inferiority criteria were met in patients on dialysis but not in all cardiovascular safety analyses of patients not on dialysis.7 In the United States, concerns for increased risk of major cardiovascular events led to the limited regulatory approval of daprodustat and vadadustat for only those patients on maintenance dialysis (a minimum of 4 and 3 months of dialysis therapy, respectively).8,9
Thromboembolic events
As with ESA therapy, patients receiving HIF-PHIs can experience serious thrombotic vascular events. Roxadustat has been associated with an increased risk of thrombotic events compared to ESAs or placebo.4,6 Nonfatal thromboembolic events, such as deep vein thrombosis, pulmonary embolism, and vascular access thrombosis, were reported in 171 patients on dialysis who were treated with daprodustat (11.5%) vs 203 patients (13.7%) in the ESA treatment cohort.12 In global phase 3 trials, 23 patients on vadadustat (1.2%) had thromboembolic events excluding dialysis access failure vs 26 patients receiving darbepoetin alfa (1.4%), and 112 patients (5.7%) vs 88 patients (4.5%) developed arteriovenous fistula thrombosis, respectively.15 Underlying mechanisms are not well understood and may be related to the rate of rise in Hb or drug dosing.6 HIF effects on iron metabolism, such as the upregulation of transferrin,30 or on the coagulation system—eg, such as via plasminogen activator inhibitor (PAI-1),31 may be contributory. Further studies are needed to better understand the mechanisms by which HIF-PHIs or ESAs affect thrombotic risk.
Cancer risk
Hypoxia is a common and salient feature of the tumor microenvironment leading to HIF activation in tumor cells, which results in transcriptional changes that regulate key features of tumorigenesis, including reprogramming of glucose, fatty acid and amino acid metabolism, angiogenesis, cell migration, proliferation and differentiation, and metastasis. The degree of HIF activation correlates with tumor progression and poor prognosis.32,33 These findings have raised concerns that pharmacological HIF activation with HIF-PHIs may promote cancer growth and/or metastases. Recently, the HIF-2 inhibitor belzutifan, which prevents the dimerization of HIF-2α with HIF-β and generates effects opposite to those of HIF-PHIs, was approved for sporadic advanced clear cell renal cancer and tumors associated with VHL disease, which are both characterized by persistent and high levels of HIF activation.34,35 However, low levels of systemic HIF activation in nontransformed cells are unlikely to cause cancer. Specifically, patients with Chuvash polycythemia, who are homozygous for specific VHL germline mutations leading to low levels of HIF activation, exhibit increased erythropoiesis and are prone to arterial and venous thromboses, as well as cerebrovascular events, but do not have an increased cancer risk.36 Animal studies have shown no evidence that prolonged exposure to approved HIF-PHIs is oncogenic.37-39 Furthermore, a cancer signal was not detected in global phase 3 safety trials, except for the ASCEND-ND trial (nondialysis), where cancer-related death, tumor progression, or tumor recurrence was more frequently observed in the daprodustat cohort (72 patients, 3.7% vs 49 2.5%, for darbepoetin alfa; relative risk, 1.47; 95% CI, 1.03-2.10).10 However, this relative risk increase was mitigated by accounting for the different half-lives and dosing frequencies of daprodustat and darbepoetin alfa during a post-hoc analysis. Nonetheless, longer drug exposure and extended follow-up of patients treated with HIF-PHIs are necessary for more conclusive safety assessments regarding cancer risk.
