Abstract
Intensive chemotherapy in combination with allogeneic hematopoietic cell transplantation and supportive care can induce long-term remissions in around 50% of acute myeloid leukemia patients eligible for intensive treatment. Several treatment optimization trials helped to refine schedule and dosing of the historic “7 + 3” combination. Together with the addition of novel agents, increased efficacy and tolerability led to improved long-term outcomes. Unsatisfactory outcomes in fit elderly patients and unfavorable genetic subgroups have raised the question of whether less-intensive venetoclax-based approaches may be beneficial as an alternative. Although tempting and worth exploring, this issue will remain controversial until the results of randomized comparisons appear. To date, intensive chemotherapy remains the only evident curative treatment option for long-term disease eradication in a fixed treatment time. With the advent of more novel agents and advances in minimal residual disease (MRD) detection and maintenance approaches, the face of intensive treatment could change in many ways. Several are being explored in clinical trials, such as (1) combinations of more than 1 novel agent with the intensive backbone, (2) head-to-head comparisons of novel agents, (3) replacement or dose reduction of cytotoxic components such as anthracyclines, and (4) MRD-guided escalation and de-escalation strategies. The combination of intensive treatment with individualized tailored innovative strategies will most certainly reduce treatment-related toxicities and increase the chances for long-term remission in the future.
Learning Objectives
Outline the development and describe current standards of intensive chemotherapy in AML
Compare strengths and shortcomings of intensive induction versus nonintensive approaches
Explain novel approaches and sketch future scenarios for intensive AML treatment
CLINICAL CASE 1
After 2 weeks of progressive weakness, a 67-year-old woman notices recurring nose bleeds and consults her general practitioner. She has well-controlled arterial hypertension and is slightly obese. In addition to general paleness and petechiae around the ankles, the lab result reveals pancytopenia. After referral to a hematologist, bone marrow assessment shows 45% myeloid blasts, resulting in the diagnosis of acute myeloid leukemia (AML) according to both World Health Organization (WHO) and International Consensus Classification (ICC) criteria. What to do next?
CLINICAL CASE 2
A 70-year-old man seeks medical advice in the emergency department for fever and shortness of breath that did not get better despite over-the-counter self-medication. He has a slight leukocytosis with 25% atypical immature cells, anemia, and thrombocytopenia. Diagnostic workup shows an AML. Our patient has only one kidney due to congenital unilateral renal agenesis but is otherwise healthy. He is an active, well-informed pensioner who enjoys working in his garden and spending lots of time doing sports with his grandchildren. How shall we advise him?
Let's first look at the reasons why this woman and this man should be treated with intensive chemotherapy before we move on to the optimal regimen and potential study options.
Why should we use intensive chemo for fit patients?
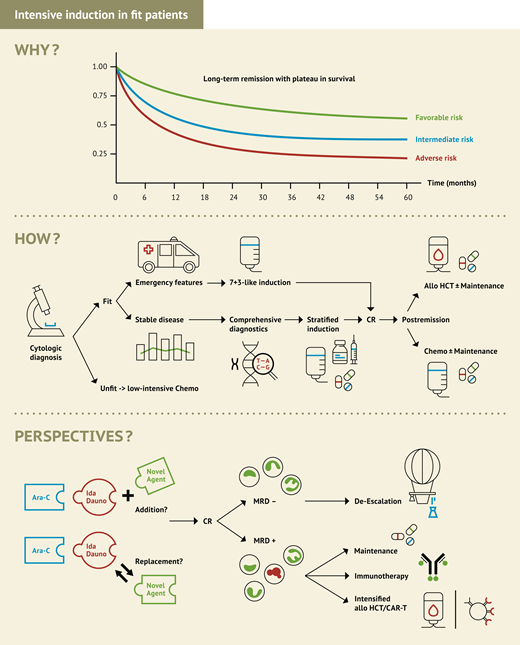
Before the 1960s with no effective cytoreductive treatment options, our patients would have to face a life expectancy of only around 2 to 4 months as shown from historic reports1 or recent outcomes in elderly patients treated with best supportive care only.2,3 A couple of decades ago, the advent of intensive chemotherapy led to a significant proportion of AML patients being cured. In the late 1960s, cytarabine and daunorubicin were identified as most effective to induce complete remissions even as single agents.4,5 Since the establishment of their combination in the 7 + 3 schedule in the 1980s,6 cure rates and life expectancy in patients fit for intensive treatment have continued to go up, mainly based on significant progress in supportive care and allogeneic hematopoietic cell transplant (HCT) technology, but also based on treatment optimization in the use of the cytostatic components (Figure 1). Recent data from clinical trials in younger fit AML patients across genetic subgroups show long-term remission rates around 60%, whereas 50% long-term remission can be achieved in combination with allogeneic HCT even in elderly fit patients with secondary AML.7,8 Therefore, based on high evidence from thousands of treated patients, intensive chemotherapy is the most certain way to cure.
Overall survival in relation to age and period of treatment. Data based on clinical trials of the Study Alliance Leukemia Group (SAL) and the SAL AML registry.
Overall survival in relation to age and period of treatment. Data based on clinical trials of the Study Alliance Leukemia Group (SAL) and the SAL AML registry.
Whereas treatment is more manageable and delivers better results in younger patients, increased toxicity and fewer long-term remissions with intensive chemo in fit elderly patients led to an ongoing debate about its value. However, numerous arguments support the intensive approach also in this patient group.
There is no alternative treatment achieving better long-term remission results than intensive chemo in first-line treatment.
Depending on genetic risk, more than 50% of elderly patients can be cured with a combination of intensive induction and postremission treatment including allogeneic HCT.9-11
Disease eradication and prognostically relevant MRD reduction is quickly and profoundly achieved after 1 to 2 cycles of intensive induction with 60% to 80% of patients becoming MRD negative, providing a stronger disease control before postremission treatment or allogeneic HCT.12-16
Early mortality rates in intensively treated elderly AML patients have continually decreased throughout the past decades due to better supportive care.17
Intensive chemotherapy is generally a time-limited treatment, ie, patients will become treatment-free within a few months.
