Abstract
Hodgkin lymphoma (HL) is a rare type of B-cell malignancy with bimodal age distribution targeting young adults and elderly. Prognostic models are available to identify risk of recurrence and response to treatment. Currently, positron emission tomography scanning is most useful in optimizing therapy. Outcomes are generally excellent with standard chemotherapy or combined modality therapy. Balancing efficacy and the risk of late effects in Hodgkin lymphoma is essential, including early detection of potential complications. Incorporation of novel therapies such as brentuximab vedotin and checkpoint inhibitors are being explored in the frontline setting, having already demonstrated improved survival and tolerable toxicity in advanced HL. Furthermore, the addition of these agents have the potential to transform treatment paradigms for early-stage HL and may result in improved outcomes with decreased risks of late toxicities that continue to afflict long-term survivors. However, the patient population, sequencing, and combinations with cytotoxic chemotherapy all remain still standing questions as results of current and upcoming randomized trials are awaited. In this article, we discuss the current data on the approach to initial treatment of early-stage classical HL, review toxicity profiles, and examine upcoming novel therapy trials.
Learning Objectives
Review the impact of prognostic scoring in early-stage Hodgkin lymphoma
Examine the role of chemotherapy alone and combined with radiotherapy; explore novel agent combinations in early-stage Hodgkin lymphoma
Discuss late effects stratified by treatment modality and early detection strategies in survivorship
Introduction
Hodgkin lymphoma (HL) is a rare malignancy that has a bimodal distribution with increased incidence in young adults as well as in patients aged 55 and older.1,2 Its unique biology includes an inflamed microenvironment, dysfunctional immune response, and relatively low presence of malignant cells (pathognomonic Reed-Sternberg cells).3-6 Clinically, disease often presents as supradiaphragmatic lymphadenopathy that spreads contiguously and is occasionally bulky; extranodal involvement at initial presentation is unusual. Patients may be asymptomatic, develop symptoms related to mass effect on surrounding tissue or constitutional symptoms like night sweats, fever, and weight loss (which are referred to as “B symptoms”), and play a role in risk stratification.7
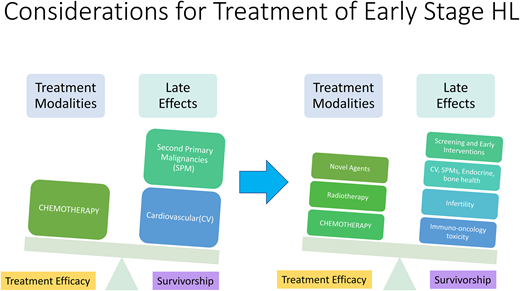
While the treatment of early-stage HL continues to evolve, current approaches are based on multiple large, randomized controlled studies that differ in the designation of pretreatment risk factors, eligibility criteria, and the definition of response based on interim positron emission tomography (PET). In the current era, well more than 70% of advanced-stage patients are cured while advancements in therapy have increased cure rates above 90% for early-stage HL.2 Unfortunately, many survivors continue to experience late effects of radiation and chemotherapy, including second primary malignancies, cardiovascular disease, and endocrine dysfunction, despite reductions and advancements in therapy. Consequently, modern treatment algorithms need to weigh the competing risks of excellent therapeutic efficacy in addition to decreasing late toxicity.
We review the management of newly diagnosed limited/ early-stage HL, including the role of combined modality treatment (CMT), chemotherapy, and radiation therapy (RT), and discuss data incorporating brentuximab vedotin and/or checkpoint inhibitors.8-10 We do not discuss the rare subtype of nodular lymphocyte predominant HL.
CLINICAL CASE
A 21-year-old previously healthy woman presented with progressive shortness of breath over weeks with intermittent chest pressure and a new palpable lump on the left side of the neck. Excisional biopsy of the neck node reveals classical HL, nodular sclerosing subtype. On PET/CT imaging, a 8.5 cm mediastinal mass is seen with standardized uptake value (SUV) of 12, in addition to a left posterior cervical node of 2.1 × 1 cm with SUV of 7.3 and a right supraclavicular lymph node measuring 1.2 cm SUV 6.7. No intra-abdominal or splenic uptake was noted. Her laboratory testing demonstrated a white blood cell count of 8000/microliter, hemoglobin of 11.9 g/dL, platelet count of 371 000, and an elevated erythrocyte sedimentation rate of 60 mm/h. She therefore has nonbulky stage IIA classic HL.
Risk stratification in early-stage HL
The current management approach in early HL is risk adaptive by using several known prognostic factors.11-13 The most important step is accurate disease staging, and HL follows the Ann Arbor staging system for lymphoma that divides patients into four stages depending on where malignant nodes lie in relation to the diaphragm and if extranodal organs are involved.14,15 Recently, data have demonstrated that bone marrow biopsies may be omitted if PET imaging is available.16 Most guidelines approach stage I and II in the same manner, and they are referred to as limited or early-stage disease and further split into two groups: favorable and unfavorable, based on age, presence of bulky lesions (usually defined as 10 cm or more in size), presence of B symptoms, erythrocyte sedimentation rate (ESR), and number of nodal areas involved (Table 1). Different cooperative groups have used varying combinations of these risk factors, which makes comparisons across studies challenging. Nevertheless, the most commonly used prognostic scores are the European Organization of Research and Therapy in Cancer (EORTC/LYSA) score, the German Hodgkin Study Group (GHSG) score, and the National Comprehensive Cancer Network (NCCN) score (Table 1). Generally speaking, ESR >50, presence of B symptoms, and ≥3 involved nodal areas are considered high-risk features in these scoring systems. It is important to highlight that these prognostic scores have limitations, such as their arbitrary delineations developed in older HL studies, and they are rarely used in current studies to stratify patients. Furthermore, while more modern prediction models have been validated in advanced HL, such as the advanced-stage Hodgkin lymphoma International Prognostic Index, no such model is available for early-stage HL.17 In the modern era, interim and end-of-treatment PET results carry the highest level of prognostic yield, with the caveat that PET-based adaptive approaches were developed before targeted drugs were incorporated into frontline therapy.18-22 Studies are needed to assess if PET- based adaptive methodologies are still valid prognostication tools for patients who receive chemoimmunotherapy.