Subpopulations of special interest
Among subpopulations of patients with CKD in whom the safety and efficacy of HIF-PHIs have not been sufficiently investigated are those with polycystic kidney disease (PKD). HIF pathway activation occurs in polycystic kidneys, and experimental manipulation of the HIF pathway can enhance cyst expansion in animal models.40 Whether HIF-PHIs enhance cyst growth in PKD patients is unclear. Without sufficient long-term safety data, the authors advise against using HIF-PHIs in patients with PKD.40 Other subpopulations of patients with CKD where safety and efficacy data are lacking include children and patients post kidney transplant.4,6 Although the role of the HIF pathway in development has been studied in genetic animal models, little is known about the effects of HIF-PHIs during pregnancy. They are likely to cause fetal harm during pregnancy and should also be avoided in women who are pregnant or expect to be pregnant and during breastfeeding.41
Caution regarding the use of HIF-PHIs is also advised for patients with pulmonary arterial hypertension and diabetic retinopathy. Both animal and human genetic studies suggest that sustained HIF activation can worsen pulmonary arterial hypertension.42 Proliferative retinal diseases, such as diabetic retinopathy and age-related macular degeneration, are associated with increased HIF activity and VEGF expression.43,44 Although serum VEGF levels were not increased in patients participating in phase 2 and 3 HIF-PHI trials, localized retinal activation of the HIF-VEGF axis may accelerate disease progression. Notwithstanding this concern, worsening of retinopathy or other adverse ocular events have not been reported in clinical trials.4,6
In sum, oral HIF-PHI therapy may benefit some patients but also increase risk for adverse events, and they should be used with caution or avoided in certain subpopulations of patients with CKD until further safety data are available.4,7Table 2 compares risks and benefits of HIF-PHIs in anemic patients with CKD.
Risk and benefit considerations for use of HIF-PHIs in patients with CKD
| Potential benefits . | Potential disadvantages including theoretical risks . |
|---|---|
| Oral dosing for patients not on dialysis (indication not yet approved in United States), patients on home HD or peritoneal dialysis | In NDD-CKD cardiovascular safety trials, lack of non-inferiority compared to ESAs (dependent on type of analysis and geographical location) |
| No cold storage needed | Potential drug-drug interactions due to polypharmacy |
| Beneficial effects on iron metabolism (absorption and utilization) | Increased pill burden, potential for error due to different strength pills and overdosing, narrow therapeutic window |
| Effective in patients with chronic inflammationa | Difficult compliance monitoring |
| Potential cytoprotective effects | Risk of promoting malignancy or kidney cyst growth |
| Risk of promoting proliferative retinopathy | |
| Risk of promoting pulmonary arterial hypertension | |
| Lack of studies on use in children and patients post renal transplantation |
| Potential benefits . | Potential disadvantages including theoretical risks . |
|---|---|
| Oral dosing for patients not on dialysis (indication not yet approved in United States), patients on home HD or peritoneal dialysis | In NDD-CKD cardiovascular safety trials, lack of non-inferiority compared to ESAs (dependent on type of analysis and geographical location) |
| No cold storage needed | Potential drug-drug interactions due to polypharmacy |
| Beneficial effects on iron metabolism (absorption and utilization) | Increased pill burden, potential for error due to different strength pills and overdosing, narrow therapeutic window |
| Effective in patients with chronic inflammationa | Difficult compliance monitoring |
| Potential cytoprotective effects | Risk of promoting malignancy or kidney cyst growth |
| Risk of promoting proliferative retinopathy | |
| Risk of promoting pulmonary arterial hypertension | |
| Lack of studies on use in children and patients post renal transplantation |
It is unclear whether HIF-PHIs are more effective in patients with inflammation at the recommended dose levels; further studies are pending.
NDD, non–dialysis-dependent.
ESA hyporesponsiveness in CKD
Patients with anemia of CKD on ESA therapy who do not achieve target Hb levels despite significant increases in ESA dosing or continue to require high ESA doses to maintain target Hb are considered ESA hyporesponders. Although ESA hyporesponsiveness affects both patients on dialysis and those not receiving dialysis, it occurs more frequently in dialyzed patients, who have a much greater prevalence of anemia. ESA hyporesponsiveness is often transient due to an underlying and often treatable disease, including dialysis catheter or other bacterial infections, noninfectious inflammation, or malignancy. ESA hyporesponsiveness prevalence varies geographically, ranging from 12.5% to 30.3%,45-50 and affected patients have increased risk for cardiovascular events, ESKD, and death.46,47,49,51-54 Numerical values that define ESA hyporesponsiveness are mostly based on clinical experience rather than randomized controlled studies that consider clinical outcomes relative to ESA responsiveness (Table 3).