Based on these arguments, I would recommend intensive induction therapy to our case patients. Although there is no general consented definition of fitness, our two patients would be considered fit according to all existing sets of criteria, which generally incorporate performance status, adequate end-organ function, age, and geriatric assessment. Assuming patients or family had a medical background, we may anticipate several questions regarding clonal disease evolution under cytostatic drugs and the alternative use of venetoclax plus hypomethylating agents (HMA). However, despite preclinical data supporting clonal evolution and selection processes,18 its clinical impact on long-term outcomes and the need for continuous treatment is less clear. And although the combination of venetoclax plus HMA means an outstanding leap forward for patients who cannot tolerate intensive chemotherapy, its capacity to achieve cure or even superiority over intensive induction has not been demonstrated in prospective randomized comparisons in fit patients. Retrospective analyses are prone to relevant issues around selection bias and statistical matching methodology, leading to mixed and partly contradictory results.
Therefore, while venetoclax will certainly play a role in the future of AML treatment, possibly also in fit patients in the context of combination approaches, for the time being, the value of HMA plus venetoclax compared to intensive treatment in fit patients has not been shown convincingly.
Several clinical trials explore the role of HMA plus venetoclax compared with standard intensive chemotherapy, as shown in Table 1. In addition, the alternative use of venetoclax not instead of but with intensive chemo is being explored in clinical trials (see section “The future of intensive chemotherapy in AML” and Table 2).
Selection of RCTs comparing intensive chemotherapy with venetoclax-based nonintensive treatment in newly diagnosed fit patients
| Target population/AML subgroup* . | Age (years) . | Experimental agent/intervention . | Experimental arm . | Control arm . | Primary endpoint† . | Planned patient number . | Name PI, country (cooperative group) . | Registry number . |
|---|---|---|---|---|---|---|---|---|
| • All comers • No CBF • No NPM1 mut in <60 y • No FLT3-ITD or –TKD | ≥18 | Venetoclax + azacitidine | Venetoclax + azacitidine | 7 + 3‡ or CPX-351 | EFS | 172 | Fathi, USA | NCT04801797 |
| • NPM1 mut • No FLT3-ITD | ≥60 or relevant comorbidity | Venetoclax + LDAC | Venetoclax + LDAC | DA+GO | Modified EFS | 186 | Dillon, UK (NCRI) | EudraCT 2020-000273-24, ISRCTN 15567173 |
| • NPM1 mut • No FLT3-ITD | 18-70 | Venetoclax + azacitidine | Venetoclax + azacitidine | DA + GO | Modified EFS | 146 | Röllig, Germany (SAL-AMLCG) | EudraCT 2021-00348-26, NCT05904106 |
| • Adverse risk (ELN2017) • No FLT3-ITD or –TKD • No t(9;22) | 18-59 | 7 + 3/CPX-351 + venetoclax or azacitidine + venetoclax | 7 + 3/CPX-351 + venetoclax or azacitidine + venetoclax | 7 + 3 or CPX-351 | MRD after induction | 268 | Shami/NCI, USA, Canada (SWOG) | NCT05554406 |
| • Intermediate risk • No FLT3-ITD or -TKD | 18-59 | 7 + 3 + venetoclax or azacitidine + venetoclax | 7 + 3 + venetoclax or azacitidine + venetoclax | 7 + 3 | MRD after induction | 153 | Savoie/NCI, Canada, USA (CCTG) | NCT05554393 |
| • All comers | 18-59 | Venetoclax | Venetoclax + decitabine | 7 + 3 | ORR§ | 188 | Suning, China | NCT05177731 |
| • TP53 mut | ≥18 | Magrolimab | Magrolimab + azacitidine | Venetoclax + azacitidine or 7 + 3 | OS | 356 | Gilead, USA | NCT04778397 |
| • Adverse risk, intermediate risk, and 50-70 y • No FLT3-ITD | 18-70 | Magrolimab | Magrolimab + venetoclax + azacitidine | DA, DA + GO, CPX-351, or FLAG-Ida | EFS | 164 | Craddock, UK (NCRI) | ISRCTN71474257 |
| Target population/AML subgroup* . | Age (years) . | Experimental agent/intervention . | Experimental arm . | Control arm . | Primary endpoint† . | Planned patient number . | Name PI, country (cooperative group) . | Registry number . |
|---|---|---|---|---|---|---|---|---|
| • All comers • No CBF • No NPM1 mut in <60 y • No FLT3-ITD or –TKD | ≥18 | Venetoclax + azacitidine | Venetoclax + azacitidine | 7 + 3‡ or CPX-351 | EFS | 172 | Fathi, USA | NCT04801797 |
| • NPM1 mut • No FLT3-ITD | ≥60 or relevant comorbidity | Venetoclax + LDAC | Venetoclax + LDAC | DA+GO | Modified EFS | 186 | Dillon, UK (NCRI) | EudraCT 2020-000273-24, ISRCTN 15567173 |
| • NPM1 mut • No FLT3-ITD | 18-70 | Venetoclax + azacitidine | Venetoclax + azacitidine | DA + GO | Modified EFS | 146 | Röllig, Germany (SAL-AMLCG) | EudraCT 2021-00348-26, NCT05904106 |
| • Adverse risk (ELN2017) • No FLT3-ITD or –TKD • No t(9;22) | 18-59 | 7 + 3/CPX-351 + venetoclax or azacitidine + venetoclax | 7 + 3/CPX-351 + venetoclax or azacitidine + venetoclax | 7 + 3 or CPX-351 | MRD after induction | 268 | Shami/NCI, USA, Canada (SWOG) | NCT05554406 |
| • Intermediate risk • No FLT3-ITD or -TKD | 18-59 | 7 + 3 + venetoclax or azacitidine + venetoclax | 7 + 3 + venetoclax or azacitidine + venetoclax | 7 + 3 | MRD after induction | 153 | Savoie/NCI, Canada, USA (CCTG) | NCT05554393 |
| • All comers | 18-59 | Venetoclax | Venetoclax + decitabine | 7 + 3 | ORR§ | 188 | Suning, China | NCT05177731 |
| • TP53 mut | ≥18 | Magrolimab | Magrolimab + azacitidine | Venetoclax + azacitidine or 7 + 3 | OS | 356 | Gilead, USA | NCT04778397 |
| • Adverse risk, intermediate risk, and 50-70 y • No FLT3-ITD | 18-70 | Magrolimab | Magrolimab + venetoclax + azacitidine | DA, DA + GO, CPX-351, or FLAG-Ida | EFS | 164 | Craddock, UK (NCRI) | ISRCTN71474257 |
Trials for unfit patients, children, APL, relapsed or refractory disease and with purely maintenance or conditioning questions were excluded.