Unfavorable risk factors in early-stage HL per cooperative groups
| GHSG . | EORTC/LYSA . | NCCN . |
|---|---|---|
| Bulky mediastinal mass MMR >0.33 | Bulky mediastinal mass MTR >0.35 | Bulky mediastinal mass MMR >0.33 |
| Extranodal site | Presence of B symptoms | Adenopathy 10 cm in size or more |
| Nodal sites >2 | Nodal sites >3 | Nodal sites >3 |
| ESR >50 (or >30 if B symptoms) | ESR >50 (or >30 if B symptoms) | ESR ≥50 or any B symptoms |
| Age >50 |
| GHSG . | EORTC/LYSA . | NCCN . |
|---|---|---|
| Bulky mediastinal mass MMR >0.33 | Bulky mediastinal mass MTR >0.35 | Bulky mediastinal mass MMR >0.33 |
| Extranodal site | Presence of B symptoms | Adenopathy 10 cm in size or more |
| Nodal sites >2 | Nodal sites >3 | Nodal sites >3 |
| ESR >50 (or >30 if B symptoms) | ESR >50 (or >30 if B symptoms) | ESR ≥50 or any B symptoms |
| Age >50 |
EORTC, European Organization for Research and Treatment of Cancer; ESR, erythrocyte sedimentation rate; GHSG, German Hodgkin Study Group; LYSA, Lymphoma Study Association; MMR, mediastinal mass ratio, maximum width of mass/maximum intrathoracic diameter; MTR, mediastinal thoracic ratio, maximum width of mediastinal mass/intrathoracic diameter at T5-6; NCCN, National Comprehensive Cancer Network.
Role of chemotherapy vs combined modality treatment
The general treatment approach in early-stage disease has historically been 2-4 cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) followed by consolidative involved-field radiotherapy (IFRT) ideally using a PET-based risk-adapted strategy. The actual number of cycles and even the dose of IFRT depend on whether the disease is favorable or not and the results of interim PET. The role of radiation after chemotherapy continues to prompt debate in the field, and many trials have attempted to explore this issue. It appears that omitting radiation may slightly increase recurrence risk but without clear detriment in overall survival. Many oncologists prefer chemotherapy alone approaches to avoid distant toxicity from radiation, such as an increased risk of second primary malignancies and cardiovascular disease; however, it is important to highlight that current radiation modalities are safer, which may partially mitigate risk of toxicity.23,24
Treatment of favorable vs unfavorable disease
Patients with favorable early-stage HL have an excellent prognosis and benefit from a shorter course of chemotherapy when combined with radiation. Several large prospective studies using PET-adapted approaches have evaluated the number of cycles of therapy and the role of RT, including UK RAPID, EORTC H10, and GHSG HD16 in Europe and the US-led CALGB 50604.19,25-27 Of note, European studies considered a Deauville score (DS) of 1-2 as negative, while CALGB considers a score of 3 as negative as well, which is in line with most clinical practice. Table 2 contains selected clinical trials in early-stage HL of both PET-adapted and non-adapted approaches.
Selected therapeutic trials in early-stage HL
| Trial . | Trial design . | PET negative definition . | Disease stage/ characteristics . | N . | Median follow-up . | PFS . | OS . |
|---|---|---|---|---|---|---|---|
| RAPID25 | ABVD × 3 - > PET neg: no further treatment or 30 Gy IFRT PET pos: ABVD × 1 plus 30 Gy IFRT | DS 1-2 | Stage IA or IIA Nonbulky | 602 | 5 yrs | 3-yr 90.8% 3-yr 94.6% 3-yr 83% | 3-yr 99.0% 3-yr 97.1% 3-yr 87.6% |
| EORTC H1019 | Favorable ABVD × 2- > ABVD × 1 plus INRT Or ABVD × 2 - > PET PET neg: ABVD × 2 PET pos: escBEACOPP × 2 plus INRT Unfavorable ABVD × 2- > ABVD × 2 plus INRT Or ABVD × 2- > PET PET neg: ABVD × 4 PET pos: escBEACOPP × 2 plus INRT | DS 1-2 | Stage I or II Favorable or unfavorable | 1950 | 4.5 yrs | (F) PET neg control = 5-yr 99% (F) PET neg trial = 5-yr 87.1% (U) PET neg control = 5-yr 92% (U) PET neg trial = 5-yr 89.6% (F/U) PET pos control = 5-yr 77.4% (F/U) PET pos trial = 5-yr 90.6% | 5-yr 100% 5-yr 99.6% 5-yr 96.7% 5-yr 98.3% 5-yr 89.3% 5-yr 96.0% |
