Learning Objectives
Discuss the clinical benefit of allogeneic hematopoietic stem cell transplantation (HSCT) in older patients with MDS
Describe the clinical relevance of genomic profiling in MDS
Provide evidence for the utility of genomic information to define eligibility for and the optimal timing of transplantation
CLINICAL CASE
Two patients aged 72 and 71 years, referred to as patient A and patient B, respectively, were admitted to our institution in March 2022. Both presented with blood cytopenia and were diagnosed with myelodysplastic syndrome (MDS) with multilineage dysplasia according to World Health Organization 2016 criteria. Bone marrow blasts were less than 5%, and karyotype was normal in both cases. Mutation screening revealed an isolated TP53 gene mutation in patient A and mutations in TET2 and SRSF2 genes in patient B. Accordingly, both patients were classified as intermediate Revised International Prognostic Scoring System (IPSS-R) risk and as moderate-low risk according to the Molecular IPSS (IPSS-M) score. After 6 months, patient A acquired a 7q deletion and an additional TP53 gene mutation, thereby identifying an MDS with biallelic TP53 inactivation. These molecular changes did not affect IPSS-R risk assessment; however, the patient was shifted to a very high risk of disease evolution by IPSS-M. In contrast, patient B had a stable disease without acquiring additional genomic lesions (Table 1).
Clinical and molecular characteristics of patient A and patient B and treatment decisions
| . | Time of diagnosis . | After 6 months of follow-up . | ||
|---|---|---|---|---|
| . | Patient A . | Patient B . | Patient A . | Patient B . |
| Age | 72 y | 71 y | 72 y | 72 y |
| Performance status by KS | >80% | >80% | >80% | >80% |
| Comorbidities by HCT-CI | Low | Low | Low | Low |
| ANC × 109/L | 0.78 | 1.5 | 1 | 1.8 |
| Hb g/dL | 8.2 | 8 | 8.1 | 8.3 |
| PLT × 109/L | 135 | 95 | 167 | 91 |
| % of marrow blasts | 4 | 3 | 4 | 3 |
| Cytogenetics by IPSS-R | Normal karyotype (good risk) | Normal karyotype (good risk) | Isolated del(7q) (intermediate risk) | Normal karyotype (good risk) |
| Gene mutations (VAF) | TP53 (5%) | TET2 (43%), SRSF2 (31%) | Additional TP53 (4%) | TET2 (42%), SRSF2 (32%) |
| IPSS-R risk | Intermediate | Intermediate | Intermediate | Intermediate |
| IPSS-M risk | Moderate low | Moderate low | Very high | Moderate low |
| Treatment decision | Watch and wait; consider ESA if moderate to severe anemia persists | Watch and wait; consider ESA if moderate to severe anemia persists | HSCT up-front | To continue ESA |
| . | Time of diagnosis . | After 6 months of follow-up . | ||
|---|---|---|---|---|
| . | Patient A . | Patient B . | Patient A . | Patient B . |
| Age | 72 y | 71 y | 72 y | 72 y |
| Performance status by KS | >80% | >80% | >80% | >80% |
| Comorbidities by HCT-CI | Low | Low | Low | Low |
| ANC × 109/L | 0.78 | 1.5 | 1 | 1.8 |
| Hb g/dL | 8.2 | 8 | 8.1 | 8.3 |
| PLT × 109/L | 135 | 95 | 167 | 91 |
| % of marrow blasts | 4 | 3 | 4 | 3 |
| Cytogenetics by IPSS-R | Normal karyotype (good risk) | Normal karyotype (good risk) | Isolated del(7q) (intermediate risk) | Normal karyotype (good risk) |
| Gene mutations (VAF) | TP53 (5%) | TET2 (43%), SRSF2 (31%) | Additional TP53 (4%) | TET2 (42%), SRSF2 (32%) |
| IPSS-R risk | Intermediate | Intermediate | Intermediate | Intermediate |
| IPSS-M risk | Moderate low | Moderate low | Very high | Moderate low |
| Treatment decision | Watch and wait; consider ESA if moderate to severe anemia persists | Watch and wait; consider ESA if moderate to severe anemia persists | HSCT up-front | To continue ESA |
ANC, absolute neutrophil count; ESA, erythropoiesis stimulating agents; Hb, hemoglobin; HCT-CI, HSCT-specific comorbidity index; IPSS-M, Molecular International Prognostic Scoring System; IPSS-R, Revised International Prognostic Scoring System; KS, Karnofsky scale; PLT, platelet; VAF, variant allele frequency.
