Abstract
Healthy volunteer donors are committed to contributing key medical resources. Repeated, regular donation of whole blood represents a specific trigger of hematopoietic stress. Hematopoietic stem cells (HSCs) are known to respond to environmental triggers by altering their differentiation and/or proliferative behavior. This can manifest in long-term changes in the clonal dynamics of HSCs, such as the age-associated expansion of HSCs carrying somatic mutations in genes associated with hematologic cancers—that is, clonal hematopoiesis (CH). A recent study revealed a higher prevalence of CH in frequent donors driven by low-risk mutations in genes encoding for epigenetic modifiers, with DNMT3A and TET2 being the most common. No difference in the prevalence of known preleukemic driver mutations was detected between the cohorts, underscoring the safety of repetitive blood donations. Functional analyses suggest a link between the presence of selected DNMT3A mutations found in the frequent donor group and the responsiveness of the cells to the molecular mediator of bleeding stress, erythropoietin (EPO), but not inflammation. These findings define EPO as one of the environmental factors that provide a fitness advantage to specific mutant HSCs. Analyzing CH prevalence and characteristics in other donor cohorts will be important to comprehensively assess the health risks associated with the different types of donation.
Learning Objectives
Discuss the long-term effects and safety of large-volume phlebotomy in healthy individuals
Review adaptive clonal hematopoiesis in the context of erythropoietic stress
CLINICAL CASE
A 57-year-old man who has been a volunteer blood donor (ID:101X) at the German Red Cross Blood Donor Service for the past 35 years and given a total of 116 donations came in for his third whole-blood donation in 2022. His hemoglobin (Hb) was 15.8 g/dL, and his standard donor questionnaire did not raise any concerns. Donor 101X has no history of cardiovascular disease. Before proceeding with the donation, he asked to speak to a physician to discuss his family's medical condition. His older brother (aged 64) had been diagnosed with acute myeloid leukemia (AML) a month earlier. Donor 101X and his younger brother (aged 55) had undergone screening as potential stem cell donors for a matched related allogeneic hematopoietic stem cell transplant (alloHSCT). HLA typing revealed both healthy brothers to be a full match. Donor 101X was in an overall better health condition compared to the younger brother based on the general assessment. Interestingly, the transplant center at which donor 101X's brother was scheduled for the procedure had implemented mutational analysis as part of the stem cell donor workup a few years prior. A targeted sequencing panel covering 49 genes recurrently mutated in myeloid neoplasms was used. Donor 101X was found to carry 2 mutations in the gene encoding for the enzyme DNA methyltransferase 3A (DNMT3A), corresponding to 2 hematopoietic clones, a small population representing 2% of the cells and a larger one comprising 6% of the cells. This finding prompted the treating transplant physician to defer the blood donor and choose the younger brother instead as the stem cell donor. Donor 101X learned about the concerns associated with the engraftment of (his) mutant HSCs, such as a higher risk for the cells to expand after the transplant and to develop into donor-derived leukemia, as well as their hyperinflammatory profile and therefore higher risk of causing graft-versus-host disease. However, he was given no information about the potential personal consequences of being a DNMT3A mutation carrier. He was wondering about the origin of the mutations and their pathogenic potential, whether external factors including his extensive history of whole-blood donation may have contributed to the emergence of the clones, whether blood donation was safe for him as well as the recipients, and whether he should take any measures in the future to avoid the acquisition of additional clones as well as the current ones developing into leukemia.