CBF, core binding factor; CRi, complete hematologic remission with incomplete hematologic recovery; DA, daunorubicin plus cytarabine (ara-c); EFS, event-free survival; GO, gemtuzumab ozogamicin; LDAC, low-dose cytarabine; MRD, measurable residual disease; mut, mutation; OS, overall survival; PI, principal investigator.
sAML/tAML/HMA pretreatment not accounted for/mentioned in table. †Secondary end points for all trials include response rates, MRD, tolerability, rate of allogeneic HCT, patient-reported outcomes, and survival end points. ‡“7 + 3” stands for all variations of standard-dose cytarabine plus anthracycline/mitoxantrone and includes intensive consolidation for patients ineligible for allogeneic HCT. §ORR, overall response rate (CR+CRi+morphologic leukemia-free state MLFS).
Selection of RCTs randomly evaluating modifications in intensive chemotherapy in newly diagnosed fit patients
| Target population/AML subgroup* . | Age (years) . | Experimental agent/intervention . | Experimental arm . | Control arm . | Primary endpoint† . | Planned patient number . | PI, country (cooperative group) . | Registry number . |
|---|---|---|---|---|---|---|---|---|
| • All comers | 18-65 | Venetoclax | Venetoclax + 7 + 3 | 7 + 3 | EFS | 300 | Wang, China | NCT05356169 |
| • Intermediate or favorable cytogenetics • In CR/CRi after intensive induction | ≥60 | Venetoclax | Chemo consolidation with venetoclax + cytarabine | Chemo consolidation with idarubicin + cytarabine | RFS | 134 | Pigneux, France (FILO) | NCT04968015 |
| • AML/MDS-EB2, • No FLT3 mut | ≥18 | Venetoclax | Venetoclax + 7 + 3 | 7 + 3 | EFS | 650 | Döhner, Germany (AMLSG/HOVON) | NCT04628026 |
| • Favorable/intermediate risk • No CBF-AML | 18-60 | Venetoclax | Venetoclax + IDAC as consolidation | IDAC as consolidation | RFS | 200 | Peterlin/Gastaud, France (FILO/ALFA) | NCT02416388 |
| • All comers • No CBF-AML and FLT3 mut | 18-65 | Venetoclax | Venetoclax + DAC | Placebo + DAC | EFS | 311 | Wierzbowska, Poland, (PALG) | EudraCT 2023-503394-37-00 |
| • AML or MDS-EB2 with FLT3-ITD and/or FLT3-TKD | ≥18 | Gilteritinib | Gilteritinib + 7 + 3 | Midostaurin + 7 + 3 | EFS | 768 | Raajimakers, Netherlands (HOVON/AMLSG/SAKK, ALFA, FILO, ALLG, CETLAM) | NCT04027309 |
| • FLT3 mut • No CBF-AML | 18-70 | Gilteritinib | Gilteritinib + 7 + 3 | Midostaurin + 7 + 3 | CR/CRi FLT3 negative | 181 | Luger, USA (PrECOG) | NCT03836209 |
| • FLT3-ITD or FLT3-TKD AML | 18-60 | Crenolanib | Crenolanib + 7 + 3 | Midostaurin + 7 + 3 | EFS | 510 | AROG, USA | NCT03258931 |
| • CBF-AML | 18-65 | Sorafenib | Sorafenib + 7 + 3‡ | 7 + 3 | CRmol | 88 | Shi, China | NCT05404516 |
| • CBF-AML | 18-70 | Midostaurin | Midostaurin + GO + 7 + 3 | GO + 7 + 3 | EFS | 66 | Röllig, Germany (SAL-AMLCG) | NCT04385290 |
| • AML or MDS-EB2 with IDH1 or IDH2 mut | ≥18 | Ivosidenib, enasidenib | Ivosidenib or enasidenib + 7 + 3 | Placebo + 7 + 3 | EFS | 968 | Wouters, Netherlands (HOVON/AMLSG/SAKK, ALFA, FILO, ALLG, CETLAM) | NCT03839771 |
| • Favorable/intermediate risk (ELN2017) • No FLT3-ITD, -TKD | 18-60 | Glasdegib | 7 + 3 + GO and postremission treatment, followed by glasdegib maintenance | 7 + 3 + GO and postremission treatment | DFS | 414 | Venditti, Italy (GIMEMA) | NCT04168502 |
| • FLT3 mut AML | 18-70 | GO | GO + midostaurin +7 + 3 | Midostaurin +7 + 3 | EFS | 130 | Röllig, Germany (SAL-AMLCG) | NCT04385290 |
| • All comers • No CBF-AML • No -5 or -7 • No FLT3-ITD or –TKD | ≥18 | Selinexor | Selinexor + 7 + 3 | 7 + 3 | OS | 100 | Pardee/NCI, USA | NCT02835222 |
| • All comers • No FLT3-ITD or –TKD | 18-75 | Pembrolizumab | Pembrolizumab + 7 + 3 | 7 + 3 | MRDneg CR | 124 | Zeidan/NCI, USA | NCT04214249 |
| • AML MR (WHO 2022) or AML with MR genetic changes (ICC 2022) or AML from MDS/MPN | 18- 75 | Pomalidomide | Pomalidomide + CPX-351 | CPX-351 | CR/CRi | 78 | Zeidner/NCI, USA | NCT04802161 |
| • MDS-IB2, MDS/AML, AML • Increased TRM score (less fit) | ≥18 | CPX-351 | CPX-351 | CLAG-M | OS | 60 | Walter, USA | NCT04195945 |