| CALGB 5060427 | ABVD × 2 - > PET PET neg: ABVD × 2 PET pos: escBEACOPP × 2 plus IFRT | DS 1-3 | Stage I or II Nonbulky | 164 | 3.8 yrs | 3-yr 91% 3-yr 66% | |
| GHSG HD16 26 | CMT arm: ABVD × 2 plus 20 Gy IFRT ABVD × 2 - > PET neg: no further treatment PET pos: 20 Gy IFRT | DS 1-2 | Stage I or II – Favorable Nonbulky | 1150 | 3.8 yrs | 5-yr 93.4% 5-yr 86.1% 5-yr 88.4% | 5-yr 98.1% 5-yr 98.4% 5-yr 97.9% |
| GHSG HD1728 | CMT arm: escBEACOPP/ABVD × 4 plus 30 Gy IFRT PET-4 neg: no further treatment PET-4 pos: 30 Gy IFRT | DS 1-2 | Stage I or II – Unfavorable Bulky | 1100 | 3.9 yrs | 5-yr 97.7% 5-yr 95.9% 5-yr 94% | 5-yr 98.7% 5-yr 98.8% 5-yr 99.2% |
| CALGB 5080139 | ABVD × 2 - > PET PET neg: ABVD × 4 PET pos: escBEACOPP × 4 plus 30 Gy ISRT | DS 1-3 | Stage IA-IIB Bulky only | 94 | 5.5 yrs | 3-yr 89.7% 3-yr 92% | 3-yr 94.4% 3-yr 97.7% |
| RATHL29 | ABVD × 2 - > PET PET neg: ABVD × 4 or AVD × 4 PET pos: BEACOPP × 4 | DS 1-3 | Stage IIB-IV or IIA with adverse features | 1203 | 3.4 yrs | 3-yr 85.7%, ABVD 3-yr 84.4%, ABVD- > AVD 3-yr 67.5% | 3-yr 97.2% 3-yr 97.6% 3-yr 87.8% |
| HD1040 | ABVD × 4 plus 30 Gy IFRT ABVD × 4 plus 20 Gy IFRT ABVD × 2 plus 30 Gy IFRT ABVD × 2 plus 20 Gy IFRT | Not PET adapted | Stage I or II Favorable | 1370 | 7.5 yrs | 8-yr 88.4% 8-yr 90.0% 8-yr 85.4% 8-yr 86.5% | 8-yr 94.4% 8-yr 94.7% 8-yr 93.6% 8-yr 95.1% |
| Trial . | Trial design . | PET negative definition . | Disease stage/ characteristics . | N . | Median follow-up . | PFS . | OS . |
|---|---|---|---|---|---|---|---|
| RAPID25 | ABVD × 3 - > PET neg: no further treatment or 30 Gy IFRT PET pos: ABVD × 1 plus 30 Gy IFRT | DS 1-2 | Stage IA or IIA Nonbulky | 602 | 5 yrs | 3-yr 90.8% 3-yr 94.6% 3-yr 83% | 3-yr 99.0% 3-yr 97.1% 3-yr 87.6% |
| EORTC H1019 | Favorable ABVD × 2- > ABVD × 1 plus INRT Or ABVD × 2 - > PET PET neg: ABVD × 2 PET pos: escBEACOPP × 2 plus INRT Unfavorable ABVD × 2- > ABVD × 2 plus INRT Or ABVD × 2- > PET PET neg: ABVD × 4 PET pos: escBEACOPP × 2 plus INRT | DS 1-2 | Stage I or II Favorable or unfavorable | 1950 | 4.5 yrs | (F) PET neg control = 5-yr 99% (F) PET neg trial = 5-yr 87.1% (U) PET neg control = 5-yr 92% (U) PET neg trial = 5-yr 89.6% (F/U) PET pos control = 5-yr 77.4% (F/U) PET pos trial = 5-yr 90.6% | 5-yr 100% 5-yr 99.6% 5-yr 96.7% 5-yr 98.3% 5-yr 89.3% 5-yr 96.0% |
| CALGB 5060427 | ABVD × 2 - > PET PET neg: ABVD × 2 PET pos: escBEACOPP × 2 plus IFRT | DS 1-3 | Stage I or II Nonbulky | 164 | 3.8 yrs | 3-yr 91% 3-yr 66% | |
| GHSG HD16 26 | CMT arm: ABVD × 2 plus 20 Gy IFRT ABVD × 2 - > PET neg: no further treatment PET pos: 20 Gy IFRT | DS 1-2 | Stage I or II – Favorable Nonbulky | 1150 | 3.8 yrs | 5-yr 93.4% 5-yr 86.1% 5-yr 88.4% | 5-yr 98.1% 5-yr 98.4% 5-yr 97.9% |
| GHSG HD1728 | CMT arm: escBEACOPP/ABVD × 4 plus 30 Gy IFRT PET-4 neg: no further treatment PET-4 pos: 30 Gy IFRT | DS 1-2 | Stage I or II – Unfavorable Bulky | 1100 | 3.9 yrs | 5-yr 97.7% 5-yr 95.9% 5-yr 94% | 5-yr 98.7% 5-yr 98.8% 5-yr 99.2% |
| CALGB 5080139 | ABVD × 2 - > PET PET neg: ABVD × 4 PET pos: escBEACOPP × 4 plus 30 Gy ISRT | DS 1-3 | Stage IA-IIB Bulky only | 94 | 5.5 yrs | 3-yr 89.7% 3-yr 92% | 3-yr 94.4% 3-yr 97.7% |
| RATHL29 | ABVD × 2 - > PET PET neg: ABVD × 4 or AVD × 4 PET pos: BEACOPP × 4 | DS 1-3 | Stage IIB-IV or IIA with adverse features | 1203 | 3.4 yrs | 3-yr 85.7%, ABVD 3-yr 84.4%, ABVD- > AVD 3-yr 67.5% | 3-yr 97.2% 3-yr 97.6% 3-yr 87.8% |
| HD1040 | ABVD × 4 plus 30 Gy IFRT ABVD × 4 plus 20 Gy IFRT ABVD × 2 plus 30 Gy IFRT ABVD × 2 plus 20 Gy IFRT | Not PET adapted | Stage I or II Favorable | 1370 | 7.5 yrs | 8-yr 88.4% 8-yr 90.0% 8-yr 85.4% 8-yr 86.5% | 8-yr 94.4% 8-yr 94.7% 8-yr 93.6% 8-yr 95.1% |
ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; CMT, combined modality treatment; DS, Deauville score; escBEACOPP, escalated BEACOPP; F, favorable; IFRT, involved-field radiotherapy; INRT, involved nodal radiotherapy; ISRT, involved site radiotherapy; neg, negative; OS, overall survival; PFS, progression-free survival; pos, positive; U, unfavorable; − >, followed by.