Advances in MDS risk assessment
MDSs have extreme clinical heterogeneity, and a risk-adapted treatment strategy is needed.1 The IPSS-R is used to assess disease-related risk based on the percentage of marrow blasts, blood cytopenias, and specific clonal cytogenetic abnormalities. While IPSS-R is an excellent tool for clinical decision-making, this scoring system may fail to capture reliable prognostic information at an individual patient level.1
MDS development is driven by mutations on genes involved in RNA splicing, DNA methylation, chromatin modification and signal transduction; the identification of these driver genomic lesions can provide valuable information on disease evolution, treatment response, and outcome prediction.2,3 As a result, conventional MDS classifications and prognostic systems are being complemented by the introduction of genomic features that better capture clinical-pathological entities and predict clinical outcome. Recently, a clinical-molecular prognostic model (IPSS-M) was developed using hematologic parameters, cytogenetic abnormalities, and the mutations of 31 MDS-related genes. IPSS-M improved prognostic discrimination across all clinical end points compared to IPSS-R.4
At diagnosis, more than 70% of MDS patients belong to very low, low, and intermediate IPSS-R risk,1 and only a minority of these patients experience leukemic evolution. These individuals are commonly referred to as “low-risk” MDS, in which the treatment goal would be to improve cytopenias and quality of life. In contrast, patients with an advanced disease stage (“high-risk” MDS) require therapeutic interventions to prevent disease evolution and prolong survival. Despite recent therapeutic progress, hematopoietic stem cell transplantation (HSCT) is the only potentially curative treatment for MDS. However, the effectiveness of transplantation is considerably limited by morbidity and mortality, and therefore an accurate patient selection is needed.5 Several factors must be considered in the decision process of how to select MDS patients as suitable candidates for HSCT: these consist of age, performance status, comorbidity, disease stage, and status after nontransplant interventions.5 The complexity of this decision is further highlighted by the fact that many eligible MDS patients do not receive appropriate assessment or consideration for transplantation, particularly outside academic centers (Table 2).
Prognostic risk factors relevant for HSCT eligibility, for outcome after HSCT, and for optimal timing of the procedure
| Prognostic risk factor . | Tools to measure risk factors in patients with MDS . | Outcome after HSCT in patients with MDS . |
|---|---|---|
| Patient-related features | ||
| Age | Calendar, age-adjusted IPSS-R | Worse outcome in elderly patients |
| Performance status | KS | KS >80% associated with better outcome |
| Comorbidities | HCT-CI | Low CI associated with better outcome |
| Disease-related features | ||
| % of marrow blasts | IPSS-R | <5% blasts associated with better outcome |
| Cytogenetics | IPSS-R | Poor-risk cytogenetics and monosomal karyotype associated with higher risk of relapse |
| Gene mutations | IPSS-M | IPSS-M high/very high risk (often including TP53 mutations) associated with poor outcome and high risk of relapse |
| Disease status after treatment interventions | ||
| ESA-lenalidomide failure | IWG criteria | No direct impact reported |
| HMAs-ICT failure | IWG criteria | HSCT is the best available treatment after HMAs-ICT failure, but response status is a prognostic factor |
| Prognostic risk factor . | Tools to measure risk factors in patients with MDS . | Impact on timing of HSCT . |
| Disease-related risk (without molecular information) | IPSS-R | Immediate transplantation is associated with maximal life expectancy in patients with early disease (IPSS-R ≤ 3.5), while for those with higher risk delayed transplantation offers optimal survival benefit. |
| Disease-related risk (including molecular information) | IPSS-M | Patients with higher risk according to IPSS-M should be considered for HSCT earlier than the conventional scoring system (IPSS-R) would dictate. |
| Prognostic risk factor . | Tools to measure risk factors in patients with MDS . | Outcome after HSCT in patients with MDS . |
|---|---|---|
| Patient-related features | ||
| Age | Calendar, age-adjusted IPSS-R | Worse outcome in elderly patients |
| Performance status | KS | KS >80% associated with better outcome |
| Comorbidities | HCT-CI | Low CI associated with better outcome |
| Disease-related features | ||
| % of marrow blasts | IPSS-R | <5% blasts associated with better outcome |
| Cytogenetics | IPSS-R | Poor-risk cytogenetics and monosomal karyotype associated with higher risk of relapse |
| Gene mutations | IPSS-M | IPSS-M high/very high risk (often including TP53 mutations) associated with poor outcome and high risk of relapse |
| Disease status after treatment interventions | ||
| ESA-lenalidomide failure | IWG criteria | No direct impact reported |
| HMAs-ICT failure | IWG criteria | HSCT is the best available treatment after HMAs-ICT failure, but response status is a prognostic factor |
| Prognostic risk factor . | Tools to measure risk factors in patients with MDS . | Impact on timing of HSCT . |
| Disease-related risk (without molecular information) | IPSS-R | Immediate transplantation is associated with maximal life expectancy in patients with early disease (IPSS-R ≤ 3.5), while for those with higher risk delayed transplantation offers optimal survival benefit. |
| Disease-related risk (including molecular information) | IPSS-M | Patients with higher risk according to IPSS-M should be considered for HSCT earlier than the conventional scoring system (IPSS-R) would dictate. |
ESA, erythropoiesis stimulating agent; HCT-CI, HSCT-specific comorbidity index; ICT, intensive chemotherapy; IPSS-M, Molecular International Prognostic Scoring System; IPSS-R, Revised International Prognostic Scoring System; IWG, International Working Group; KS, Karnofsky scale.