Definitions of hyporesponsiveness to ESAs
| Definitions of ESA hyporesponsiveness . | Organization or study . |
|---|---|
| Failure to attain the target Hb concentration while receiving >300 IU/kg/wk (20 000 IU/wk) of epoetin or 1.5 µg/kg of darbepoetin alfa (100 µg/wk), or a continued need for such high dosages to maintain the target | Revised EBPG, ERA-EDTA, 200468 |
| Initial ESA hyporesponsiveness: • if no increase in Hb concentration from baseline after the first month of ESA treatment on appropriate weight-based dosing Subsequent ESA hyporesponsiveness: • classify patients as having acquired ESA hyporesponsiveness if after treatment with stable doses of ESA, they require 2 increases in ESA doses up to 50% beyond the dose at which they had maintained a stable Hb concentration | KDIGO, 20122 |
| Failure to achieve Hb target: HD patients: despite 3000 IU dose of IV rHuEPO 3 × wk (9000 IU/wk) or 60 µg/wk of IV darbepoetin alfa once per week PD patients: despite 6000 IU dose of SC rHuEPO once per week (6000 IU/wk) or 60 µg/wk of IV darbepoetin alfa once per week Predialysis CKD patients: despite 6000 IU dose of SC HuEPO once per week (6000 IU/wk) | Japanese Society for Dialysis Therapy, 201569 |
| Failure to achieve target Hb levels with epoetin doses > • IV EPO 450 IU/kg/wk • SC EPO: 300 IU/kg/wk • darbepoetin dose >1.5 µg/kg/wk | The Renal Association, UK, 2017 and 202070 |
| Weight-adjusted ERI [weekly ESA dose/(body weight × Hb)] ESA resistance: ERI >15.4 IU/kg × g/dL (quartile IV)a | Panichi et al, RISCAVID study, 201152 |
| Definitions of ESA hyporesponsiveness . | Organization or study . |
|---|---|
| Failure to attain the target Hb concentration while receiving >300 IU/kg/wk (20 000 IU/wk) of epoetin or 1.5 µg/kg of darbepoetin alfa (100 µg/wk), or a continued need for such high dosages to maintain the target | Revised EBPG, ERA-EDTA, 200468 |
| Initial ESA hyporesponsiveness: • if no increase in Hb concentration from baseline after the first month of ESA treatment on appropriate weight-based dosing Subsequent ESA hyporesponsiveness: • classify patients as having acquired ESA hyporesponsiveness if after treatment with stable doses of ESA, they require 2 increases in ESA doses up to 50% beyond the dose at which they had maintained a stable Hb concentration | KDIGO, 20122 |
| Failure to achieve Hb target: HD patients: despite 3000 IU dose of IV rHuEPO 3 × wk (9000 IU/wk) or 60 µg/wk of IV darbepoetin alfa once per week PD patients: despite 6000 IU dose of SC rHuEPO once per week (6000 IU/wk) or 60 µg/wk of IV darbepoetin alfa once per week Predialysis CKD patients: despite 6000 IU dose of SC HuEPO once per week (6000 IU/wk) | Japanese Society for Dialysis Therapy, 201569 |
| Failure to achieve target Hb levels with epoetin doses > • IV EPO 450 IU/kg/wk • SC EPO: 300 IU/kg/wk • darbepoetin dose >1.5 µg/kg/wk | The Renal Association, UK, 2017 and 202070 |
| Weight-adjusted ERI [weekly ESA dose/(body weight × Hb)] ESA resistance: ERI >15.4 IU/kg × g/dL (quartile IV)a | Panichi et al, RISCAVID study, 201152 |
Shown are examples of regional guideline definitions based on numerical threshold values. Also shown is a hyporesponsiveness definition based on the ERI. In the RISCAVID study, IL-6 was the best predictor for ESA resistance. Patients in quartile 4 were defined as hyporesponders. In this study, quartile 4 was associated with worse cardiovascular outcome and higher mortality.