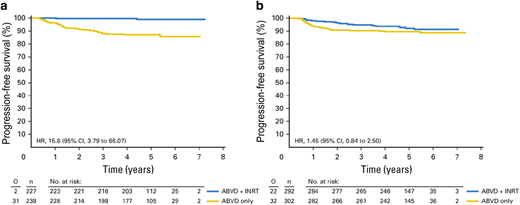
The EORTC H10 study is the largest PET-adapted study in early-stage HL to date and enrolled 1950 patients with either stage I or II (754 had favorable, and 1196 had unfavorable disease). In the control arms, treatment consisted of 3 (favorable) or 4 (unfavorable) ABVD cycles and involved nodal radiotherapy (INRT), regardless of PET results. Patients in the experimental arm received 2 cycles of ABVD followed by PET imaging, and subsequently, if PET was negative (DS 1-2), they received either 2 cycles of ABVD for favorable or 4 cycles if unfavorable. Patients with positive PET received a more intensified BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) regimen in addition to 30 Gray (Gy) INRT. In the favorable disease group with negative interim PET, 5-year progression-free survival (PFS) was 87% with ABVD × 4 vs 99% with ABVD × 3 plus 30 Gy INRT while 5-year overall survival (OS) was 99.6% and 100%, respectively.19 Of note, the EORTC H10 study was closed prematurely after exceeding the boundary limit of 10% relapse or progression in the non-RT arm. (Figure 1)
Progression-free survival of 1059 early PET-negative patients who were treated according to the initial protocol. Shown are the rates of progression-free survival of the (a) favorable (F) groups of patients randomly assigned to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) plus involved-node radiotherapy (INRT; n = 227) or ABVD only (n = 238) and of the (b) unfavorable (U) groups randomly assigned to ABVD plus INRT (n = 292) or ABVD only (n = 302). HR = hazard ratio; O = observed; n = number of patients. Previously published in: André MPE, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35(16):1786-1794.19 doi: 10.1200/JCO.2016.68.6394. Reproduced with permission from the American Society of Clinical Oncology.
Progression-free survival of 1059 early PET-negative patients who were treated according to the initial protocol. Shown are the rates of progression-free survival of the (a) favorable (F) groups of patients randomly assigned to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) plus involved-node radiotherapy (INRT; n = 227) or ABVD only (n = 238) and of the (b) unfavorable (U) groups randomly assigned to ABVD plus INRT (n = 292) or ABVD only (n = 302). HR = hazard ratio; O = observed; n = number of patients. Previously published in: André MPE, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35(16):1786-1794.19 doi: 10.1200/JCO.2016.68.6394. Reproduced with permission from the American Society of Clinical Oncology.
The RAPID and GHSG HD16 studies demonstrated CMT improved PFS compared to chemotherapy alone, but the PFS benefit was small without corresponding improvement in OS and thus might not be clinically significant when factoring in late toxicities from RT. In the CALGB study, 164 patients were enrolled and received ABVD × 2 cycles followed by PET imaging. If PET was negative, patients received 2 additional cycles of ABVD without radiation; for PET-positive disease, therapy was changed to BEACOPP followed by 30 Gy consolidative RT. In this study, 91% of patients had a negative interim PET and received 2 additional cycles of AVBD resulting in a 3-year PFS of 91%. The 3-year PFS in interim PET-positive group after escalation of therapy was 66%.27
Patients with one or more of the risk factors cited above are considered to have unfavorable disease and were treated with a multimodality approach with CMT. Generally speaking, 4 cycles of ABVD followed by 30 Gy of RT is recommended for these patients based on results of the H10 study, where 1196 out of 1950 patients had unfavorable disease. It also demonstrated those achieving negative PET after 2 cycles of ABVD had a 5-year PFS of 89.6% with ABVD × 6 cycles vs 92% with ABVD × 4 cycles plus 30 Gy consolidative RT, while 5-year OS was 98.3% and 96.7%, respectively. Other studies have attempted to omit RT. For example, in the GHSG HD17 phase 3 study, 1100 patients with unfavorable stage I/II HL were randomized to either 2 cycles of escalated BEACOPP in addition to 2 cycles of ABVD (2 + 2) followed by 30 Gy consolidative RT (standard CMT arm) or the RT was omitted if patients achieved a negative PET (DS 1-2) after 4 cycles of chemotherapy (experimental arm). Five-year PFS was 97.3% in the standard CMT arm vs 95.1% in the chemotherapy-only arm, representing a 2.2% difference that excluded their noninferiority margin of 8%.28 Another strategy is the PET-adapted approach following the response-adjusted therapy for advanced Hodgkin lymphoma (RATHL) study, in which 40% of patients in both ABVD and AVD arms had stage II disease. The omission of bleomycin if interim PET-CT was negative (DS 1-3) after 2 cycles of ABVD did not affect outcomes, with a 3-year and a 7-year PFS in the AVD group of 84.4% and 79.2%, respectively.29,30 While the RAPID, EORTC H10, and GHSG HD16 trials all failed to show noninferiority in PFS with PET-adapted omission of RT compared with CMT, particularly in favorable patients, there was no OS benefit with inclusion of radiation. Alternatively, CALGB 50604 demonstrated an inferior PFS of 77% in patients treated with chemotherapy alone with a DS 3 on interim PET.
The outcomes of patients with poor response on interim PET (DS 4 or 5) are significantly worse, which represents an unmet need, and studies have explored intensification of therapy to improve response rates, such as changing ABVD to BEACOPP and adding RT as in the CALGB study. In the H10 study, patients with positive interim PET either received ABVD × 4 and 30 Gy INRT, and their 5-year PFS and OS were 77.4% and 89.3%, respectively or for patients who received ABVD × 2 followed by BEACOPP × 2 and 30 Gy INRT, 5-year PFS and OS were 90.6% and 96%, respectively.