Addressing several critical questions is crucial to increase the implementation of HSCT as a curative option in MDS.
Benefit of reduced-intensity conditioning regimen in elderly patients with MDS
Given that most patients are over 60 years old, it is important to define whether reduced-intensity conditioning (RIC) can provide a valuable clinical benefit in this specific patient population. The rationale for using RIC before HSCT is to shift from high-dose chemotherapy that is aimed at maximizing cytotoxic leukemia killing to a more immune-mediated effect by harvesting the graft- versus-tumor effect to eradicate the disease.5 The European Society for Blood and Marrow Transplantation conducted a prospective study (RICMAC trial) comparing RIC with a myeloablative conditioning regimen (MAC) in 129 patients with MDS or acute leukemia from MDS. The 2 conditioning regimens showed comparable 2-year relapse-free and overall survival rates (62% and 76%, respectively, after RIC and 58% and 63%, respectively, after MAC; P = .58 and P = .08). The only risk factor for relapse in a multivariable analysis was advanced disease status.6 Based on this evidence, RIC can be offered as an alternative to a MAC regimen in MDS patients, especially in those subjects without advanced disease and/or high-risk cytogenetics (recommendation grade 2A).
Benefit of HSCT vs hypomethylating agents
The second relevant question is whether HSCT can improve the survival of older MDS patients in comparison with nontransplant strategies (hypomethylating agents [HMAs]).
This issue was addressed by 2 prospective studies using a treatment biologic-assignment. The VidazaAllo study enrolled 190 patients receiving 4 to 6 cycles of HMAs followed by human leukocyte antigen (HLA)–matched HSCT or by continuous HMAs if no donor was identified. Some methodological considerations regarding immortal time bias for HCST in the study should be taken into account (patients must survive long enough to receive a transplant; that is, they are immortal until they receive a transplant. Since the clock to estimate patient survival starts long before transplant, this can lead to a bias in favor of HCST). Despite these concerns, the analysis on 108 out of 190 patients revealed that after a 2-year period HSCT led to an improvement in event-free survival (though not necessarily overall survival) when compared to continuous HMAs (event-free and overall survival were 34% and 50% after HSCT and 0% and 32% after continuous HMAs treatment, respectively; P < .0001 and P = .12).7 Another study, the CIBMTR 1102 trial, compared reduced- intensity HSCT to HMAs or best supportive care in 384 high-risk MDS patients aged 50 to 75. Subjects were assigned to the donor vs no-donor arms according to the availability of a matched donor within 90 days of study registration. After 3 years, the overall survival rate in the donor arm was 47.9% compared with 26.6% in the no-donor arm (P = .0001), and the leukemia-free survival was also greater in the donor arm (35.8% vs 20.6%; P = .003).8 Overall, available evidence suggests that HSCT should be considered as a reasonable treatment option for high-risk older MDS patients with HLA-matched donors (recommendation grade 1B).