Thresholds to meet the definition of ESA hyporesponsiveness vary between different studies that use the ERI.
EBPG, European best practice guidelines; ERI, ESA resistance index; ERA-EDTA, European Renal Association-European Dialysis and Transplantation Association; KDIGO, Kidney Disease: Improving Global Outcomes; PD, peritoneal dialysis; rHuEPO, recombinant human erythropoietin; SC, subcutaneous.
Although multiple factors can contribute to ESA hyporesponsiveness, specific causes cannot be identified in approximately 30% of cases (Table 4).55 The most common causes and strongest predictors of ESA hyporesponsiveness are inflammation and iron deficiency. Inflammation inhibits erythropoiesis via cytokine- mediated suppression of EPO synthesis, EPO receptor signaling, iron mobilization and utilization, and other mechanisms (Figure 2).56 Patients with ESA hyporesponsiveness have elevated serum levels of CRP, interleukin 6 (IL-6), and hepcidin, which are strong predictors of increased ESA requirements.52,57-64 Patient H receives high doses of epoetin alfa and has multiple risk factors for ESA-hyporesponsiveness. He is acutely ill, requiring hospitalization, has inadequately controlled secondary hyperparathyroidism, and his laboratory findings are consistent with severe inflammation.
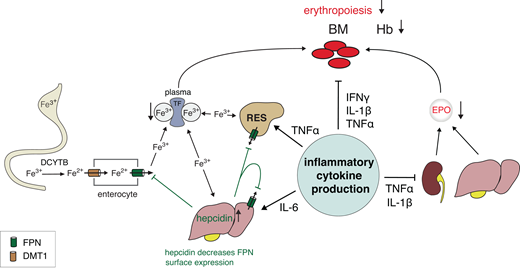
Mechanisms of ESA hyporesponsiveness. Overview of pathogenic mechanisms by which inflammatory cytokines suppress iron uptake and mobilization and erythropoiesis, thereby worsening anemia in patients with CKD. IL-6 stimulates liver production of hepcidin, which downregulates ferroportin (FPN) on all cells and thereby restricts iron availability resulting in functional iron deficiency. In the duodenum, DCYTB reduces ferric iron (Fe3+) to its ferrous form (Fe2+), which is then transported into the cytosol of enterocytes by DMT1. DCYTB and DMT1 are mostly HIF-2-regulated. Absorbed iron is released into the circulation by FPN, the only known cellular iron exporter, the expression of which is regulated by HIF-2 and hepcidin. FPN exports Fe2+, which is oxidized to Fe3+ by enterocyte hephaestin or, in the case of hepatocytes and RES cells, by plasma ceruloplasmin before transport in a complex with TF to the bone marrow and other organs. TF-Fe3+ complexes bind specific cell surface TF receptors and then enter cells via endocytosis of TF-receptors to which they are bound. TF is HIF-regulated, and hypoxia and HIF-PHIs increase TF plasma levels and total iron-binding capacity. Increased hepcidin synthesis in the liver due to inflammation results in decreased FPN cell surface expression, as hepcidin promotes FPN degradation and lowers its cell surface expression. As a result, less iron is released from enterocytes, hepatocytes, and RES cells. In CKD, plasma hepcidin levels are increased at baseline due to diminished renal clearance. Functional iron deficiency is exacerbated further by inflammation. Tumor necrosis factor α (TNF-α) decreases EPO synthesis in the kidney, further exacerbating relative EPO-deficiency in patients with CKD anemia, and, together with IL-1β and interferon γ (IFNγ), suppresses erythroid maturation and proliferation. Although not shown, TNF-α also increases hemophagocytosis of RBCs by macrophages, thereby decreasing RBC life span and exacerbating iron sequestration in RES cells. BM, bone marrow; DCYTB, duodenal cytochrome b; DMT1, divalent metal transporter 1; RES, reticuloendothelial system; TF, transferrin.