Clonal hematopoiesis and HSC turnover
As we age, hematopoietic stem cells accumulate mutations.1 The older an individual, the more cycling an HSC has undergone and hence the higher the likelihood of having acquired a driver mutation that changes the cellular phenotype.1 The bone marrow is an inherently competitive environment, as the space is limited. If a mutant HSC turns out to be “fitter” than the others, it can clonally expand. Clonal hematopoiesis (CH) refers to an age-associated overrepresentation of HSCs carrying certain genetic aberrations within the healthy blood system.2-4 That is, instead of 50 000 HSCs contributing to healthy hematopoiesis, selected clones of mutated HSCs and their progeny contribute disproportionally and thus become detectable by sequencing.2-4 Originally, the sequencing techniques used required a variant allele fraction of at least 2% for reliable detection of a clone. Technical advances have lowered the detection limit down to 0.01%, although the biological significance of hematopoietic clones that small remains to be determined.5
The competitive advantage of a mutation can be apparent at a steady state, for example, due to improved self-renewal and/or become more pronounced under specific conditions, such as exposure to cytotoxic signals, growth factors, proinflammatory stimuli, and so on. Depending on the type of selection pressure, the advantages of particular types of mutations have been demonstrated; mutations in genes involved in DNA damage response show superior fitness in the context of cytotoxic therapy.6-8 Mutations in DNMT3A are acquired very early in life and are by far the most common in older healthy individuals.2-4 They typically have a slow rate of expansion9,10 ; however, an altered responsiveness of DNMT3A-mutant cells has been demonstrated under inflammatory conditions.11 On the molecular level, aging-induced tumor necrosis factor-α as well as inflammation-associated interferon and IL-6 have been demonstrated to selectively promote the growth of DNMT3A mutant vs wild-type cells.11,12
Clonal hematopoiesis and disease risks
CH clones driven by certain mutations have been demonstrated to have a high likelihood of undergoing subsequent leukemic transformation.13-15 Hence, individuals with CH show an increased risk of developing hematologic malignancies.2,4 Moreover, the pathologic conditions associated with CH are not limited to primarily hematopoietic diseases. Thus, CH has been linked to an increased risk and severity of cardiovascular and chronic obstructive pulmonary disease,16,17 gout,18 chronic liver disease,19 and many more. Additionally, as the presence of CH is increasingly recognized as a likely rather than aberrant path of HSC development, conditions in which it can benefit patients' outcomes have been identified, such as Alzheimer's disease and in fact matched related alloHSCT.20-22
The acquisition of CH mutations is not associated with immediate changes in the differentiation potential of the HSCs and is very difficult to identify by standard laboratory analysis until close to the diagnosis of malignancy.13,14 Significant effort has been dedicated to devising a scoring system to help predict the risk of malignant transformation in CH-positive individuals and allow for an early intervention.13-15,23 Genes encoding for splicing factors (SRSF2, SF3B1, U2AF1, ZRSR2) and the AML-associated genes IDH1/2 and RUNX1 as well as JAK2 and TP53 have been identified as high-risk genes as opposed to low-risk genes, which include the epigenetic modifiers DNMT3A and TET2. Association with the different malignant progression risk groups, defined based on genetic and laboratory parameters, was shown to also correlate with nonhematologic CH comorbidities—in particular, cardiovascular events.23 This is remarkable since the pathophysiology of the latter has been best described for TET2-mutant CH, whereas TET2 mutations were not included in the risk-stratification as a separate variable.
Whole-blood donation
Fewer than 5% of all eligible donors contribute over 90% of all blood products worldwide. As a result, instead of donating once every 10 years, committed healthy individuals donate several times per year to meet the steadily increasing demand for these lifesaving resources. Most of the studies of long-term outcomes in frequent blood donors have focused on the consequences of iron depletion for donor safety.24,25 Only one recent study assessed the incidence of hematologic malignancies in frequent blood donors and reported a subtle decrease in AML risk.26
Whole-blood donation of 500 mL corresponds to a loss of approximately 10% to 15% of the total blood volume. This represents a considerable erythropoietic stress that manifests in short term as well as more protracted changes in the blood parameters.24,27 Thus, about 2-fold elevated levels of the hematopoietic cytokine erythropoietin (EPO), produced by the kidneys following blood donation to replenish the lost cells, have been detected up to 56 days after a whole-blood donation and could conceivably have an impact on HSC dynamics.28
Frequent blood donation as a model of erythropoietic stress
Repeated large-volume phlebotomy represents a unique model to study the impact of erythropoietic stress on the clonal dynamics of the hematopoietic system. By contrast, investigations performed in pathologic erythropoietic stress conditions, in particular anemia (sickle cell disease,29,30 acquired aplastic anemia31 ), are confounded by multiple systemic characteristics, including increased inflammation, autoimmune selection, multisystem organ damage, and so on, as well as long-term, systemic medications.
