Abstract
Marginal zone lymphomas (MZLs) represent about 7% of B-cell non-Hodgkin lymphomas and include 3 different subtypes—namely, extranodal (EMZL), nodal, and splenic (SMZL). The initial assessment requires specific diagnostic and staging procedures depending on organ-related peculiarities. In particular, although positron emission tomography/computed tomography was not initially recommended, recent data have reassessed its role in the routine staging of MZL, especially when only localized treatment is planned or there is a suspicion of histologic transformation. Recent findings have improved the risk stratification of MZL patients, highlighting the association of early progression after frontline therapy with worse overall survival. A significant fraction of MZL cases may be related to specific bacterial (ie, Helicobacter pylori in gastric EMZL) or viral infections (hepatis C virus), and in the earlier phases of disease, a variable percentage of patients may respond to anti-infective therapy. Involved-site radiotherapy has a central role in the management of localized EMZL not amenable to or not responding to anti-infective therapy. Although rituximab-based treatments (bendamustine- rituximab in advanced EMZL or rituximab monotherapy in SMZL) have demonstrated favorable results, the current therapeutic scenario is predicted to rapidly change as emerging novel agents, especially Bruton's tyrosine kinase inhibitors, have demonstrated promising efficacy and safety profiles, leading to their approval in the relapsed setting. Moreover, a large variety of novel agents (phosphatidylinositol 3-kinase inhibitors, chimeric antigen receptor T-cells, bispecific antibodies) are being tested in MZL patients with encouraging preliminary results.
Learning Objectives
Describe the essential requirements for initial assessment of patients with MZLs and review prognostic assessment in MZLs
Discuss approaches to anti-infective treatments in MZLs
Review therapeutic options in the first-line and relapsed/refractory setting
Introduction
Marginal zone lymphomas (MZLs) are the third most common subtype of B-cell non-Hodgkin lymphomas (NHL). They account for about 7% of all NHLs and include extranodal MZL (EMZL), nodal MZL (NMZL), and splenic MZL (SMZL).1,2 In the upcoming fifth edition of the World Health Organization's classification, primary cutaneous MZL will be listed as a separate entity.3 A premalignant condition defined as clonal B-cell lymphocytosis of marginal zone origin and characterized by lymphocytosis, bone marrow (BM) infiltration by B cells with an immunophenotype consistent with MZL, and no associated organomegaly may precede overt SMZL.4 According to the US SEER-18 program (2001-2017), the age- standardized incidence rate for MZL is 1.96 per 100 000 person-years while in Europe 3.44 new cases per 100 000 inhabitants per year are reported.1,5
MZLs are characterized by slow growth and often do not require immediate therapy. When treatment is needed, excellent and durable responses are achieved. The overall outcome is favorable: gastric EMZL exhibits an overall survival (OS) rate at 10 years of about 83%, similar to the matched general population; nongastric EMZL, 77%6,7 ; and SMZL, 68%.8
Moreover, similar to other indolent lymphomas, a small but significant group of patients present a worse prognosis9–11 ; in particular, it has recently ben reported that early progression is related to a worse outcome.12–15
The number of clinical, biological, and pathological studies specifically devoted to MZL is increasing, confirming the unique biology of the disease(s) and the activity of immunochemotherapy and of novel agents.
CLINICAL CASE 1
A 52-year-old man presented with abdominal discomfort, night sweats, splenomegaly, and superficial adenopathies. At physical examination, splenomegaly was detected 2 cm under the umbilical line, with no hepatomegaly. Inguinal and neck adenopathies were found (max diameter, 1 cm). The blood count showed a white blood cell count of 20.6 × 109/L (neutrophils, 18%; lymphocytes, 80%), a hemoglobin level of 9.7 g/dL, and a platelet count of 70 × 109/L. Flow cytometry on peripheral blood showed a B-cell monoclonal population of 63% (CD20+, CD10−, CD5−, CD23−, CD103−). Hepatitis C virus (HCV) serology was negative. Lab exams showed a lactate dehydrogenase (LDH) level of 822 mU/mL (upper limit of normal, 220 mU/mL) and an albumin level of 2.9 g/dL (reference range, 3.5-5.5 g/dL). BM histology showed a B-cell infiltrate (70%) with an interstitial pattern and intrasinusoidal localization. Cytogenetics analysis of BM showed a complex karyotype. A total-body computed tomography (CT) scan evidenced splenomegaly with nodular lesions and extrahilar adenopathies, while positron emission tomography/CT (PET/CT) showed focal lesions in the spleen with a maximum standardized uptake value (SUVmax) of 20 and multiple nodal uptakes with inferior SUVmax.
A diagnosis of SMZL, stage IV, symptomatic for systemic symptoms and abdominal discomfort, was established. However, the high SUVmax of the splenic lesions raised a suspicion of histologic transformation (HT).
Initial assessment of MZLs
The initial evaluation in MZLs must assess disease dissemination in EMZLs, define spleen size and spleen lesions and extrahilar nodal spread in SMZL, and define the size and the number of lymph nodes and not clinically evident extranodal localizations in NMZL.
According to European Society for Medical Oncology and National Comprehensive Cancer Network (NCCN) guidelines, CT with contrast is the gold-standard approach for staging and assessing treatment response in MZL,16,17 while magnetic resonance imaging (MRI) is useful in specific sites such as the ocular adnexa, breasts, salivary glands, and central nervous system (dura mater). Specific criteria for assessment of response in SMZL have been proposed.18
Regarding the use of PET/CT in MZL staging and response assessment, Lugano classification originally listed MZLs as nonfluorodeoxyglucose (FDG)-avid diseases without recommending PET/CT. Nevertheless, PET/CT is being implemented more and more in routine MZL assessment.19
FDG avidity is variable in extranodal sites involving EMZL: better detection rates have been described in certain locations, such as the lungs or head and neck compared with the ocular adnexa and stomach.20,21 On the other hand, in NMZL and SMZL FDG avidity is reported in around 75% of cases.22,23
PET/CT is able to detect BM involvement in MZL in only around one-third of cases: this implies that BM biopsy is still needed, if relevant, for therapeutic decisions.24 However, a recent retrospective study showed that BM biopsy may not be required because it does not affect lymphoma-related outcomes in patients with clinically/imaging-based localized EMZL treated with radiotherapy (RT).25
NCCN guidelines list PET/CT as useful in a selected fraction of patients,17 and according to ESMO guidelines, its use is now being reconsidered as a result of the increased sensitivity of modern techniques. Particularly, it may be useful when only localized treatment is planned, when clinical and/or laboratory data suggest HT, and to guide decisions for biopsy. Indeed, SUV standard cutoffs are not established, and biopsy is still mandatory to rule out HT.