The use of RT should be discussed with the patient and a multidisciplinary team highlighting the potential late effects specific to that particular patient and their inherent risk factors (i.e., a different discussion may take place for a young woman with stage IIB anatomically central disease as compared with a singular node in the periphery). While there is no OS benefit observed across these studies for patients with negative interim PET scans, consideration of toxicity and patient preference should guide treatment decisions. Particularly in early-stage HL, it is crucial to include nuclear medicine colleagues in the discussion because many trials used DS 1-2 as their PET-negative arms in contrast to clinical practice, where DS 1-3 is considered negative. Within the confines of the various trials and specific eligibility, for early-stage disease we recommend CMT with the above caveats. In particularly favorable disease, clinicians could consider the HD10 approach of ABVD × 2 plus 20 Gy IFRT, although given the ubiquitous use of PET2 imaging, we generally use PET results when presenting patients with therapy choices. In our practice, we do not escalate to BEACOPP in patients with a positive interim PET scan because of the potential risks of infertility and second primary malignancies with the regimen; instead, we repeat the biopsy to confirm disease and proceed to salvage chemoimmunotherapy followed by consolidative autologous stem cell transplant in chemosensitive disease, particularly given the impressive response rates with modern salvage regimens.31-33
Bulky disease
While patients with bulky mediastinal masses are generally treated with CMT, there are more studies that suggest that radiation can be eliminated in PET-negative patients without compromising outcomes. Older studies demonstrated that 4 cycles of combination chemotherapy with IFRT have improved local control.34-36 A large Canadian study omitted RT in patients with early-stage bulky HL who were PET negative (DS 1-3); 84% achieved PET negativity and did not receive radiation. In PET-negative patients with bulk (n = 112), 5-year freedom from treatment failure was 89% compared with 88.5% for PET-negative nonbulky disease (n = 152).37 CALGB 50801 treated 101 bulky stage I to II HL patients with 2 cycles of ABVD, and those with PET-negative (DS 1-3) disease received 4 additional cycles of ABVD and no radiation, while PET-positive patients received 4 cycles of escalated BEACOPP and 30 Gy involved-site RT (ISRT). In this study, 78% were PET negative with a 3-year PFS of 93.1% compared with 89.7% for PET-positive patients, and the majority of patients were spared of radiation exposure.38 Additionally, the RATHL study included 500 patients with bulky or high-risk stage II HL and demonstrated a 90.9% PFS in these patients if PET was negative after 6 cycles of ABVD.29 In practice, we generally prefer CMT for bulky disease based on the data available; however, it is also reasonable to omit radiation in patients with an interim negative PET scan after a multidisciplinary team meeting with radiation oncology colleagues for a discussion of risk vs benefit.
Older patients
Older patients, commonly defined as ≥60 years of age, historically have lower survival rates compared with younger patients, which may be due to a different disease biology, including increased incidence of mixed cellularity histology, Epstein-Barr virus–related, and advanced-stage disease.41 Additionally older patients are more likely to be unable to tolerate chemotherapy at full dose and schedule and have increased treatment-related toxicity (including bleomycin pulmonary toxicity) and mortality.42 Novel agents have been studied in older patients with early-stage disease, and given the toxicity profile and improvement in outcomes, our practice is generally to treat with sequential Bv-AVD in robust patients whereas we use Bv-AD, Bv-DTIC, immunotherapy in combination, or single agent in more frail patients.43-45 (Table 3)
Selected clinical trials incorporating Bv and immunotherapy in early-stage HL
| Trial . | Trial design . | Disease stage . | N . | Median follow-up . | Outcomes . | PFS . | OS . |
|---|---|---|---|---|---|---|---|
| Pembrolizumab followed by AVD51 | Pembro × 3 ⟶ AVD × 4-6 (4 cycles for early stage, 6 cycles for advanced-stage or early-stage bulky) | Stage I/II unfavorable Stage III/IV | 30 | 22.5 months | CMR 55% with pembro alone, but reached 100% after AVD × 2 | Median PFS not reached, 2-year PFS 100% | Median OS not reached, 2-year OS 100% |
| Nivolumab and AVD54 | Nivo-AVD × 4 plus 30 Gy ISRT Sequential therapy: nivo × 4 doses ⟶ nivo-AVD × 2 ⟶ AVD × 2 plus 30 Gy ISRT | Early-stage unfavorable | 109 | 13 months | CMR Group 1: 83% Group 2: CR 84% | 12-month PFS: Group 1: 100% Group 2: 98% | 12-month OS 100% in both groups |
| Bv-AVD vs ABVD, followed by 30 Gy INRT53 | Bv-AVD × 4 or ABVD × 4 ⟶ 30 Gy INRT | Early-stage unfavorable | 170 | 45 months | CMR Bv-AVD 86.7% ABVD 78.9% | 2-year PFS: 97.3% with Bv-AVD vs 92.6% with ABVD | Median not reached |
| Bv-AD45 | Bv - AD × 2 ⟶ PET 1. If PET neg, then Bv - AD × 2 (total 4) 2. If PET pos, then Bv - AD × 4 (total 6) | Non-bulky early-stage favorable or unfavorable | 34 | 53 months | CMR 97% | Estimated 5-year PFS of 91% | Estimated 5-year OS of 96% |