In the first—and so far, only—comprehensive assessment of the long-term effects of frequent whole-blood donations,32 a cohort of healthy male blood donors (median age 66 years, 120 lifetime donations, 3.2 donations per year every 114.5 days over the past 3 or 4 decades; n = 105) was compared to age-matched male sporadic blood donors (median age 63 years, 5 lifetime donations; n = 103). The prevalence and spectrum of CH was assessed with error-corrected sequencing using a broad panel (141 genes) of genes associated with hematologic malignancies (Figure 1).
Effects of large-volume phlebotomy on clonal hematopoiesis. Study setup and analysis performed in blood donors. CRISPR, clustered regularly interspaced short palindromic repeats; HDR, homology directed repair; PBMC, peripheral blood mononuclear cells. Figure created using BioRender.32
Effects of large-volume phlebotomy on clonal hematopoiesis. Study setup and analysis performed in blood donors. CRISPR, clustered regularly interspaced short palindromic repeats; HDR, homology directed repair; PBMC, peripheral blood mononuclear cells. Figure created using BioRender.32
The prevalence of CH was not significantly different between frequent and control donors (54.2% vs 39.8% and 32.4% vs 20.3% in frequent vs control donors using a detection limit of 0.5% and 2%, respectively) and well within the range of mathematical modeling–based predictions for a given age and sequencing detection range.33
Furthermore, the spectrum of drivers was similar in that, in agreement with all CH studies in individuals without hematologic malignancies published to date,2-4,13,14 DNMT3A and TET2 were by far the 2 most commonly mutated genes in both cohorts (Table 1). However, frequent donors showed an overall significant increase in CH driven by mutations in genes encoding for epigenetic modifiers (44.7% vs 22.3%), including DNMT3A and TET2.
Spectrum of mutations detected in blood donors
| Cohort . | Frequent donor . | Control donor . |
|---|---|---|
| Characteristics | n = 105, median age, 66 y Median # donations, 120 | n = 103, median age, 63 y Median # donations, 5 |
| Low-risk mutations13 | DNMT3A (34)a TET2 (14) KRAS (2) CBL (1) ASXL1 (1) | DNMT3A (23)a TET2 (7) CBL (1) ASXL1 (1) |
| High-risk mutations23 | RUNX1 (1) SRSF2 (1) | RUNX1 (1) U2AF1 (1) |
| Unclear risk mutations34 | KMT2C (5), MPL (3), SH2B3 (2), SF3B1 (2)b, BCR (2), EED (2), and 1 each of SMC1A, TERT, WAS, HNRNPK, JAK3, ATM, ASXL2, STAT3, LRRC4, KAT6A, KDM6A, IKZF1, FAM154B, EP300, ELANE, DNMT1, CTCF, CRLF2, and BRINP3 | SMC1A (2), HNRNPK (2), FAM47A (2), DNM2 (2), BRCA2 (2), and 1 each of KMT2C, SH2B3, SF3B1b, BCR, WAS, TERT, JAK3, ATM, ASXL2, TAL1, SETBP1, NPAT, NOTCH1, IL7R, GJB3, FIP1L1, DDX41, CUX1, CHEK2, BRCA1, ANKRD26, and ABL1 |
| Cohort . | Frequent donor . | Control donor . |
|---|---|---|
| Characteristics | n = 105, median age, 66 y Median # donations, 120 | n = 103, median age, 63 y Median # donations, 5 |
| Low-risk mutations13 | DNMT3A (34)a TET2 (14) KRAS (2) CBL (1) ASXL1 (1) | DNMT3A (23)a TET2 (7) CBL (1) ASXL1 (1) |
| High-risk mutations23 | RUNX1 (1) SRSF2 (1) | RUNX1 (1) U2AF1 (1) |
| Unclear risk mutations34 | KMT2C (5), MPL (3), SH2B3 (2), SF3B1 (2)b, BCR (2), EED (2), and 1 each of SMC1A, TERT, WAS, HNRNPK, JAK3, ATM, ASXL2, STAT3, LRRC4, KAT6A, KDM6A, IKZF1, FAM154B, EP300, ELANE, DNMT1, CTCF, CRLF2, and BRINP3 | SMC1A (2), HNRNPK (2), FAM47A (2), DNM2 (2), BRCA2 (2), and 1 each of KMT2C, SH2B3, SF3B1b, BCR, WAS, TERT, JAK3, ATM, ASXL2, TAL1, SETBP1, NPAT, NOTCH1, IL7R, GJB3, FIP1L1, DDX41, CUX1, CHEK2, BRCA1, ANKRD26, and ABL1 |
Including 3 × and 2 × R882 mutations in frequent and control donors, respectively.