Mechanisms of ESA hyporesponsiveness. Overview of pathogenic mechanisms by which inflammatory cytokines suppress iron uptake and mobilization and erythropoiesis, thereby worsening anemia in patients with CKD. IL-6 stimulates liver production of hepcidin, which downregulates ferroportin (FPN) on all cells and thereby restricts iron availability resulting in functional iron deficiency. In the duodenum, DCYTB reduces ferric iron (Fe3+) to its ferrous form (Fe2+), which is then transported into the cytosol of enterocytes by DMT1. DCYTB and DMT1 are mostly HIF-2-regulated. Absorbed iron is released into the circulation by FPN, the only known cellular iron exporter, the expression of which is regulated by HIF-2 and hepcidin. FPN exports Fe2+, which is oxidized to Fe3+ by enterocyte hephaestin or, in the case of hepatocytes and RES cells, by plasma ceruloplasmin before transport in a complex with TF to the bone marrow and other organs. TF-Fe3+ complexes bind specific cell surface TF receptors and then enter cells via endocytosis of TF-receptors to which they are bound. TF is HIF-regulated, and hypoxia and HIF-PHIs increase TF plasma levels and total iron-binding capacity. Increased hepcidin synthesis in the liver due to inflammation results in decreased FPN cell surface expression, as hepcidin promotes FPN degradation and lowers its cell surface expression. As a result, less iron is released from enterocytes, hepatocytes, and RES cells. In CKD, plasma hepcidin levels are increased at baseline due to diminished renal clearance. Functional iron deficiency is exacerbated further by inflammation. Tumor necrosis factor α (TNF-α) decreases EPO synthesis in the kidney, further exacerbating relative EPO-deficiency in patients with CKD anemia, and, together with IL-1β and interferon γ (IFNγ), suppresses erythroid maturation and proliferation. Although not shown, TNF-α also increases hemophagocytosis of RBCs by macrophages, thereby decreasing RBC life span and exacerbating iron sequestration in RES cells. BM, bone marrow; DCYTB, duodenal cytochrome b; DMT1, divalent metal transporter 1; RES, reticuloendothelial system; TF, transferrin.
ESA hyporesponsiveness
| Causes of ESA hyporesponsiveness . |
|---|
| Iron deficiency |
| Inflammation (infections, dialysis catheter use, autoimmune disease) |
| Hyperparathyroidism |
| Blood loss (gastrointestinal tract bleeding, dialysis procedure, menses, and hemolysis) |
| Inadequate dialysis |
| Malignancy |
| Marrow disorders (hemoglobinopathies, multiple myeloma, myelodysplasia, antibody-mediated pure red cell aplasia) |
| Nutritional deficiencies (copper, zinc, folic acid, vitamin B12, carnitine, vitamin E) |
| Medications (eg, renin-angiotensin system [Ras] inhibitors) |
| Unexplained (~30%) |
| Causes of ESA hyporesponsiveness . |
|---|
| Iron deficiency |
| Inflammation (infections, dialysis catheter use, autoimmune disease) |
| Hyperparathyroidism |
| Blood loss (gastrointestinal tract bleeding, dialysis procedure, menses, and hemolysis) |
| Inadequate dialysis |
| Malignancy |
| Marrow disorders (hemoglobinopathies, multiple myeloma, myelodysplasia, antibody-mediated pure red cell aplasia) |
| Nutritional deficiencies (copper, zinc, folic acid, vitamin B12, carnitine, vitamin E) |
| Medications (eg, renin-angiotensin system [Ras] inhibitors) |
| Unexplained (~30%) |
Are HIF-PHIs more effective than ESAs in patients with ESA hyporesponsiveness?