A summary of initial approaches in MZLs is reported in Table 1, and principal prognostic scores proposed for MZLs are summarized in Table 2.
Initial assessment of MZLs
| MZL subtype and anatomical site . | Staging . |
|---|---|
| All MZLs | Familial and individual history Physical examination with PS and B symptoms Lab: complete blood and differential counts, lactate dehydrogenase, beta-2-microglobulin, renal and liver function, protein electrophoresis, serum and urine immunofixation Viral serologies: complete HBV markers, HIV and HCV serologies (if HCV positive, HCV-RNA and genotype; cryoglobulins and cryocrit) Direct Coombs' test (if clinically indicated, especially in SMZL) Bone marrow biopsy in SMZL and NMZL (recommended in EMZL, particularly nongastric, if cytopenias are present or only local treatment is planned) CT of the neck, chest, abdomen, and pelvis MRI in specific situations PET/CT when only local treatment is planned and to identify biopsy site if clinical or laboratory data suggest histologic transformation |
| NMZL | Flow cytometry (bone marrow and peripheral blood) |
| SMZL | Flow cytometry (bone marrow and peripheral blood) |
| Stomach | Esophagogastroduodenoscopy Endoscopic ultrasonography (optional) Helicobacter pylori status by IHC (mandatory) (if IHC negative fecal antigen or breath test and serology studies) FISH or PCR assay for the t(11;18) translocation (optional) |
| Small intestine | Campylobacter jejuni status (PCR, IHC, or ISH) |
| Colon | Colonoscopy Esophagogastroduodenoscopy |
| Salivary glands | MRI of head and neck ENT examination and ultrasonography Esophagogastroduodenoscopy anti-SSA/Ro and anti-SSB/La antibodies |
| Ocular adnexa | Head and neck and orbit imaging Ophthalmologic examination Chlamydophila psittaci status (PCR) in the tumor biopsy, conjunctival swab, and PBMCs (optional, geographic variability) anti-SSA/Ro and anti-SSB/La antibodies (lacrimal gland) |
| Lung | Bronchoscopy and bronchoalveolar lavage Esophagogastroduodenoscopy (optional) |
| Breast | Mammography Breast ultrasonography MRI (or CT scan) of breast |
| Thyroid | Thyroid ultrasonography Thyroid function tests |
| Central nervous system (dura mater) | MRI of brain |
| Skin | Borrelia burgdorferi status (PCR) in the tumor biopsy (optional) |
| MZL subtype and anatomical site . | Staging . |
|---|---|
| All MZLs | Familial and individual history Physical examination with PS and B symptoms Lab: complete blood and differential counts, lactate dehydrogenase, beta-2-microglobulin, renal and liver function, protein electrophoresis, serum and urine immunofixation Viral serologies: complete HBV markers, HIV and HCV serologies (if HCV positive, HCV-RNA and genotype; cryoglobulins and cryocrit) Direct Coombs' test (if clinically indicated, especially in SMZL) Bone marrow biopsy in SMZL and NMZL (recommended in EMZL, particularly nongastric, if cytopenias are present or only local treatment is planned) CT of the neck, chest, abdomen, and pelvis MRI in specific situations PET/CT when only local treatment is planned and to identify biopsy site if clinical or laboratory data suggest histologic transformation |
| NMZL | Flow cytometry (bone marrow and peripheral blood) |
| SMZL | Flow cytometry (bone marrow and peripheral blood) |
| Stomach | Esophagogastroduodenoscopy Endoscopic ultrasonography (optional) Helicobacter pylori status by IHC (mandatory) (if IHC negative fecal antigen or breath test and serology studies) FISH or PCR assay for the t(11;18) translocation (optional) |
| Small intestine | Campylobacter jejuni status (PCR, IHC, or ISH) |
| Colon | Colonoscopy Esophagogastroduodenoscopy |
| Salivary glands | MRI of head and neck ENT examination and ultrasonography Esophagogastroduodenoscopy anti-SSA/Ro and anti-SSB/La antibodies |
| Ocular adnexa | Head and neck and orbit imaging Ophthalmologic examination Chlamydophila psittaci status (PCR) in the tumor biopsy, conjunctival swab, and PBMCs (optional, geographic variability) anti-SSA/Ro and anti-SSB/La antibodies (lacrimal gland) |
| Lung | Bronchoscopy and bronchoalveolar lavage Esophagogastroduodenoscopy (optional) |
| Breast | Mammography Breast ultrasonography MRI (or CT scan) of breast |
| Thyroid | Thyroid ultrasonography Thyroid function tests |
| Central nervous system (dura mater) | MRI of brain |
| Skin | Borrelia burgdorferi status (PCR) in the tumor biopsy (optional) |
ENT, ear, nose, and throat; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; PBMC, peripheral blood mononuclear cells; PS, performance status; SSA, Sjogren's syndrome.