| • HR-MDS, AML ≤30% blasts | 18-75 | CPX-351 | CPX-351 before alloHCT | 7 + 3 or Aza before alloHCT | EFS | 150 | Platzbecker, Germany (SAL) | NCT04061239 |
| • All comers with • No FLT3-ITD or –TKD, no NPM1 • No CBF or APL • No AML-MRC | ≥50 | CPX-351 | CPX-351 | 7 + 3 | MRDneg CR/CRi | 210 | Foussat, France (ALFA) | NCT05260528 |
| • Intermediate/adverse risk (ELN2017) including AML-MRC and tAML | ≥18 | CPX-351 | CPX-351 | 7 + 3 | OS in de novo AML | 882 | Döhner, Germany (AMLSG) | NCT03897127 |
| • All comers in CR after intensive induction | 60-75 | Idarubicin | Idarubicin + IDAC§ for consolidation | IDAC for consolidation | RFS | 320 | Hu, China | NCT04216771 |
| • FLT3-ITD AML | 18-65 | Treatment intensification based on early blast clearance during 7 + 3 + midostaurin | HIDAC-based second induction and early alloHCT | Standard second induction and postremission treatment | EFS | 172 | Vannucchi, Italy (GIMEMA) | NCT04174612 |
| • Intermediate risk in CR after induction | 14-60 | Decitabine | Decitabine + IDAC consolidation | IDAC consolidation | MRD | 100 | Jiang, China | NCT03417427 |
| • CBF-AML in CR after intensive induction | 18-60 | Fludarabine | Fludarabine + IDAC for consolidation | HDAC for consolidation | Relapse rate | 200 | Song, China | NCT02926586 |
| Target population/AML subgroup* . | Age (years) . | Experimental agent/intervention . | Experimental arm . | Control arm . | Primary endpoint† . | Planned patient number . | PI, country (cooperative group) . | Registry number . |
|---|---|---|---|---|---|---|---|---|
| • All comers | 18-65 | Venetoclax | Venetoclax + 7 + 3 | 7 + 3 | EFS | 300 | Wang, China | NCT05356169 |
| • Intermediate or favorable cytogenetics • In CR/CRi after intensive induction | ≥60 | Venetoclax | Chemo consolidation with venetoclax + cytarabine | Chemo consolidation with idarubicin + cytarabine | RFS | 134 | Pigneux, France (FILO) | NCT04968015 |
| • AML/MDS-EB2, • No FLT3 mut | ≥18 | Venetoclax | Venetoclax + 7 + 3 | 7 + 3 | EFS | 650 | Döhner, Germany (AMLSG/HOVON) | NCT04628026 |
| • Favorable/intermediate risk • No CBF-AML | 18-60 | Venetoclax | Venetoclax + IDAC as consolidation | IDAC as consolidation | RFS | 200 | Peterlin/Gastaud, France (FILO/ALFA) | NCT02416388 |
| • All comers • No CBF-AML and FLT3 mut | 18-65 | Venetoclax | Venetoclax + DAC | Placebo + DAC | EFS | 311 | Wierzbowska, Poland, (PALG) | EudraCT 2023-503394-37-00 |
| • AML or MDS-EB2 with FLT3-ITD and/or FLT3-TKD | ≥18 | Gilteritinib | Gilteritinib + 7 + 3 | Midostaurin + 7 + 3 | EFS | 768 | Raajimakers, Netherlands (HOVON/AMLSG/SAKK, ALFA, FILO, ALLG, CETLAM) | NCT04027309 |
| • FLT3 mut • No CBF-AML | 18-70 | Gilteritinib | Gilteritinib + 7 + 3 | Midostaurin + 7 + 3 | CR/CRi FLT3 negative | 181 | Luger, USA (PrECOG) | NCT03836209 |
| • FLT3-ITD or FLT3-TKD AML | 18-60 | Crenolanib | Crenolanib + 7 + 3 | Midostaurin + 7 + 3 | EFS | 510 | AROG, USA | NCT03258931 |
| • CBF-AML | 18-65 | Sorafenib | Sorafenib + 7 + 3‡ | 7 + 3 | CRmol | 88 | Shi, China | NCT05404516 |
| • CBF-AML | 18-70 | Midostaurin | Midostaurin + GO + 7 + 3 | GO + 7 + 3 | EFS | 66 | Röllig, Germany (SAL-AMLCG) | NCT04385290 |
| • AML or MDS-EB2 with IDH1 or IDH2 mut | ≥18 | Ivosidenib, enasidenib | Ivosidenib or enasidenib + 7 + 3 | Placebo + 7 + 3 | EFS | 968 | Wouters, Netherlands (HOVON/AMLSG/SAKK, ALFA, FILO, ALLG, CETLAM) | NCT03839771 |
| • Favorable/intermediate risk (ELN2017) • No FLT3-ITD, -TKD | 18-60 | Glasdegib | 7 + 3 + GO and postremission treatment, followed by glasdegib maintenance | 7 + 3 + GO and postremission treatment | DFS | 414 | Venditti, Italy (GIMEMA) | NCT04168502 |
| • FLT3 mut AML | 18-70 | GO | GO + midostaurin +7 + 3 | Midostaurin +7 + 3 | EFS | 130 | Röllig, Germany (SAL-AMLCG) | NCT04385290 |
| • All comers • No CBF-AML • No -5 or -7 • No FLT3-ITD or –TKD | ≥18 | Selinexor | Selinexor + 7 + 3 | 7 + 3 | OS | 100 | Pardee/NCI, USA | NCT02835222 |
| • All comers • No FLT3-ITD or –TKD | 18-75 | Pembrolizumab | Pembrolizumab + 7 + 3 | 7 + 3 | MRDneg CR | 124 | Zeidan/NCI, USA | NCT04214249 |