Assigned as unclear risk mutation due to controversial predictions in the literature.
Data compiled from Karpova et al.32
In the context of malignant transformation, mutations in epigenetic modifiers represent the so-called very early events that do not cause immediate, stark changes in the proliferative behavior or in the differentiation potential of the cells. Accordingly, while many CH mutations have been directly linked to hematologic malignancies, with the exception of IDH1/2, epigenetic modifier mutations were associated with a low hazard ratio when the risk of malignant transformation vs persistence in the form of a CH clone was assessed in large cohorts.13,14,23 Moreover, as mentioned above, mutations in DNMT3A alone display a markedly benign profile—that is, a low risk of myeloid neoplasms compared to other CH genotypes.13,14,23
Adaptive hematopoiesis in frequent blood donors
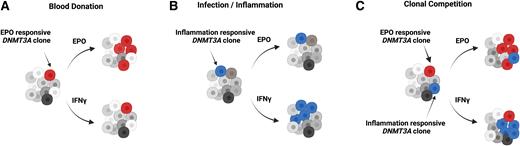
In addition to the differences in the overall characteristics of CH between frequent donors and controls, the type and distribution of DNMT3A mutations also differed between the groups (Figure 1).32 When selected mutations were analyzed functionally (Figure 1), 3 out of 3 DNMT3A mutations chosen from the frequent donors showed responsiveness toward EPO but not to inflammatory stimuli. This is in striking contrast to the proliferative behavior of the well-known preleukemic DNMT3A mutations, R882H and R882C, that expand under inflammatory conditions but not with EPO (Figure 2).11,32 Importantly, there was no difference in the incidence of R882 DNMT3A mutations between frequent and control donors.
Clonal dynamics of human hematopoiesis under different conditions. Figure created using BioRender.
Clonal dynamics of human hematopoiesis under different conditions. Figure created using BioRender.
This observation implies several new and important insights. First, EPO is identified as a novel factor shaping the clonal dynamics of the human hematopoietic system at the level of the HSCs. Second, it defines a new class of DNMT3A mutations that promote a proliferative advantage to HSCs in EPO-rich as opposed to inflammatory environments known to favor preleukemic R882 hits. And third, it suggests that in frequent blood donors, in addition to the spectrum of age-associated mutations, CH is likely driven by mutations with a competitive advantage in EPO-rich environments. Given that the acquisition and accumulation of mutations in HSCs is an inevitable process, it is tempting to speculate that repeated large-volume phlebotomy favors the benign, lower-risk mutations such as the EPO-responsive DNMT3A variants.
Blood donation is safe
Overall, the assessment of clonal dynamics in individuals with a lifelong blood donation history confirmed that blood donation is safe. Even decades of donating whole blood several times per year do not result in a significant overall transformation of the clonal hematopoietic composition. An increase in CH driven by mutations in genes encoding for epigenetic modifiers was observed in frequent compared to control donors. Given the mild pathophysiological profile of these mutations, it is unlikely these outgrowths will lead to myeloid malignancy over a lifetime. Moreover, no increase in CH driven by established preleukemic hits is found in frequent blood donors. With regard to nonhematologic comorbidities, detailed risk-assessment studies assigning CH-positive individuals into high- vs low-risk categories have not yet been performed. Despite the overall CH prevalence and mutational spectrum being well in agreement with mathematical models and previously published cohorts,2,23,33 the link between CH and cardiovascular pathologies remains to be verified in frequent blood donors, a cohort preselected for exclusively healthy individuals.