HSCT from mismatched HLA-related donor
An HLA-matched donor is available for fewer than 50% of elderly patients. The use of HLA-mismatched related donors (especially HLA haploidentical-related donors) significantly increased in the last few years, taking advantage of substantial improvements such as the administration of posttransplant cyclophosphamide as a graft-versus-host disease prophylaxis. Recently, the EBMT reported the outcome of 228 MDS patients transplanted from a mismatched HLA-related donor. One-third of patients were alive and free of disease 3 years after HSCT, and the use of posttransplant cyclophosphamide was found to improve the effectiveness of the procedure, suggesting that haploidentical HSCT is a suitable option for MDS patients lacking an HLA-matched donor (recommendation grade 2C).9
Impact of genomic screening on the optimal timing of HSCT
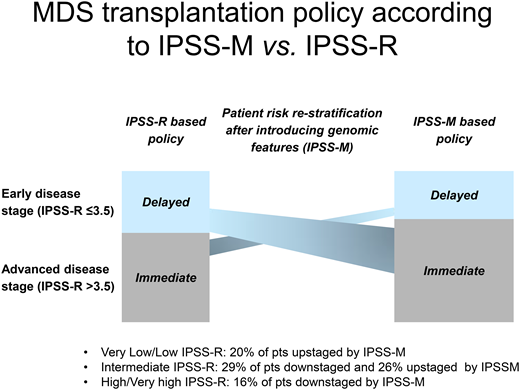
Finally, a crucial issue is to define the optimal timing of HSCT, which would be a disease stage that provides the best life expectancy accounting for both pretransplantation and posttransplantation survival. Patients at early stages may experience long periods with stable disease after diagnosis, and the risks of morbidity/mortality related to HSCT would be unacceptably high for many of them.1,5 However, a number of studies have shown that advanced disease stage at transplantation is associated with inferior overall survival.5 Previous decision analyses concluded that delayed transplantation is associated with maximal life expectancy in patients with a lower IPSS-R risk (≤3.5 score points), while for those with high/very high IPSS-R risk immediate transplantation offers the optimal survival benefit.10 Although these studies provided clinicians with useful information, there are still areas of uncertainty that affect decision-making.
A precise risk score is essential to improve personalized medicine strategies for MDS. The accurate definition of the probability of leukemic evolution is particularly important for lower-risk patients, in whom treatment approaches, including HSCT, may be addressed in a refined manner.1,5
The IPSS-M reflects the relevance that molecular characterization can provide on clinical outcomes. Importantly, in the original study in the groups with very low, low, and intermediate IPSS-R risk, 20% of patients were reclassified into a less favorable prognostic category, with over 90% having one or more mutated IPSS-M genes.4 Therefore, the clinical implementation of IPSS-M is expected to result in a more effective selection of candidates to disease-modifying therapies (including HSCT) among patients with early-stage disease. Accordingly, patients with higher risk according to IPSS-M should be considered for transplantation earlier than the conventional scoring system (IPSS-R) would dictate (Table 2).
Genomic features also impact the posttransplantation prognosis of MDS. TP53 mutations, especially when combined with complex karyotype, resulted in very poor outcomes after HSCT.3,4,11 Recently, it was observed that IPSS-M significantly improved prediction of the probability of survival with respect to IPSS-R in MDS treated with HSCT. In particular, IPSS-M was able to efficiently capture the probability of relapse, potentially refining the choice of the optimal conditioning regiment at individual patient level and improving the identification of patients who can be considered for preemptive treatments of disease recurrence.12
While prospective studies are needed and the optimal pre-HSCT therapy at individual patient level remains to be clarified (especially in high-risk MDS), genomic screening improves MDS prognostication and may result in a more effective selection of candidates for HSCT and a better definition of the optimal timing of the procedure (recommendation grade 2C).
CLINICAL CASE (continued)
Both patients required only sporadic red blood cell transfusions and had fewer than 5% bone marrow blasts. However, different therapeutic decisions were made based on their genomic profile. Patient A underwent up-front HSCT from an HLA-haploidentical family donor after RIC, followed by posttransplant cyclophosphamide. Meanwhile, patient B continued to receive regular follow-up and erythropoiesis stimulating agents. As of October 2023, patient A is in good general condition with full donor chimerism; patient B still presents mild anemia without transfusion dependence and no evidence of disease progression. Although a formal validation of the clinical value of a dynamic IPSS-M assessment is still pending, the use of the molecular score may provide a proof of concept to objectively measure (in an easily understandable way for clinicians) the change in patent prognosis when a modification of the genomic profile occurs.
Conflict-of-interest disclosure
Alessia Campagna: no competing financial interests to declare.
Matteo G. Della Porta: no competing financial interests to declare.
Off-label drug use
Alessia Campagna: Nothing to disclose.
Matteo G. Della Porta: Nothing to disclose.