Investigations in animals suggested that HIF-PHIs may be more effective than ESAs in experimental inflammation.4 Phase 2 and 3 trials demonstrated that HIF-PHIs are efficacious in patients with elevated baseline CRP.4 However, whether HIF-PHIs are more effective than ESAs in patients with CKD is not clear. Clinical studies, mostly with roxadustat, suggested that for Hb maintenance in patients with inflammation, an increase in HIF-PHI doses may not be necessary or needed to the same degree as for ESAs. For example, in the SIERRAS study (mostly maintenance dialysis patients), weekly doses of roxadustat were similar for patients with normal-baseline high-sensitivity (hs) CRP and those with above-normal hsCRP, whereas the weekly epoetin alfa doses needed to maintain Hb targets were higher in patients with above-normal baseline hsCRP compared to those with normal-baseline hsCPR.65 However, hsCRP levels at efficacy end points and the number of patients who actually met guideline definitions for ESA hyporesponsiveness were not reported.65 In contrast, post hoc analyses did not suggest that dialysis patients with significantly elevated CRP were more responsive to daprodustat than epoetin alfa.12,13 Comparable efficacy was also observed between darbepoetin alfa and vadadustat regardless of baseline ESA dose, including patients receiving more than 300 IU/kg/wk at baseline.66 Conversely, a small uncontrolled prospective study reported that 15 of 32 dialysis patients diagnosed with ESA hyporesponsiveness who met their Hb target level when treated with roxadustat had lower baseline serum levels of hsCRP and IL-6 compared to nonresponders prescribed the maximum recommended dose of roxadustat.67 These results would suggest that HIF-PHIs at the approved dosing levels will not be effective in patients with severe inflammation. Furthermore, patients with long-standing ESA hyporesponsiveness due to certain hemoglobinopathies, myelofibrosis, or other marrow-based diseases will most likely not respond to HIF-PHI therapy. Cardiovascular safety data from large global HIF-PHI trials raise concerns that HIF-PHI use in patients with significant inflammation may further increase their preexisting risk for serious cardiovascular events. To clarify whether HIF-PHIs are beneficial to patients at risk for ESA hyporesponsiveness, randomized controlled trials need to be conducted to address therapeutic efficacy, impact on quality of life, drug dosing, and most importantly, cardiovascular safety.
CLINICAL CASE (continued)
Patient H's anemia was multifactorial, involving not only relative EPO deficiency due to CKD but also intermittent blood loss, worsening inflammation, and hyperparathyroidism. Acutely, he received transfusions of packed RBCs and urological evaluation for macroscopic hematuria, which identified prostatic disease with accompanying inflammation. Patient H was treated for his secondary hyperparathyroidism and subsequently underwent carotid endarterectomy followed by prostatic enucleation. EPO therapy was suspended during his hospitalization but resumed by his outside nephrologist. His ESA resistance improved, and he received IV iron. Had he remained resistant to ESA therapy, a trial of HIF-PHI could have been considered.
Summary and future directions
HIF-PHIs are a new class of oral drugs that effectively treat anemia in patients with CKD. In addition to stimulating endogenous EPO production, they have beneficial effects on iron utilization. Because of cardiovascular safety concerns, regulatory approval has been restricted to patients on dialysis in the United States and other countries. The decision to treat anemia with HIF-PHIs should be based on careful patient selection weighing potential risks vs benefits. Given the limited therapeutic window, staying within the recommended dosing ranges and using the lowest possible HIF-PHI dose is of paramount importance to avoid undesirable on- and off-target effects. Postmarketing studies and surveillance are needed to address the remaining safety concerns.