| • AML MR (WHO 2022) or AML with MR genetic changes (ICC 2022) or AML from MDS/MPN | 18- 75 | Pomalidomide | Pomalidomide + CPX-351 | CPX-351 | CR/CRi | 78 | Zeidner/NCI, USA | NCT04802161 |
| • MDS-IB2, MDS/AML, AML • Increased TRM score (less fit) | ≥18 | CPX-351 | CPX-351 | CLAG-M | OS | 60 | Walter, USA | NCT04195945 |
| • HR-MDS, AML ≤30% blasts | 18-75 | CPX-351 | CPX-351 before alloHCT | 7 + 3 or Aza before alloHCT | EFS | 150 | Platzbecker, Germany (SAL) | NCT04061239 |
| • All comers with • No FLT3-ITD or –TKD, no NPM1 • No CBF or APL • No AML-MRC | ≥50 | CPX-351 | CPX-351 | 7 + 3 | MRDneg CR/CRi | 210 | Foussat, France (ALFA) | NCT05260528 |
| • Intermediate/adverse risk (ELN2017) including AML-MRC and tAML | ≥18 | CPX-351 | CPX-351 | 7 + 3 | OS in de novo AML | 882 | Döhner, Germany (AMLSG) | NCT03897127 |
| • All comers in CR after intensive induction | 60-75 | Idarubicin | Idarubicin + IDAC§ for consolidation | IDAC for consolidation | RFS | 320 | Hu, China | NCT04216771 |
| • FLT3-ITD AML | 18-65 | Treatment intensification based on early blast clearance during 7 + 3 + midostaurin | HIDAC-based second induction and early alloHCT | Standard second induction and postremission treatment | EFS | 172 | Vannucchi, Italy (GIMEMA) | NCT04174612 |
| • Intermediate risk in CR after induction | 14-60 | Decitabine | Decitabine + IDAC consolidation | IDAC consolidation | MRD | 100 | Jiang, China | NCT03417427 |
| • CBF-AML in CR after intensive induction | 18-60 | Fludarabine | Fludarabine + IDAC for consolidation | HDAC for consolidation | Relapse rate | 200 | Song, China | NCT02926586 |
Selection of trials currently recruiting or planned at the time of writing. Trials for unfit patients, children, APL, relapsed or refractory disease and with purely maintenance or conditioning questions were excluded.
CBF, core binding factor; CRi, complete hematologic remission with incomplete hematologic recovery; CRmol, molecular CR; DA, daunorubicin plus cytarabine (ara-c); EFS, event-free survival; GO, gemtuzumab ozogamicin; HDAC, high-dose cytarabine; IDAC, intermediate-dose cytarabine; LDAC, low-dose cytarabine; MRD, measurable residual disease; MRDneg, MRD negativity; mut, mutation; OS, overall survival; PI, principal investigator; RFS, relapse-free survival; TRM, treatment-related mortality.
sAML/tAML/HMA pretreatment not accounted for/mentioned in table.
“7 + 3” stands for all variations of standard-dose cytarabine plus anthracycline/mitoxantrone and includes intensive consolidation for patients ineligible for allogeneic HCT. †Secondary end points for all trials include response rates, MRD, tolerability, rate of allogeneic HCT, patient-reported outcomes, and survival end points. §IDAC = intermediate-dose cytarabine.
Since our two case patients are convinced by our arguments and agree with intensive induction, let us move on to see what we have learned about its use and what we should pay attention to during their induction.
How to use intensive induction
General aspects of induction
There are several ways to combine cytarabine and anthracyclines, and there are many variations of 7 + 3. They should all lead to similar outcomes when these combinations are used correctly. A few points are of general importance:
Cytarabine dose and schedule
Randomized comparisons suggest that 100 and 200 mg of continuous daily cytarabine are equally efficacious,19,20 and there is no evidence on whether 7 or 10 or 5 days are better. However, the most extensive body of knowledge exists for 7 days of continuous infusion as it has been most widely used, also in establishing novel agent combinations. Higher doses of cytarabine have resulted in higher remission rates and superior relapse-free survival (RFS) than standard doses, but long-term beneficial effects on overall survival (OS) across all subgroups could not be shown.21-24 Time-sequential splitting of induction shortens the critical leukopenia time, but its antileukemic efficacy is not significantly better than that of standard induction.25 Based on this data, I use a dose of 100-200 mg/m2 cytarabine as continuous infusion over 7 days in my clinical practice.