In the specific case of donor 101X, both the presence of 2 (most likely independent) DNMT3A clones as well as the fact that neither of the clones is driven by an R882 mutation is in line with what one would expect for a blood donor with a decades-long history of regular whole-blood donations in general and given the low frequency of R882 mutations (only 10%) found in blood donors in particular.32 As a relative of a patient with a myeloid malignancy, donor 101X is more likely to be a myeloid CH carrier,21 yet the acquisition of DNMT3A mutations per se must have occurred in his 30s and was not triggered by regular blood donation.9,10
Naturally, the presence of DNMT3A-mutant CH does not preclude donor 101X from further donation since (as expected) no blood count abnormalities (signs of cytopenia, aberrant red blood cell parameters, low Hb) have ever been detected in the donor. Moreover, if he continues to donate, his blood parameters can and should be monitored closely as changes in Hb, hematocrit, mean corpuscular volume, and red-cell distribution width represent very early phenotypic abnormalities that occur in individuals with CH years prior to developing myeloid neoplasms.13 Additionally, clonal composition can be assessed via sequencing at intervals of 2 to 3 years. Preleukemic clones have been shown to have markedly augmented growth kinetics; that is, they grow quickly and continuously as compared to benign CH.13-15,33 By contrast, DNMT3A-driven clones show very slow growth kinetics compared to other CH drivers,13,14 consistent with their benign profile.23 If the hypothesis is true and the donor's EPO-responsive clones indeed emerge and persist at the expense of preleukemic ones, one would predict the 2 DNMT3A clones to remain at a stable size within the next decades. Lastly, the decision of the treating physician to exclude donor 101X as a stem cell donor, primarily due to the concern that the CH clones would undergo disproportional expansion in the recipient (as has been shown for DNMT3A-mutant CH21,22 ) was debatable. In the HLA-matched related alloHSCT setting, AML/myelodysplastic syndrome patients who received DNMT3A CH-positive grafts have been demonstrated to have a lower risk of relapse/disease progression due to higher rates of chronic graft-versus-host disease, in particular when transplantation was performed in a noncomplete remission state.21 Moreover, low rates of donor-derived myelodysplastic syndrome/AML in recipients of DNMT3A mutant grafts have been reported.23
Outlook
Healthy volunteer donors, including whole-blood, platelet, and stem cell donors, selflessly provide lifesaving resources. A recent study of whole-blood donors with a lifelong history of regular donations is a first comprehensive sequencing-based analysis of the clonal dynamics in this group of donors. The marks of continued significant erythropoietic stress were apparent in the form of the increased prevalence of CH driven by epigenetic modifier mutations. However, this finding does not suggest an elevated risk of developing myeloid neoplasms in frequent blood donors as the detected CH clones, especially those driven by DNMT3A mutations, are likely benign. While the suggested direct effect of EPO on HSPC dynamics is novel, the role of another hematopoietic cytokine, thrombopoietin, in HSC biology is well documented.36 Furthermore, platelet donation is associated with a significant increase in thrombopoietin levels,37 whereas the maximum total number of platelet donations (up to 24) is 6 times higher compared to whole-blood donations.38 Frequent platelet donors therefore represent the next obvious donor cohort to be analyzed with regard to CH prevalence and characteristics. Though clinical actionability in the context of CH detection remains a controversial topic, recent analysis of big cohorts, including the invaluable UK biobank resource, have further advanced our interpretation of risks linked to mutations in specific genes. The assessment of CH is critical for donor safety and helps provide insight into the long-term effects of certain types of selection pressure in humans without any confounding pathologic conditions.
Acknowledgments
I would like to thank Dr. Elisa Donato, Dr. Dilan Patel, and Dr. Terrence N. Wong for revising the manuscript and providing helpful comments and discussion. Also, I kindly thank Dr. Silvia Calderazzo for her help with statistical analysis.
Conflict-of-interest disclosure
Darja Karpova: no competing financial interests to declare.
Off-label drug use
Darja Karpova: nothing to disclose.