Acknowledgments
The authors regret that due to the constraints on manuscript length and the number of allowable citations, it was not possible to reference all original works. They apologize to those colleagues whose original contributions were not cited.
Volker H. Haase is supported by the Krick-Brooks Chair in Nephrology at Vanderbilt University, by NIH grants R01-DK081646, R01-DK135308, R21-AG082416, R35-GM145375, and U24-DK128851, and by Department of Veterans Affairs Merit Award I01-BX002348. Tetsuhiro Tanaka is supported by JSPS KAKENHI Grant Number 23K07713. Information about research conducted in the Haase laboratory can be found at https://www.haaselab.org.
Conflict-of-interest disclosure
Volker H. Haase has received honoraria for consulting from Akebia Therapeutics Inc. and GlaxoSmithKline.
Tetsuhiro Tanaka has received honoraria from Astellas, Bayer, Kyowa-Kirin, Mitsubishi-Tanabe and Torii.
Mark J. Koury has received consulting fees from Akebia Therapeutics Inc., GlaxoSmithKline, and Alexion Pharmaceuticals.
Off-label drug use
Volker H. Haase: nothing to disclose.
Tetsuhiro Tanaka: nothing to disclose.
Mark J. Koury: nothing to disclose.

![Mechanisms of action of HIF-PHIs. Schematic overview of HIF activity regulation by PHD dioxygenases. The oxygen-sensitive HIF-α subunit is constitutively synthesized and rapidly degraded under normoxic conditions. Proteasomal degradation of HIF-α is initiated by prolyl-hydroxylation and mediated by the von Hippel-Lindau (VHL)-E3-ubiquitin ligase complex. PHD1, PHD2, and PHD3 utilize molecular oxygen and 2-oxoglutarate (2OG) for HIF-α hydroxylation. PHD2 is the main regulator of HIF activity in most cells. Hypoxia or exposure to HIF-PHIs reduces PHD catalytic activity, which results in intracellular accumulation of HIF-α and its nuclear translocation. In the nucleus, HIF-α heterodimerizes with constitutively expressed HIF-β, forming the HIF transcription factor, which increases the expression of HIF target genes such as EPO, ceruloplasmin (CP), divalent metal transporter 1 (DMT1), duodenal cytochrome b (DCYTB), ferroportin (FPN), transferrin (TF), lactate dehydrogenase (LDH), VEGF, and others. Also shown are examples of HIF-regulated biological processes. HIF-2 induces renal and hepatic EPO synthesis in response to hypoxia or HIF-PHI administration. Chemical structures of HIF-PHIs daprodustat (2-[(1,3-dicyclohexyl-2,4,6-trioxo-1,3-diazinane-5-carbonyl)amino]acetic acid) and vadadustat (2-[[5-(3-chlorophenyl)-3-hydroxypyridine-2-carbonyl]amino]acetic acid) are shown at the top. A common feature of HIF-PHIs is the presence of a carbonylglycine side chain, which is structurally analogous to 2-OG.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2024/1/10.1182_hematology.2024000655/8/m_hem2024000655figure1.png?Expires=1769846864&Signature=UXa0FGr2-W7r92ph1fTcY2sMzqocmP0-auemV~2q3c7VWlW01I1kW0HaoKqwvEtSeVqRI3iVQRDZWaLgk8M6tIF29QOdyu3WFV3ELQaEyOf0SQCPNlJWyNiYIneih-DtF6myds0YHidgoy2km7TSMzeVd0lJIEsmVnHrAlaPJfzeRb4laenYfldbIw1IkjDRRm6DkCG1za~hZgjN7iJkBTh7M1o2-ElrB29EDzHLhEhsinms2FP4hiGcr0WwUGGHIF-mbR8Kx6uZkwo1XKwZ07JR2ChDH2uThNwD-o8d-dmiXmBN~jGtBN4PMVK0ZqytyUrTsyV7xTUbLE~fdtxQow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)