A selection of prognostic scores proposed for MZLs
| Score . | Subtype . | Parameters . | Categories . |
|---|---|---|---|
| IIL score9 | SMZL | Hemoglobin <12 g/dL LDH > ULN Albumin <3.5 g/dL | Low-risk (0 factor): 5-y CSS 88% Intermediate-risk (1 factor): 5-y CSS 73% High-risk: (2-3 factors): 5-y CSS 50% |
| HPLL score76 | SMZL | Hemoglobin Platelets LDH Extrahilar lymphadenopathy | Low-risk (0 factor): 5-y LSS 94% Intermediate-risk (1 factor): 5-y LSS 78% High-risk group (2-3 factors): 5-y LSS 69% |
| HPLL score simplified11 | SMZL | Hemoglobin <9.5 g/dL Platelets <80 × 109/l LDH > ULN Extrahilar lymphadenopathy | Group A (0 factor): 5-y LSS 95% Group B (1-2 factors): 5-y LSS 87% Group C (3-4 factors): 5-y LSS 68% |
| MALT-IPI10 | EMZL | Age >70 y Stage III/IV LDH > ULN | Low-risk (0 factor): 5-y EFS 70% Intermediate-risk (1 factor): 5-y EFS 78% High-risk group (2-3 factors): 5-y EFS 69 |
| POD24 (early POD)12,13 | EMZL SMZL Disseminated MZL | Lymphoma progression within 24 mo from diagnosis (yes or no) | FIL study (all MZL)12 : No: 3-y OS 88% Yes: 3-y OS 53% IELSG study (EMZL)13 : No: 10-y OS 85% Yes: 10-y OS 64% |
| Score . | Subtype . | Parameters . | Categories . |
|---|---|---|---|
| IIL score9 | SMZL | Hemoglobin <12 g/dL LDH > ULN Albumin <3.5 g/dL | Low-risk (0 factor): 5-y CSS 88% Intermediate-risk (1 factor): 5-y CSS 73% High-risk: (2-3 factors): 5-y CSS 50% |
| HPLL score76 | SMZL | Hemoglobin Platelets LDH Extrahilar lymphadenopathy | Low-risk (0 factor): 5-y LSS 94% Intermediate-risk (1 factor): 5-y LSS 78% High-risk group (2-3 factors): 5-y LSS 69% |
| HPLL score simplified11 | SMZL | Hemoglobin <9.5 g/dL Platelets <80 × 109/l LDH > ULN Extrahilar lymphadenopathy | Group A (0 factor): 5-y LSS 95% Group B (1-2 factors): 5-y LSS 87% Group C (3-4 factors): 5-y LSS 68% |
| MALT-IPI10 | EMZL | Age >70 y Stage III/IV LDH > ULN | Low-risk (0 factor): 5-y EFS 70% Intermediate-risk (1 factor): 5-y EFS 78% High-risk group (2-3 factors): 5-y EFS 69 |
| POD24 (early POD)12,13 | EMZL SMZL Disseminated MZL | Lymphoma progression within 24 mo from diagnosis (yes or no) | FIL study (all MZL)12 : No: 3-y OS 88% Yes: 3-y OS 53% IELSG study (EMZL)13 : No: 10-y OS 85% Yes: 10-y OS 64% |
CSS, cause-specific survival; EFS, event-free survival; HPLL, hemoglobin, platelet count, LDH, extrahilar lymphadenopathy; IIL, Integruppo Italiano Linfomi; LSS, lymphoma-specific survival; POD, progression of disease; ULN, upper level of normal.
CLINICAL CASE 1 (Continued)
Our patient was classified into the high-risk group according to the Integruppo Italiano Linfomi score and into the C group according to the HPPLs/ABC score.9,11 In order to rule out HT, we decided to perform a laparoscopic hand-assisted splenectomy, and a diagnosis of SMZL with an area of HT into diffuse large B-cell lymphoma (DLBCL) was established. The patient was treated with 6 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone, and after 2 years he is still in complete remission (CR).
Conconi et al reported HT in 3.8% of 340 MZL patients26 ; an elevated LDH at diagnosis was associated with the risk of HT (5% at 5 and 10 years and 10% at 12 years). At the time of HT, most patients had high LDH and systemic symptoms. Alderuccio et al reported 453 MZLs27 : 7.5% had biopsy-proven HT to DLBCL, a fifth diagnosed at the time of initial MZL diagnosis. A failure to achieve CR after initial treatment, an elevated LDH, and more than 4 nodal sites at the time of diagnosis are the main predictors of an increased risk of HT. Patients who presented with HT at diagnosis or more than 12 months since diagnosis had a longer OS than those with HT within 12 months after MZL diagnosis. In addition, the same group suggested that EMZL patients with multiple mucosa-associated lymphoid tissue (MALT) sites represent a novel clinical subset associated with worse outcome and a higher incidence of HT.15 Finally, Bastidas-Mora et al described 36 patients with SMZL28 : the only independent predictor for HT in multivariate analysis was complex karyotype.
CLINICAL CASE 2
A 72-year-old female presented with persistent right parotid and conjunctival swelling. A conjunctival biopsy revealed EMZL. A CT scan showed additional cervical right lymph node enlargement (2.5 cm). A BM biopsy excluded lymphoma infiltration. HCV serology was positive, and HCV-RNA was 4.5 × 106 UI/mL (genotype 2). Hepatitis B virus (HBV) and HIV serology were negative. A liver fibroscan showed a moderate liver fibrosis of 8.5 KpA (F3).
Infectious agents and MZLs: the case of HCV
HCV-associated MZL represents an antigen-driven model of lymphomagenesis. HCV infection has been associated with SMZL and other MZL subtypes. The prevalence of HCV infection among MZL cases varies widely across different geographical areas due to the specific epidemiology of the infection.31
In the last 2 decades, retrospective observations reported that antiviral therapy with either interferon-based or, more recently, interferon-free combinations is able to induce a significant rate of lymphoma regression in HCV-positive MZLs.32–35 A recent study from Fondazione Italiana Linfomi prospectively investigated the use of direct-acting antivirals (DAAs) as a primary treatment in patients with HCV-positive indolent lymphomas not requiring immediate conventional therapy (40 patients, 27 MZL).36 The overall response rate (ORR) and CR rates among MZL patients were 48% and 26%, respectively, and 3-year progression-free survival (PFS) was 73% in MZL patients.