Anthracycline type, dose, and schedule
As the oldest anthracycline, experience and data are most robust for daunorubicin, but the anthracycline idarubicin and the anthracenedione mitoxantrone are equally effective according to randomized comparisons.26,27 There is a lack of convincing randomized evidence for the acridine derivative m-amsacrine in induction treatment, not even in patients with impaired cardiac function.28 To reduce the risk of cardiotoxicity, a cumulative dose threshold around 500 mg/m2 daunorubicin equivalents or preexisting cardiac insufficiency should be considered as relative contraindication for anthracycline use. There are no comparisons indicating that application on days 1-3 versus 3-5 is more efficacious. The optimal dose of daunorubicin has been the subject of several randomized controlled trials (RCTs). Whereas 90 mg/m2 is more efficacious than 45 mg/m2 based on 3 randomized trials,29-31 60 mg/m2 is equally effective as 90 mg/m2, as shown in 2 randomized studies.7,32 The NCRI-AML17 study showed a significant benefit of 90 mg in FLT3-ITD-mutated patients,33 but this could not be reproduced in the DaunoDouble trial, possibly due to partial use of the FLT3 inhibitor midostaurin after its approval in the latter study.7 Considering these results, I recommend 60 mg/m2 daunorubicin over 3 days or, if not available, idarubicin 12 mg/m2 as an alternative.
Additional nontargeted agents
Several attempts have been made to improve the efficacy of the cytarabine-anthracycline doublet by adding a third cytostatic drug. Thioguanine has historically been part of several induction regimens, but its potential additional benefit has never been assessed in randomized comparisons. The addition of fludarabine does not improve the outcome of newly diagnosed patients.34 It is part of the high-dose cytarabine-based relapse combination FLAG-Ida, also used in first line in the MRC-AML15 and NCRI-AML19 trials, showing lower relapse rates with increased hematotoxicity,35 and suggesting a higher survival efficacy than cytarabine plus daunorubicin in some forms of secondary AML and NPM1-FLT3-mutated AML without the use of midostaurin.36,37 However, as fludarabine is a fixed component of FLAG-Ida, its additional cytoreductive effect cannot be clearly isolated from the other components. Cladribine was able to increase the complete remission (CR) rates from 56% to 68% in the Polish PALG study, leading to similar RFS but significantly longer OS in the cladribine arm.34 A similar trial in elderly patients from 60 years could show a significant CR improvement in the age subgroup 60-65 years and improved OS in patients with favorable and intermediate karyotypes, but not in the entire study population.38 When lomustin was combined with a chemotherapy backbone comprising induction, consolidation with low-dose cytarabine plus idarubicin and 6 reinduction cycles of low-dose cytarabine plus idarubicin, and maintenance with 6-MP and MTX, the OS was significantly longer than without lomustin in a French RCT on elderly patients without unfavorable cytogenetics.39 The addition of clofarabine to standard induction significantly reduced the time to CR and the relapse risk, but due to similar CR rates and increased toxicity, OS was similar compared to standard induction alone, with the exception of European LeukemiaNet (ELN) 2010 intermediate- risk patients, who had a significant survival prolongation.40 Trials testing all-trans retinoic acid (ATRA) in combination intensive chemotherapy have delivered heterogeneous results. A significant survival benefit was detected in 1 trial with elderly AML patients,41 whereas a different RCT in younger patients showed improved OS in the per-protocol population but not in the whole intent-to-treat population.42 However, 2 other randomized studies could not show a beneficial effect of ATRA.43,44 Last but not least, attempts to improve leukemia outcomes by adding etoposide35 or priming leukemic blasts by granulocyte colony-stimulating factor were not successful.43-46
Number of induction cycles
Double induction in younger AML patients was introduced in the 1980s mainly in Europe to establish a standardized dose intensity without treatment delay caused by response assessments. A recent randomized comparison of patients with a good early response to induction cycle 1 showed no relevant differences in CR rates or survival for single versus double induction.47 Regimens containing higher doses of cytarabine should be considered for fit patients not responding to a first cycle of 7 + 3.48,49
Specific modification in patient subgroups
With the development and approval of novel targeted agents, comprehensive pretherapeutic diagnostics have become very relevant and should be available preferably within 5 to 7 days. In clinically stable patients such as our 2 case patients, waiting for diagnostic results for several days does not negatively affect the prognosis, whereas AML-associated complications, namely leukostasis, tumor lysis syndrome, or disseminated intravascular coagulation, must trigger an immediate start of specific treatment.50,51
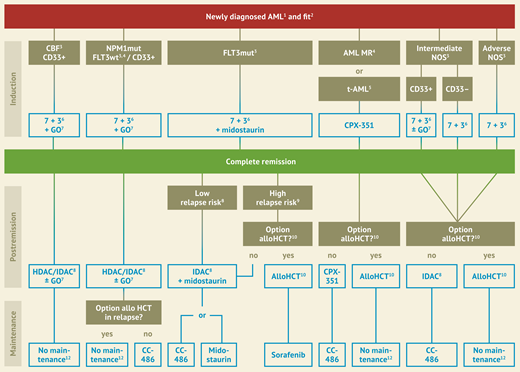
We could offer our case patients an approved specific combinational treatment of a 7 + 3 backbone with novel specific agents if initial diagnostics showed either CBF-AML, NPM1-mutated AML, or FLT3-mutated AML, whereas in the case of treatment-related or myelodysplasia-associated AML, the liposomal formulation of daunorubicin and cytarabine CPX-351 would be offered instead of 7 + 3. Treatment options are shown in Figure 2.
Treatment stratification for newly diagnosed patients fit for intensive treatment, modified after Onkopedia Guidelines for AML (www.onkopedia.com).1 APL, acute promyelocytic leukemia excluded. 2 Fit for intensive therapy, based on ECOG status and comorbidity. 3 Genetically defined subgroups according to ELN 2022. 4 AML MR, AML with myelodysplasia-related changes (WHO) or entities “AML with myelodysplasia-related gene mutations”, “AML with myelodysplasia-related cytogenetic abnormalities” and AML with the diagnostic qualifier “Progressed form MDS or MDS/MPN” (ICC). 5 t-AML, therapy-associated AML. 6 7+3, therapy regimen with cytarabine (Ara-C) on 7 days, daunorubicin on 3 days. 7 GO, gemtuzumab ozogamicin, recommended in patients up to 70 years. 8 HDAC, high-dose Ara-C; IDAC, intermediate-dose Ara-C. 9 Low risk of recurrence: NPM1-mut without relevant MRD. High risk of recurrence: NPM1 wildtype or relevant MRD. 10 Allo HCT, allogeneic hematopoietic cell transplantation. 11 This recommendation includes bZIP inframeCEBPA mutated patients. 12 MRD monitoring recommended.