| Bv-AVD +/- RT (4 cohorts)52 | BV-AVD × 4 ⟶ PET If PET neg, then 1. 30 Gy ISRT 2. 20 Gy ISRT 3. 30 consolidation volume radiotherapy 4. No radiotherapy | Early-stage unfavorable | 117 | 45.6 months | CMR 1. 93% 2. 100% 3. 93% 4. 97% | Overall 2-year PFS 94% 2-year PFS for 4 cohorts 1. 93.1% 2. 97% 3. 90% 4. 97% | Overall 2-year OS 99.1% |
| ABVD followed by Bv consolidation56 | ABVD × 2 ⟶PET Favorable and PET neg, then BV consolidation Favorable and PET pos or unfavorable and PET neg, then ABVD × 2 plus BV consolidation Unfavorable and PET pos, then ABVD × 4 plus BV consolidation | Nonbulky early-stage favorable and unfavorable | 41 | 47 months | CMR 95% | 3-year PFS of 92% 3-year PFS 100% for PET neg after Bv | OS was 97% 3 year OS 100% for PET neg after Bv |
| Trials with novel agents including patients older than 60 | |||||||
| Bv followed by AVD followed by Bv consolidation43 | Bv × 2 ⟶ AVD × 6 ⟶ Bv × 4 | Stage II-IV (age 60 or older) | 48 (9 stage II) | 23 months | CMR 90% after Bv-AVD | 2-year PFS of 84% | 2-year OS of 93% |
| Bv57 | Bv monotherapy × 16 cycles with PET after 4 cycles | Unfit for chemo Stage IIB or bulky, stage III/IV | 38 (7 stage II) | 36 months | CMR 25.8% | Median PFS 7.3 months | Median OS 19.5 months |
| Bv- DTIC vs Bv - bendamustine44 | Bv-DTIC × 12 Bv-benda × 6* *closed early due to toxicity | Stages I-IV | 42 | 21.6 months | CMR Bv-DTIC 62% Bv-benda 88% | Median PFS 17.9 months | Not reached |
| Pembrolizumab followed by AVD51 | Pembro × 3 ⟶ AVD × 4-6 (4 cycles for early stage, 6 cycles for advanced-stage or early-stage bulky) | Stage I/II unfavorable Stage III/IV | 30 (4 pts >60) | 22.5 months | CMR 55% with pembro alone, but reached 100% after AVD × 2 | Median PFS not reached, 2-year PFS 100% | Median OS not reached, 2-year OS 100% |
| BV plus nivolumab58 | Bv-nivo every 21 days × 8 cycles | Stages I, II, III, IV, ≥60 years or <60 and unsuitable for standard chemo | 46 | 21.2 months | CMR 48% | Median PFS 18.3 months | Median OS not reached |
| Trial . | Trial design . | Disease stage . | N . | Median follow-up . | Outcomes . | PFS . | OS . |
|---|---|---|---|---|---|---|---|
| Pembrolizumab followed by AVD51 | Pembro × 3 ⟶ AVD × 4-6 (4 cycles for early stage, 6 cycles for advanced-stage or early-stage bulky) | Stage I/II unfavorable Stage III/IV | 30 | 22.5 months | CMR 55% with pembro alone, but reached 100% after AVD × 2 | Median PFS not reached, 2-year PFS 100% | Median OS not reached, 2-year OS 100% |
| Nivolumab and AVD54 | Nivo-AVD × 4 plus 30 Gy ISRT Sequential therapy: nivo × 4 doses ⟶ nivo-AVD × 2 ⟶ AVD × 2 plus 30 Gy ISRT | Early-stage unfavorable | 109 | 13 months | CMR Group 1: 83% Group 2: CR 84% | 12-month PFS: Group 1: 100% Group 2: 98% | 12-month OS 100% in both groups |
| Bv-AVD vs ABVD, followed by 30 Gy INRT53 | Bv-AVD × 4 or ABVD × 4 ⟶ 30 Gy INRT | Early-stage unfavorable | 170 | 45 months | CMR Bv-AVD 86.7% ABVD 78.9% | 2-year PFS: 97.3% with Bv-AVD vs 92.6% with ABVD | Median not reached |
| Bv-AD45 | Bv - AD × 2 ⟶ PET 1. If PET neg, then Bv - AD × 2 (total 4) 2. If PET pos, then Bv - AD × 4 (total 6) | Non-bulky early-stage favorable or unfavorable | 34 | 53 months | CMR 97% | Estimated 5-year PFS of 91% | Estimated 5-year OS of 96% |
| Bv-AVD +/- RT (4 cohorts)52 | BV-AVD × 4 ⟶ PET If PET neg, then 1. 30 Gy ISRT 2. 20 Gy ISRT 3. 30 consolidation volume radiotherapy 4. No radiotherapy | Early-stage unfavorable | 117 | 45.6 months | CMR 1. 93% 2. 100% 3. 93% 4. 97% | Overall 2-year PFS 94% 2-year PFS for 4 cohorts 1. 93.1% 2. 97% 3. 90% 4. 97% | Overall 2-year OS 99.1% |
| ABVD followed by Bv consolidation56 | ABVD × 2 ⟶PET Favorable and PET neg, then BV consolidation Favorable and PET pos or unfavorable and PET neg, then ABVD × 2 plus BV consolidation Unfavorable and PET pos, then ABVD × 4 plus BV consolidation | Nonbulky early-stage favorable and unfavorable | 41 | 47 months | CMR 95% | 3-year PFS of 92% 3-year PFS 100% for PET neg after Bv | OS was 97% 3 year OS 100% for PET neg after Bv |
| Trials with novel agents including patients older than 60 | |||||||
| Bv followed by AVD followed by Bv consolidation43 | Bv × 2 ⟶ AVD × 6 ⟶ Bv × 4 | Stage II-IV (age 60 or older) | 48 (9 stage II) | 23 months | CMR 90% after Bv-AVD | 2-year PFS of 84% | 2-year OS of 93% |
| Bv57 | Bv monotherapy × 16 cycles with PET after 4 cycles | Unfit for chemo Stage IIB or bulky, stage III/IV | 38 (7 stage II) | 36 months | CMR 25.8% | Median PFS 7.3 months | Median OS 19.5 months |
| Bv- DTIC vs Bv - bendamustine44 | Bv-DTIC × 12 Bv-benda × 6* *closed early due to toxicity | Stages I-IV | 42 | 21.6 months | CMR Bv-DTIC 62% Bv-benda 88% | Median PFS 17.9 months | Not reached |
| Pembrolizumab followed by AVD51 | Pembro × 3 ⟶ AVD × 4-6 (4 cycles for early stage, 6 cycles for advanced-stage or early-stage bulky) | Stage I/II unfavorable Stage III/IV | 30 (4 pts >60) | 22.5 months | CMR 55% with pembro alone, but reached 100% after AVD × 2 | Median PFS not reached, 2-year PFS 100% | Median OS not reached, 2-year OS 100% |
| BV plus nivolumab58 | Bv-nivo every 21 days × 8 cycles | Stages I, II, III, IV, ≥60 years or <60 and unsuitable for standard chemo | 46 | 21.2 months | CMR 48% | Median PFS 18.3 months | Median OS not reached |
ABVD, doxorubicin hydrochloride, bleomycin, vinblastine sulfate, dacarbazine (DTIC); Bv, brentuximab vedotin; CMR, complete metabolic response; INRT, involved nodal radiotherapy; ISRT, involved site radiotherapy; OS, overall survival; PFS, progression free survival.