The currently recommended DAA regimens include the 2 available pangenotypic combinations—that is, sofosbuvir- velpatasvir for 12 weeks and glecaprevir/pibrentasvir for 8 to 12 weeks (Table 3).37
Use of anti-infectious regimens in patients with MZLs
| Pathogen . | MZL subtype, organ . | Prevalence range (%) . | Anti-infectious regimen . | Type of evidence . | ORR (CR) . | PFS . | Notes . |
|---|---|---|---|---|---|---|---|
| Helicobacter pylori | EMZL, stomach | >90% | PPI, clarithromycin-based triple therapy with amoxicillin or metronidazolea | >30 retrospective or prospective studies; data from >1400 pts | 75% | 28 mo | Responses observed also in HP-negative cases (false-negative tests or other Helicobacter species) |
| Chlamydophila psittaci | EMZL, ocular adnexa | 0%-80% | Doxycyclineb or clarithromycinc | >10 retrospective and 3 prospective studies; data from >100 pts | 45%-65% | 55% at 5 y | Wide prevalence variability depending on geographical region |
| Borrelia burgdorferi | EMZL, skin | 0%-40% | Ceftriaxoned | Case reports | 40% | NA | Prevalence high in endemic areas; median 7.3% |
| Campylobacter jejuni | EMZL, small bowel (IPSID) | up to 60% | Tetracycline, metronidazole, or ampicillin | Case reports | NA | NA | Association with low socioeconomic and sanitation status |
| Achromobacter xylosoxidans | EMZL, lung | 2%-46% | NA | NA | NA | NA | Low virulence, highly resistant to antibiotics |
| Hepatitis C virus | EMZL, various nongastric sites; SMZL; NMZL | 5%-20% | DAAse | Retrospective studies, 1 prospective study | 48% (26%) | 73% at 3 y | ORR higher in EMZL |
| Pathogen . | MZL subtype, organ . | Prevalence range (%) . | Anti-infectious regimen . | Type of evidence . | ORR (CR) . | PFS . | Notes . |
|---|---|---|---|---|---|---|---|
| Helicobacter pylori | EMZL, stomach | >90% | PPI, clarithromycin-based triple therapy with amoxicillin or metronidazolea | >30 retrospective or prospective studies; data from >1400 pts | 75% | 28 mo | Responses observed also in HP-negative cases (false-negative tests or other Helicobacter species) |
| Chlamydophila psittaci | EMZL, ocular adnexa | 0%-80% | Doxycyclineb or clarithromycinc | >10 retrospective and 3 prospective studies; data from >100 pts | 45%-65% | 55% at 5 y | Wide prevalence variability depending on geographical region |
| Borrelia burgdorferi | EMZL, skin | 0%-40% | Ceftriaxoned | Case reports | 40% | NA | Prevalence high in endemic areas; median 7.3% |
| Campylobacter jejuni | EMZL, small bowel (IPSID) | up to 60% | Tetracycline, metronidazole, or ampicillin | Case reports | NA | NA | Association with low socioeconomic and sanitation status |
| Achromobacter xylosoxidans | EMZL, lung | 2%-46% | NA | NA | NA | NA | Low virulence, highly resistant to antibiotics |
| Hepatitis C virus | EMZL, various nongastric sites; SMZL; NMZL | 5%-20% | DAAse | Retrospective studies, 1 prospective study | 48% (26%) | 73% at 3 y | ORR higher in EMZL |
PPI (standard dose) twice daily + clarithromycin 500 mg twice daily + amoxicillin 1000 mg twice daily or metronidazole 500 mg twice daily, for 14 days.
Doxycycline 100 mg twice daily for 3 weeks.
Clarithromycin 500 mg twice daily for 6 months.
Ceftriaxone intravenously 2 g/d for 2 weeks.
Sofosbuvir/velpatasvir 400/100 mg/d for 12 weeks or glecaprevir/pibrentasvir 300/100 mg/d for 8 weeks (12 weeks for genotype 3).
CR, complete response; IPSID, immunoproliferative small intestine disease; NA, not available; PPI, proton pump inhibitor; pts, patients.
CLINICAL CASE 2 (Continued)
The patient received a 12-week course of sofosbuvir-velpatasvir, achieving a sustained virologic response. At lymphoma restaging, a CT scan showed a CR of both extranodal and nodal localizations. The patient was progression-free at 3.5 years since treatment start.