Treatment stratification for newly diagnosed patients fit for intensive treatment, modified after Onkopedia Guidelines for AML (www.onkopedia.com).1 APL, acute promyelocytic leukemia excluded. 2 Fit for intensive therapy, based on ECOG status and comorbidity. 3 Genetically defined subgroups according to ELN 2022. 4 AML MR, AML with myelodysplasia-related changes (WHO) or entities “AML with myelodysplasia-related gene mutations”, “AML with myelodysplasia-related cytogenetic abnormalities” and AML with the diagnostic qualifier “Progressed form MDS or MDS/MPN” (ICC). 5 t-AML, therapy-associated AML. 6 7+3, therapy regimen with cytarabine (Ara-C) on 7 days, daunorubicin on 3 days. 7 GO, gemtuzumab ozogamicin, recommended in patients up to 70 years. 8 HDAC, high-dose Ara-C; IDAC, intermediate-dose Ara-C. 9 Low risk of recurrence: NPM1-mut without relevant MRD. High risk of recurrence: NPM1 wildtype or relevant MRD. 10 Allo HCT, allogeneic hematopoietic cell transplantation. 11 This recommendation includes bZIP inframeCEBPA mutated patients. 12 MRD monitoring recommended.
Most recent data and open questions around novel agents include the second-generation tyrosinkinase inhibitor (TKI) quizartinib, the use of gemtuzumab ozogamicin in NPM1-mutated and elderly AML patients, and the value of CPX-351 and FLAG-Ida in secondary-type mutations.
Quizartinib is a second-generation type-II TKI with a high specificity for mutated KIT and FLT3-ITD, while not active in FLT3-TKD-mutated cells. In the randomized-controlled QuANTUM- First trial, quizartinib or placebo were added to standard induction and chemoconsolidation treatment and as maintenance for up to 36 cycles after chemoconsolidation or allogeneic HCT in 539 fit newly diagnosed AML patients aged 18-75 years with an FLT3-ITD-mutation. While responses and event-free survival (EFS) were similar, quizartinib led to a significant prolongation of both RFS (median 39.3 versus 13.6 months, hazard ratio [HR] 0.61) and OS (median 31.9 versus 15.1 months, HR 0.78) with a similar toxicity profile as placebo.52 In contrast to the pivotal trial for midostaurin (RATIFY),53 the QuANTUM-First trial with quizartinib also enrolled patients ≥60 years, maintenance was 36 instead of 12 cycles and also allowed after allogeneic HCT. The results of the trial do not allow a direct comparison to midostaurin, but in the younger subgroup of patients 18-59 years, the HR for OS in FLT3-ITD patients was approximately 0.80 in RATIFY, whereas it was 0.68 in QuANTUM-First, indicating at least comparable or higher efficacy of quizartinib. Based on this data, the drug was approved by the FDA in July 2023 for combination with intensive chemotherapy and as maintenance in newly diagnosed FLT3-ITD-mutated AML. By the time of writing, trial data were still under review by the EMA.
Gemtuzumab ozogamicin (GO) is a well-established standard in patients with CBF-AML based on the results of the ALFA-0701 study and the meta-analysis of 5 randomized trials, showing a considerable survival prolongation in this subgroup, while the beneficial effect is smaller in intermediate-risk and absent in adverse-risk patients. The results of the AMLSG-0909 study have shown that patients with NPM1 mutation also benefit from the addition of GO to intensive chemotherapy. A single dose of GO led to a deeper molecular remission and subsequently significantly prolonged RFS. Increased toxicity for the combination of GO with the quadruplet chemotherapy backbone (ICE plus ATRA) led to higher early mortality in patients 70 years and older and early crossing of EFS curves.54 Subgroup analyses show a significant EFS benefit in patients 18-60 and a trend for OS and EFS prolongation in patients 60-69 years. The results of the NCRI-AML18 study adding 1 versus 2 doses to induction with daunorubicin and cytarabine (DA) show higher rates of MRD negativity after 2 versus 1 cycles and an OS benefit in patients receiving allogeneic HCT as postremission treatment. The beneficial effect could not be observed in non-adverse-risk patients 70 years and older.55 Although the study designs and data on GO are quite heterogeneous, these trial results confirm its potential to increase the depth of response in non-high-risk patients, prove a dose-response relationship, and indicate a caveat to use the drug in patients older than 70 years.