The role of brentuximab vedotin and checkpoint inhibitors
Brentuximab vedotin (Bv) and checkpoint inhibitors (CPI) have revolutionized the treatment of relapsed HL, and they are increasingly incorporated in the frontline trials.46 Replacing bleomycin with Bv (CD30-directed monoclonal antibody) demonstrated improved PFS and OS in patients with advanced HL in the ECHELON-1 study and is considered the standard for advanced-stage HL.47,48 Moreover, results of a recent randomized phase 3 study combining nivolumab with AVD revealed improvement in PFS compared to Bv-AVD on interim analysis and is poised to become the new standard.49
Investigators have incorporated these agents in the treatment of early-stage HL with encouraging results (Table 3).50 Allen and colleagues conducted a single-arm phase 2 study with sequential administration of 3 cycles of pembrolizumab followed by AVD including unfavorable stage II and advanced-stage HL. The overall response rate after single agent pembrolizumab was 67% in early-stage disease, including 42% complete metabolic response but an impressive complete metabolic response (CMR) of 100% after AVD.51 GHSG conducted a randomized phase 2 study of nivolumab with AVD given either concomitantly or sequentially followed by consolidative RT with a dose of 30 Gy in early-stage unfavorable HL with excellent outcomes; PFS was 100% in the concomitant group and 98% in the sequential group.
Kumar et al published a multicenter phase 2 study with unfavorable stage I/II HL in which 117 patients received 4 cycles of Bv-AVD and, if PET negative, were assigned to one of four cohorts with different doses of consolidative RT, except cohort 4, which excluded RT. Two-year PFS ranged from 90% to 97%, and the addition of RT did not lead to further benefit.52 European investigators conducted a phase 2 study wherein patients with unfavorable early-stage HL were randomized to either Bv-AVD × 4 or standard ABVD × 4 and 30 Gy consolidative RT. The primary endpoint was the rate of negative PET after 2 cycles that was met (82% in Bv-AVD group vs 75% in ABVD group), while 2-year PFS was 97% with Bv-AVD and 93% with standard therapy.53 Collectively, these studies, though small with short follow-up, are very promising, although not without their own toxicity profile. Bv is generally well tolerated but with higher rates of peripheral neuropathy, and more febrile neutropenia/sepsis is observed when combined with chemotherapy. CPI have unique immune-mediated toxicities, including hypothyroidism, pneumonitis, and colitis.54,55 In our current practice, outside of unique situations, including older patients or those ineligible for standard chemotherapy, we do not treat patients with early-stage disease with novel agents off clinical trials.
Multiple trials investigating different combinations and strategies are currently ongoing, with details in Table 4. Of these, the North American study, which is a randomized phase 3 PET-adapted trial incorporating Bv, nivolumab, and/or RT in early- stage HL for children and adults, is particularly intriguing. It is planned to accrue 1875 patients from ages 5 to 60 with ABVD being the comparator arm to several experimental arms (NCT05675410).
Ongoing trials in early-stage HL incorporating novel agents
| Clinical trial . | Interventions . | Phase . | Anticipated enrollment . |
|---|---|---|---|
| NCT04685616 | ABVD vs Bv-AVD | III, randomized | 1042 |
| NCT05675410 | Bv-nivo vs standard ABVD +/- radiotherapy | III, randomized | 1875 |
| NCT05627115 | Tislelizumab +/- AVD and radiotherapy | II, single arm | 80 |
| NCT05900765 | Zimberelimab | II, single arm | 54 |
| NCT05404945 | Pembro plus Bv +/- AVD | II, multicohort | 44 |
| NCT04837859 | Tiselizumab +/- AVD and radiotherapy | II, single arm | 120 |
| NCT04866654 | ABVD +/- nivolumab | II, single arm | 160 |
| NCT03712202 | Nivolumab plus Bv vs ABVD plus nivolumab vs Bv-AVD plus nivolumab | II, randomized | 264 |
| Clinical trial . | Interventions . | Phase . | Anticipated enrollment . |
|---|---|---|---|
| NCT04685616 | ABVD vs Bv-AVD | III, randomized | 1042 |
| NCT05675410 | Bv-nivo vs standard ABVD +/- radiotherapy | III, randomized | 1875 |
| NCT05627115 | Tislelizumab +/- AVD and radiotherapy | II, single arm | 80 |
| NCT05900765 | Zimberelimab | II, single arm | 54 |
| NCT05404945 | Pembro plus Bv +/- AVD | II, multicohort | 44 |
| NCT04837859 | Tiselizumab +/- AVD and radiotherapy | II, single arm | 120 |
| NCT04866654 | ABVD +/- nivolumab | II, single arm | 160 |
| NCT03712202 | Nivolumab plus Bv vs ABVD plus nivolumab vs Bv-AVD plus nivolumab | II, randomized | 264 |
ABVD, doxorubicin hydrochloride, bleomycin, vinblastine sulfate, dacarbazine (DTIC); Bv, brentuximab vedotin.