Bacteria-associated EMZLs
Helicobacter pylori (HP)–associated gastric EMZL is the best-studied model of a multistep process of infectious antigen-driven lymphoproliferation. However, in recent years a declining rate of HP-positive gastric MALT lymphoma has been observed in Italy, possibly due to higher control of HP infection in the general population.38
HP should be promptly eradicated in all cases of HP-positive gastric EMZL regardless of the stage.16 Although some features, such as the involvement of submucosa or regional lymph nodes by endoscopic ultrasound and the presence of t(11;18)(q21;q21) (BIRC3/MALT1), may predict a lower likelihood of lymphoma response after eradication,39 HP eradication is still recommended as the first treatment choice.16 In patients with localized gastric EMZL, the exclusive use of anti-HP antibiotic therapy is able to induce CR and long-term control in up to 75% of cases.40 As responses may develop slowly (up to more than 1 year), a careful watch-and-wait approach with endoscopy every 3 to 6 months is suggested in cases with asymptomatic persistence of microscopic lymphoma infiltration, waiting at least 12 months before starting a new line of therapy.16
Ocular adnexal marginal zone lymphoma (OAML) may involve the eyelids and the conjunctival, lacrimal gland, or orbital soft tissue. Beginning from the first study published in 2004,41 many reports pointed out a possible association of OAML with the obligate intracellular bacterium Chlamydophila psittaci (CP),42–44 which can be transmitted to humans after prolonged contact with infected birds, cats, or other pets; other studies suggested a wide geographic variability of this association.45 In particular, several series from the United States reported no evidence of CP infection in patients with OAML.46,47 OAML regression after CP-directed antibiotic therapy with doxycycline was demonstrated by 3 prospective studies,42–44 the most recent of which showed a 65% ORR (18% CR) in 34 patients (29 CP positive), with a 5-year PFS of 55%.44 However, studies from different regions found inferior ORR.48
Overall, although there is some controversy about the use of CP-directed antibiotics in OAML, especially when testing is not available, an empiric trial of eradication may be considered in cases in which there is no immediate concern about vision loss and in geographical regions with a high prevalence of infection.16
Data regarding the potential efficacy of antibiotics in other nongastric EMZL are scant and based only on small retrospective series or anecdotal reports. Borrelia burgdorferi has been associated with cutaneous EMZL, especially in highly endemic regions (Scottish Highlands, Austria), while in nonendemic areas, including the United States, the association was almost invariably excluded.49,50 The regression of lymphoma lesions after Borrelia-directed antibiotic therapy (ceftriaxone) has been reported in 6 of 11 cases described in the literature.51 Immunoproliferative small intestine disease, a relatively rare variant of EMZL affecting mainly young men in the Middle East and Africa, has been associated with intestinal infection by Campylobacter jejuni: a 90% rate of response has been reported using broad-spectrum antibiotics.52
Management of localized EMZL outside anti-infective treatment settings
In patients with gastric EMZL who did not achieve lymphoma regression after eradication therapy, the standard approach is represented by involved-site RT (ISRT) at the recommended dose of 24 Gy delivered over 3 to 4 weeks.16 ISRT is associated with excellent outcomes, with CR detected in almost all cases and relapse rates of only 5% to 10% over time.53 ISRT at 24 Gy is also considered the mainstay of the management of localized nongastric EMZL at various sites, leading to long-lasting local disease control in the vast majority of patients.54 In recent years, low-dose RT (4 Gy in 2 fractions) has been increasingly adopted, as it has demonstrated an ability to maintain substantial efficacy (about 70% PFS at 5 years) while reducing toxicity and treatment duration,55 although its use outside the palliative care setting is still controversial.
Although surgery is not generally recommended in the management of EMZL,16 it may be considered a sufficient therapeutic approach in selected asymptomatic patients who have had complete resection of the tumor mass for diagnostic purposes, especially at certain nongastric sites not amenable to RT (eg, duodenum, colon), with subsequent monitoring advised.17
CLINICAL CASE 3
A 62-year-old woman complained of upper respiratory obstruction. A large nasopharyngeal mass was detected by nasal endoscopy, and a biopsy revealed EMZL. PET/CT showed diffuse enlarged lymph nodes (6 cm at retroperitoneal sites) and splenomegaly (16 cm). A BM biopsy detected nodular infiltration by lymphoma (35%). The hemoglobin level was 10.2 g/dL, the platelet count was 115 × 109/L, and the leukocyte count was 9.5 × 109/L, with 65% CD19+ clonal B cells. Her LDH was elevated, resulting in MALT-IPI 2 (high risk). The HCV serology was negative. Due to advanced, high-tumor-burden disease and the absence of significant comorbidities, the patient received 6 cycles of bendamustine-rituximab (BR) and achieved CR (partial response [PR] after 4 cycles).
First-line systemic therapy in advanced-stage EMZL