After the pivotal study for CPX-351 enrolling older patients with secondary AML or MDS-like changes showed a significant improvement in OS with CPX-351 versus 7 + 3, the approved indication of CPX-351 was linked to the WHO category of AML-MRC. This category was revised both by the WHO and in a similar way by the ICC classifications in 2022.56,57 Whereas the elimination of multilineage dysplasia at diagnosis as defining criterion for MDS relatedness from the definition and treatment indication of CPX-351 is evident due to the lack of prognostic significance and lack of study representation, the value of CPX-351 in patients with secondary-type mutations (STM: ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, ZRSR2) is less clear. Retrospective analyses indicate that more than 40% of patients of the newly defined MDS relatedness categories are solely defined by STM mutations.58 Post hoc analyses of the CPX-351 pivotal study show similar response rates and a trend for longer survival for CPX-351 versus 7 + 3 in STM patients, whereas no benefit was seen for TP53-mutated patients.59 A small retrospective French analysis in CPX-351–treated patients shows superior OS in STM patients compared to other AMLs.60 Finally, a post hoc analysis of the NCRI AML19 trial comparing FLAG-Ida induction with CPX-351 in younger, fit AML patients shows significantly longer survival after CPX-351 versus 7 + 3 in a small group of patients with STM and no adverse cytogenetics or TP53 mutations.36
One day after the first bone marrow aspiration establishing the AML diagnosis in our 67-year-old woman, the RNA-based genetic result revealed the presence of a RUNX1::RUNX1T1 fusion transcript. This established the WHO and ICC diagnosis of AML with the recurrent genetic abnormality and categorized the patient as favorable risk according to current ELN criteria. Additional genetic aberrations such as +8, which was later found in classic chromosome analysis, do not change the prognostic group. Based on the above-mentioned evidence, standard intensive induction treatment plus GO was recommended. Early marrow assessment on day 15 showed 3% myeloblasts, and on day 30, a CR was confirmed. Based on risk-benefit considerations, postremission chemotherapy with 3 cycles of intermediate-dose cytarabine was suggested to the patient. With this treatment, we can expect her long-term remission probability to be around 40%. If we bear in mind that this is the outcome for the best prognostic group in non-APL AML, it becomes clear that there is still considerable room for improvement, even in patients with “good” risk, but more so in all other risk groups.
The PCR-based mutational screening of our 70-year-old male patient showed NPM1 and FLT3-ITD at an allelic ratio of 0.4, putting him in the intermediate-risk category of ELN 2022 and leading to the addition of midostaurin to 7 + 3 induction. Due to concerns about the renal function under immunosuppression and our patient's preference, chemoconsolidation plus midostaurin was chosen instead of allogeneic HCT as postremission treatment, followed by maintenance with CC-486 (oral azacitidine).
The future of intensive chemotherapy in AML
So far, we have looked at the evidence for intensive chemotherapy as the standard for curative treatment, and we have reviewed the approved novel agents used in addition to or instead of standard chemotherapy. However, since even favorable risk does not mean 90% to 100% cure, there is a clear need to develop this standard further. There are still several open questions in the context of intensive treatment ranging from eligibility for intensive treatment based on fitness versus genomics, the value of MRD guidance, optimization options for allogeneic HCT, maintenance, and combination partners of standard chemotherapy (Figure 3). Based on 7 + 3 as a standard backbone, there are several ongoing studies that either (1) test the feasibility of single-agent treatment in combination with 7 + 3 or CPX-351 and (2) compare novel agent combinations head-to-head, but also (3) explore the many permutations of possible combinations of more than 1 novel agent with standard intensive therapy.
When we focus on intensive approaches in late clinical development currently evaluated in randomized trials, the intensive treatment standard 7 + 3 is combined either with subgroup- specific targeted agents or with drugs that work across different subgroups (Table 2).
General nontargeted approaches
Liposomal versus conventional 7 + 3: As the pivotal trial for CPX-351 focused on tAML and sAML in an elderly patient population, the AMLSG 30-18 trial is assessing the value of the liposomal agent in younger patients with intermediate and adverse genetic risk compared with standard 7 + 3 induction.
Venetoclax: Similarly, venetoclax or placebo is combined with standard intensive chemotherapy in newly diagnosed patients irrespective of their genetic profile in the AMLSG31-19/HOVON 501/AbbVie B18-982 study.
Targeted approaches
Several novel agents selectively inhibiting cellular pathways in genetically defined AML subgroups are tested in combination with intensive chemotherapy. The first step usually is to test the feasibility and efficacy of 1 novel agent versus placebo in combination with intensive chemotherapy. Novel agents currently evaluated are the IDH inhibitors ivosidenib and enasidenib, several menin inhibitors, the XPO1 inhibitor selinexor, the PD1-checkpoint inhibitor pembrolizumab, or the immune modulator pomalidomide.
Once established, longer approved novel agents can also be combined in addition to intensive chemotherapy. Apart from the question of additional beneficial effects, the excess hematologic toxicity remains the most burning open question to be answered here. One example for the latter development is the combination of midostaurin plus GO in addition to intensive standard treatment.
The third comparative study pattern is the head-to-head comparison of two novel agents such as the first- and second-generation FLT3 inhibitors midostaurin and gilteritinib in two RCTs and the second-generation FLT3 inhibitor crenolanib with midostaurin.
The long-term goal of treatment evolution in AML must be to increase the rates of long-term cure by intensifying and harnessing intensive chemotherapy to reduce toxicity and increase tolerability by dose reduction or substitution of particularly toxic components such as anthracyclines, and to further individualize treatment intensity using a standardized and refined MRD technology.
Although our 67-year-old female patient belonged to the “favorable” risk group with the rare CBF fusion transcript, long-term cure rates range only around 30% to 50%,61-64 so that even she could benefit from novel options and should be offered a clinical trial. Currently, the combination of GO and midostaurin would be an option based on the finding that KIT is frequently overexpressed or mutated in CBF-AMLs and midostaurin is a KIT inhibitor. The NPM1/FLT3-ITD AML of our 70-year-old patient could be offered, eg, a trial comparing midostaurin with second-generation TKIs gilteritinib or crenolanib or combining midostaurin with GO.
Summary
With a chance for long-term remission of around 50% across all fit patients, treatment with intensive chemotherapy, followed by allogeneic stem cell transplant for appropriate patients, gives the highest potential for cure and forms the basis for further clinical development and treatment optimization in fit eligible patients.
The combination with more novel agents and advances in MRD detection and maintenance approaches will allow us to increase remission rates, deepen remission quality before postremission treatment, and prevent relapses to allow more patients to be cured in the future.
Conflict-of-interest disclosure
Christoph Röllig: honoraria: AbbVie, Astellas, Bristol Myers Squibb, Jazz, Pfizer, Servier; research funding: AbbVie, Novartis, Pfizer.
Off-label drug use
Christoph Röllig: No off-label drug use outside the cited clinical trials.