Late toxicities and survivorship
While the majority of patients with early HL can be cured of their disease with the therapies discussed above, the long-term impact of these treatments can be significant. Late effects of cytotoxic therapies and radiation are well described and include cardiovascular disease, recurrent infections, second primary malignancies, endocrine dysfunction such as infertility, early menopause, and thyroid disorders. Cumulatively, this leads to HL patients having a 5.1-fold higher risk of death due to causes other than HL.59-65 Dores et al conducted a large study that included 20 000 patients treated from 2000 to 2016 and determined that heart disease, infections, and interstitial lung disease were leading causes of noncancer-related mortality.66
In a large study over four decades with 2000 patients, Van Nimwegen et al noted the 40-year cumulative risk of cardiovascular disease was 50% higher in patients treated before age 25. Mediastinal RT increased the risk of coronary disease, valvular heart disease, and cardiomyopathy, while anthracyclines increased the risks of valvular heart disease and cardiomyopathy. Moreover, combining mediastinal RT with anthracycline and/or smoking had additive risk.60 The study highlights the need for tools to predict late toxicities in HL survivors, and researchers have been developing models to answer these questions.67,68 De Vries et al in the Netherlands established and validated a risk prediction model for heart disease in HL survivors that includes age at HL diagnosis, sex, smoking status, RT, and anthracycline treatment as predictors for coronary heart disease and heart failure, based on a multicenter cohort of 1433 patients with a median follow-up of 24 years. These models can assist in identifying HL survivors who may need closer follow-up and more intensive screening.68 Recently, a double-blind randomized clinical trial conducted in lymphoma patients to receive atorvastatin vs placebo during anthracycline chemotherapy revealed that the odds of a 10% or greater decline in ejection fraction to a final value of less than 55% after anthracycline treatment was almost 3 times greater for participants randomized to placebo compared with those randomized to atorvastatin.69 Further trials are needed to assess if early interventions or better patient selection may decrease the long-term risk of adverse cardiac events. Although optimal screening strategies are unclear for cardiovascular disease, monitoring and aggressive management of cardiovascular risk factors, including smoking, hypertension, diabetes, and hyperlipidemia, are recommended, with consideration of a baseline stress test or echocardiogram at 10-year intervals from treatment and, additionally, a carotid ultrasound at 10-year intervals if there was exposure to neck radiation.70
Second primary malignancies continue to rise, even up to 40 years from initial diagnosis, and constitute a leading cause of mortality for HL survivors.71-73 Breast, lung, and gastrointestinal carcinomas as well as non-Hodgkin lymphoma and leukemia correlate with the use of radiation and alkylator chemotherapies, and studies demonstrate a dose dependent relationship between RT and risk of malignancy.74-76 Travis et al reported that the risk of developing breast cancer in patients treated with chest radiation before age 25 was as high as 29% by age 55.74 Increased risk of hematologic malignancies such as therapy-related acute myeloid leukemia and myelodysplastic syndrome are associated with regimen intensity as rates are lower with ABVD compared to the more intensive BEACOPP regimen.61,77 Finally, endocrinopathies and thyroid disease can develop in up to 50% of patients, especially those who received radiation to the neck.78 Infertility rates are significantly higher in patients exposed to BEACOPP compared to ABVD.62,79,80 While fertility preservation options have expanded and improved over time, ongoing focus is necessary to decrease the infertility risk associated with therapy.
While there is no consensus on screening, in practice, we follow NCCN guidelines: initiate mammograms at age 40 or 8 years post-therapy, whichever comes first; if chest or axillary radiation therapy was administered for patients assigned female at birth who received RT to the chest between ages 10-30 years old, then breast MRI in addition to mammography is recommended.70 HL survivors are often followed by PCPs are not aware of the resource of NCCN guidelines for surveillance.81 Emphasis should be on developing a collaborative space between patient care teams to convey the risk of late toxicities that may emerge 10 years or more from initial diagnosis and continue to persist for decades.
CLINICAL CASE (continued)
Our patient was started on treatment with ABVD and interim PET CT and after 2 cycles showed complete metabolic response (Deauville score of 2). She was offered two options: either 4 additional cycles of AVD without bleomycin (RATHL approach) or two more cycles of ABVD followed by consolidative RT up to 30 Gy. The patient decided to go with the additional 2 cycles and then RT and was counseled heavily on late toxicities from mediastinal RT.
Conclusions
Patients with early-stage HL continue to have excellent outcomes with time-limited chemotherapy or chemoradiotherapy. We recommend a risk-adapted approach to therapy using PET imaging and consider PET to be negative if the Deauville score is 1-3. If patient develops a score of 5 while on or after treatment, we advocate for a repeat biopsy to verify disease recurrence/ progression. For patients with favorable disease and negative interim PET, we favor omitting radiotherapy in cases where the field includes breast or cardiac tissue. We recommend consultation with radiation oncology in patients with unfavorable or bulky disease to discuss the risk vs benefit of radiotherapy. Incorporating newer agents like Bv and CPI may avoid prolonged chemotherapy and/or radiotherapy and potentially the need for treatment intensification. Larger randomized studies are needed, however, before recommending practice change for early-stage disease. Finally, patient survivorship is crucial. A focus on late effects such as risk of cardiovascular disease, second primary malignancies, and endocrine dysfunction is essential, and all patients should have a fertility preservation consultation, if possible, before starting therapy.
Conflict-of-interest disclosure
Taha Al-Juhaishi: no competing financial interests to declare.
Sairah Ahmed: research support (to institution) for clinical trials: Seattle Genetics, Merck, Xencor, Chimagen, and Tessa Therapeutics; scientific advisory committee: Tessa Therapeutics, Chimagen; data safety monitoring board: Myeloid Therapeutics; consultancy: ADC therapeutics, KITE/Gilead.
Off-label drug use
Taha Al-Juhaishi: nothing to disclose.
Sairah Ahmed: nothing to disclose.