Current guidelines recommend initiating therapy in patients who have lymphoma-related symptoms; disseminated, high-tumor-burden, or bulky disease; organ function damage; a contraindication or failure of anti-infective or local therapy; and rapid disease progression.16
Rituximab plus chemotherapy is still considered the mainstay of EMZL treatment, based on the results of the randomized IELSG19 study (Table 4), which demonstrated the superiority of chlorambucil plus rituximab over either chlorambucil or rituximab or monotherapy arms (5-year PFS, 72% vs 59% vs 57%).56
Selected studies evaluating first-line treatments in MZLs
| Study . | Phase . | MZL subtype . | N pts . | Regimen . | ORR, % (CR) . | 5-y PFS, % . | Notes . |
|---|---|---|---|---|---|---|---|
| IESLG1956 | 3 | EMZL | 401 (171 gastric) | Chlorambucil alone R-chlorambucil R alone | 85.5 (73.4) 94.7 (78.8) 78.3 (55.8) | 59 72 57 | All regimens well tolerated. |
| MALT2008-0177 | 2 | EMZL | 57 (19 gastric) | R-bendamustine (4 to 6 cycles)a | 100 (98) | 92.8 (7-y) | |
| Alderuccio et al78 | Retrospective | EMZL | 237 (41 gastric) | R-bendamustine (+ R-maintenance in 48) | 93.2 (81) | 80.5 (94.4 vs 81.1) | OS not impacted by R maintenance. |
| IELSG3858 | 2 | EMZL | 112 (36 gastric) | R-chlorambucil +2 years of R-maintenance (sc) | (65) | 87 | No new safety signals. 5-y PFS compare favorably with IELSG19. |
| Kalpadakis et al61 | Retrospective | SMZL | 108 | R × 6 weekly doses (+ R-maintenance in 48) | 92 (65) | 71 (79 vs 52b ) | |
| IELSG36 (BRISMA)62 | 2 | SMZL | 56 | R-bendamustine (4 to 6 cycles)a | 91 (73) | 90 (3-y) | Grade ≥3 AEs 68%; grade ≥3 infections 5.4%. |
| STiL NHL7-08 (MAINTAIN)79 | 3 | SMZL, NMZL | 119 | R-bendamustine (6 cycles +2 R) +2 y of R-maintenance vs WW | 91 (19) | NR vs 92.2 mo (median) | OS not impacted by R-maintenance. |
| GALLIUM59 | 3 | EMZL, SMZL, NMZL | 195 (61 EMZL, 68 SMZL, 66 NMZL) | O-chemo + O-maintenance vs R-chemo + R-maintenance (chemo: B, CHOP, COP) | 81.8 (17.7) vs 81.3 (17.2) | 72.6 vs 64.1 (4-y)c |
| Study . | Phase . | MZL subtype . | N pts . | Regimen . | ORR, % (CR) . | 5-y PFS, % . | Notes . |
|---|---|---|---|---|---|---|---|
| IESLG1956 | 3 | EMZL | 401 (171 gastric) | Chlorambucil alone R-chlorambucil R alone | 85.5 (73.4) 94.7 (78.8) 78.3 (55.8) | 59 72 57 | All regimens well tolerated. |
| MALT2008-0177 | 2 | EMZL | 57 (19 gastric) | R-bendamustine (4 to 6 cycles)a | 100 (98) | 92.8 (7-y) | |
| Alderuccio et al78 | Retrospective | EMZL | 237 (41 gastric) | R-bendamustine (+ R-maintenance in 48) | 93.2 (81) | 80.5 (94.4 vs 81.1) | OS not impacted by R maintenance. |
| IELSG3858 | 2 | EMZL | 112 (36 gastric) | R-chlorambucil +2 years of R-maintenance (sc) | (65) | 87 | No new safety signals. 5-y PFS compare favorably with IELSG19. |
| Kalpadakis et al61 | Retrospective | SMZL | 108 | R × 6 weekly doses (+ R-maintenance in 48) | 92 (65) | 71 (79 vs 52b ) | |
| IELSG36 (BRISMA)62 | 2 | SMZL | 56 | R-bendamustine (4 to 6 cycles)a | 91 (73) | 90 (3-y) | Grade ≥3 AEs 68%; grade ≥3 infections 5.4%. |
| STiL NHL7-08 (MAINTAIN)79 | 3 | SMZL, NMZL | 119 | R-bendamustine (6 cycles +2 R) +2 y of R-maintenance vs WW | 91 (19) | NR vs 92.2 mo (median) | OS not impacted by R-maintenance. |
| GALLIUM59 | 3 | EMZL, SMZL, NMZL | 195 (61 EMZL, 68 SMZL, 66 NMZL) | O-chemo + O-maintenance vs R-chemo + R-maintenance (chemo: B, CHOP, COP) | 81.8 (17.7) vs 81.3 (17.2) | 72.6 vs 64.1 (4-y)c |
4 cycles if CR after 3 cycles, 6 cycles if PR.
FFP not different if R-maintenance 1 and 2y.
Not significantly different (not powered to detect PFS difference in MZL). Grade ≥3 toxicity higher in O arm.
B, bendamustine; chemo, chemotherapy; CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; COP, rituximab, cyclophosphamide, vincristine, prednisone; FFP, freedom from progression; NR, not reached; O, obinutuzumab; R, rituximab; sc, subcute; WW, watch and wait.
Despite proven efficacy, chlorambucil-rituximab has been largely replaced in real life by BR.12 A phase 2 Spanish study tested a response-adapted strategy of 4 or 6 cycles of BR in 57 patients with EMZL and reported a 7-year PFS of 92.8%.57 A recent retrospective international study evaluating a cohort of 237 patients with EMZL treated frontline with BR reported a 5-year PFS of 89.6%; notably, 48 patients received rituximab maintenance and exhibited an improved PFS (94.4% vs 81.1%), although no difference in OS was detected.15
The issue of rituximab maintenance after chlorambucil- rituximab induction was addressed by the phase 2 IELSG38 study, which showed a 5-year PFS of 87%.58 Overall these results suggest a potential favorable impact of rituximab maintenance after frontline chlorambucil-rituximab or BR in EMZL; however, this strategy needs further evaluation, as toxicity issues of rituximab maintenance after BR induction (mainly infections) should be carefully addressed.
Finally, the GALLIUM study failed to demonstrate the superiority of obinutuzumab-based over rituximab-based immunochemotherapy and maintenance in the MZL cohort.59
Many ongoing studies are evaluating chemo-free options in the frontline treatment of EMZL, with the aim to challenge the paradigm of rituximab-chemotherapy (Table 5). For instance, the IELSG47-MALIBU phase 2 study (NCT03697512) is currently testing ibrutinib for 2 years plus rituximab.
Selected phase 2 and phase 3 ongoing studies in untreated or relapsed or refractory MZL
| Compound . | Class . | Setting . | Phase . | N pts . | Primary end point . | Comparator . | ClinicalTrials.gov N (Title) . |
|---|---|---|---|---|---|---|---|
| Ibrutinib-rituximab | BTKi + anti-CD20 | EMZL first-line | 2 | 130 | CR, PFS | / | NCT03697512 (MALIBU, IELSG47 |
| Ibrutinib-rituximab | BTKi + anti-CD20 | EMZL, NMZL, SMZL first-line | 3 | 138 | CR | Placebo-rituximab | NCT04212013 |
| Copanlisib-rituximab | PI3Ki(αδ) + anti-CD20 | EMZL, NMZL, SMZL first-line | 2 | 56 | CR | / | NCT03474744 (COUP-1) |
| Obinutuzumab | Anti-CD20 | EMZL, NMZL, SMZL first-line | 2 | 56 | CR | / | NCT03322865 (OLYMP-1) |
| Pembrolizumab-rituximab | Anti-PD-1 + anti-CD20 | EMZL, NMZL, SMZL first-line | 2 | 56 | CR | / | NCT03474744 (POLE-1) |
| Venetoclax-rituximab | BCL2i + anti-CD20 | EMZL, NMZL, SMZL first-line | 2 | 47 | CR | / | NCT04416451 |
| Mosunetuzumab-lenalidomide | Bispecific (CD3 × CD20) + IMID | FL, EMZL, NMZL, SMZL first-line | 2 | 52 | CR | / | NCT04792502 (BrUOG 401) |
| Mosunetuzumab-polatuzumab vedotin-obinutuzumab | Bispecific (CD3 × CD20) + anti-CD79b + anti-CD20 | FL, EMZL, NMZL, SMZL first-line | 2 | 42 | CR | / | NCT05169658 |
| Ibrutinib-rituximab-lenalidomide | BTKi + anti-CD20 + IMID | FL, EMZL, NMZL, SMZL first-line | 2 | 46 | CR | / | NCT02532257 |
| Acalabrutinib-tafasitamab | BTKi + anti-CD19 | EMZL, NMZL, SMZL R/R (≥1 prior line) | 2 | 24 | CR | / | NCT04646395 (IELSG49) |
| Zanubrutinib-rituximab | BTKi + anti-CD20 | EMZL, NMZL, SMZL R/R (≥1 prior line) | 3 | 372 | PFS | R-lenalidomide | NCT05100862 |
| Orelabrutinib | BTKi | EMZL, NMZL, SMZL R/R (≥1 prior line) | 2 | 80 | ORR | / | NCT03797456 |
| Zandelisib | PI3Kiδ | EMZL, NMZL, SMZL R/R (≥2 prior lines) | 2 | 180 | ORR | / | NCT03768505 (TIDAL) |
| Zandelisib-rituximab | PI3Kiδ + anti-CD20 | EMZL, NMZL, SMZL R/R (≥1 prior line) | 3 | 534 | PFS | BR or R-CHOP | NCT04745832 (COASTAL) |
| SHC014748M | PI3Kiδ | FL, EMZL, NMZL, SMZL R/R (≥2 prior lines) | 2 | 122 | ORR | / | NCT04431089 |
| HMPL-689 | PI3Kiδ | FL, EMZL, NMZL, SMZL R/R (≥2 prior lines) | 2 | 81 (MZL) | ORR | / | NCT04849351 |
| Loncastuximab tesirine | Anti-CD19 | EMZL, NMZL, SMZL R/R (≥1 prior line) | 2 | 50 | CR | / | NCT05296070 |
| Lisocabtagene maraleucel | CAR T CD19 | FL, MZL R/R (≥2 prior lines) | 2 | 188 | ORR, CR | / | NCT04245839) (TRANSCEND FL) |
| Epcoritamab | Bispecific (CD3 × CD20) | MZL R/R (≥2 prior lines) | 2a (expansion cohort) | 30 | ORR | / | NCT03625037 (GCT3013-01) |
| Tafasitamab-rituximab-lenalidomide | Anti-CD19 + anti-CD20 + IMID | FL, EMZL, NMZL, SMZL R/R (≥1 prior line) | 3 | 618 | PFS | Placebo-R-lenalidomide | NCT04680052 (InMIND) |
| Compound . | Class . | Setting . | Phase . | N pts . | Primary end point . | Comparator . | ClinicalTrials.gov N (Title) . |
|---|---|---|---|---|---|---|---|
| Ibrutinib-rituximab | BTKi + anti-CD20 | EMZL first-line | 2 | 130 | CR, PFS | / | NCT03697512 (MALIBU, IELSG47 |
| Ibrutinib-rituximab | BTKi + anti-CD20 | EMZL, NMZL, SMZL first-line | 3 | 138 | CR | Placebo-rituximab | NCT04212013 |
| Copanlisib-rituximab | PI3Ki(αδ) + anti-CD20 | EMZL, NMZL, SMZL first-line | 2 | 56 | CR | / | NCT03474744 (COUP-1) |
| Obinutuzumab | Anti-CD20 | EMZL, NMZL, SMZL first-line | 2 | 56 | CR | / | NCT03322865 (OLYMP-1) |
| Pembrolizumab-rituximab | Anti-PD-1 + anti-CD20 | EMZL, NMZL, SMZL first-line | 2 | 56 | CR | / | NCT03474744 (POLE-1) |
| Venetoclax-rituximab | BCL2i + anti-CD20 | EMZL, NMZL, SMZL first-line | 2 | 47 | CR | / | NCT04416451 |
| Mosunetuzumab-lenalidomide | Bispecific (CD3 × CD20) + IMID | FL, EMZL, NMZL, SMZL first-line | 2 | 52 | CR | / | NCT04792502 (BrUOG 401) |
| Mosunetuzumab-polatuzumab vedotin-obinutuzumab | Bispecific (CD3 × CD20) + anti-CD79b + anti-CD20 | FL, EMZL, NMZL, SMZL first-line | 2 | 42 | CR | / | NCT05169658 |
| Ibrutinib-rituximab-lenalidomide | BTKi + anti-CD20 + IMID | FL, EMZL, NMZL, SMZL first-line | 2 | 46 | CR | / | NCT02532257 |
| Acalabrutinib-tafasitamab | BTKi + anti-CD19 | EMZL, NMZL, SMZL R/R (≥1 prior line) | 2 | 24 | CR | / | NCT04646395 (IELSG49) |
| Zanubrutinib-rituximab | BTKi + anti-CD20 | EMZL, NMZL, SMZL R/R (≥1 prior line) | 3 | 372 | PFS | R-lenalidomide | NCT05100862 |
| Orelabrutinib | BTKi | EMZL, NMZL, SMZL R/R (≥1 prior line) | 2 | 80 | ORR | / | NCT03797456 |
| Zandelisib | PI3Kiδ | EMZL, NMZL, SMZL R/R (≥2 prior lines) | 2 | 180 | ORR | / | NCT03768505 (TIDAL) |
| Zandelisib-rituximab | PI3Kiδ + anti-CD20 | EMZL, NMZL, SMZL R/R (≥1 prior line) | 3 | 534 | PFS | BR or R-CHOP | NCT04745832 (COASTAL) |
| SHC014748M | PI3Kiδ | FL, EMZL, NMZL, SMZL R/R (≥2 prior lines) | 2 | 122 | ORR | / | NCT04431089 |
| HMPL-689 | PI3Kiδ | FL, EMZL, NMZL, SMZL R/R (≥2 prior lines) | 2 | 81 (MZL) | ORR | / | NCT04849351 |
| Loncastuximab tesirine | Anti-CD19 | EMZL, NMZL, SMZL R/R (≥1 prior line) | 2 | 50 | CR | / | NCT05296070 |
| Lisocabtagene maraleucel | CAR T CD19 | FL, MZL R/R (≥2 prior lines) | 2 | 188 | ORR, CR | / | NCT04245839) (TRANSCEND FL) |
| Epcoritamab | Bispecific (CD3 × CD20) | MZL R/R (≥2 prior lines) | 2a (expansion cohort) | 30 | ORR | / | NCT03625037 (GCT3013-01) |
| Tafasitamab-rituximab-lenalidomide | Anti-CD19 + anti-CD20 + IMID | FL, EMZL, NMZL, SMZL R/R (≥1 prior line) | 3 | 618 | PFS | Placebo-R-lenalidomide | NCT04680052 (InMIND) |
BCL2i, BCL2 inhibitor; CR, complete response rate; IMID: immunomodulatory drug; NA, not available; PD-1, programmed cell death 1 protein; PI3Ki, phosphatidylinositol 3-kinase inhibitor; R, rituximab; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone.
First-line therapy in SMZL and NMZL
Asymptomatic patients with SMZL can be managed with active surveillance. The most recognized criteria for treatment initiation include the presence of progressive and symptomatic splenomegaly and the development of cytopenias (hemoglobin level <10 g/dL, platelet count <80 × 109/L, and absolute neutrophil count <1000 × 109/L), while autoimmune manifestations, if present, should be specifically treated.16,18
Traditionally, splenectomy has for many years been considered to be the standard option for SMZL, as, jointly with a definitive pathological diagnosis, it allows for the rapid restoration of cytopenias and a resolution of abdominal discomfort due to splenic bulk. On the other hand, splenectomy is not a curative procedure and may be associated with a nonnegligible rate of complications (thrombosis, bleeding, and infections).60
For these reasons, splenectomy has been largely replaced as a first-line option by rituximab monotherapy, although the main evidence comes from retrospective series. In the largest experiment, 108 patients received 6 weekly doses of rituximab, which was eventually followed in responding patients by rituximab maintenance (1 or 2 years): the ORR after induction was 92% while 5-year PFS was 71%. Rituximab maintenance was associated with better freedom from progression (79% vs 52%).61
First-line BR has been investigated in SMZL by the prospective IELSG36 trial (BRISMA), which included 56 patients treated in 4 to 6 cycles; the ORR was 91% and the CR rate was 73%, with a 3-year PFS and OS of 90% and 96%, respectively. Notably, toxicities of grade 3 or higher occurred in 68% of patients (neutropenia, 43%; infections, 5.4%) and led to treatment discontinuation in 9%.62 Overall, these data demonstrate that BR is a highly effective regimen in SMZL; however, its toxicity profile in this peculiar setting suggests it should not be recommended for generalized use as front-line therapy but reserved for younger, fit patients with disseminated disease.16
Few data exist on the management of NMZL, and current guidelines suggest to follow the same principles currently adopted for follicular lymphoma with regard to risk stratification (Follicular Lymphoma International Prognostic Index score), treatment initiation (Groupe d'Etude des Lymphomes Folliculaires criteria), and the selection of first-line therapy, with ISRT recommended for localized disease and BR as the most commonly adopted regimen in advanced disease.63
CLINICAL CASE 3 (Continued)
Our patient remained disease-free for 22 months, when increasing lymphocytosis was detected. Complete restaging disclosed a disseminated relapse without HT (abdominal adenopathies, splenomegaly, lung nodules, BM infiltration). She started ibrutinib at 560 mg/d and achieved a partial response at the 6-month restaging (still ongoing at the last follow-up after 12 months). Treatment was well tolerated, without significant adverse events.
Management of relapsed or refractory MZL
Disease recurrence generally requires a biopsy to rule out HT. Patients with relapsed MZL require a highly individualized approach, which should be based on stage, tumor burden, organ involved, number and type of prior treatments, and duration of previous remissions, as well as patient age, fitness, and comorbidities.
Patients with localized relapse may be considered for ISRT, while asymptomatic patients with advanced stage recurrence may be closely observed. However, most patients usually require a new systemic treatment at relapse or progression.
Immunochemotherapy could be repeated in the case of long-term remission (≥24 months), while consolidation with autologous stem cell transplantation may be considered in selected young, fit patients with an aggressive chemosensitive relapse.16 In this regard, a retrospective European Society for Blood and Marrow Transplantation study reported a 5-year OS of 73% in 199 relapsed MZL patients.64
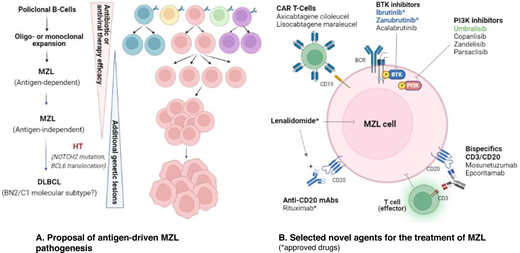
Although no phase 3 studies have been specifically conducted in relapsed MZL, an increasing number of phase 2 trials evaluating novel agents, alone or in combination, have recently been reported (Table 6). However, at the present time few compounds have received US Food and Drug Administration approval (Figure 1).
ORR observed in studies with novel agents in relapsed or refractory MZL. axi-cel, axicabtagene ciloleucel.
ORR observed in studies with novel agents in relapsed or refractory MZL. axi-cel, axicabtagene ciloleucel.
Selected phase 2 and phase 3 studies evaluating novel agents in relapsed or refractory MZL
| Study . | N pts . | MZL subtype . | N prior lines . | Agent/regimen . | ORR, % (CR) . | mPFS,mo . | mDOR,mo . | Grade 3-4 AEs . |
|---|---|---|---|---|---|---|---|---|
| BTK inhibitors | ||||||||
| Noy et al65,66 | 63 | EMZL n = 32 NMZL n = 17 SMZL n = 14 | 2 (1-9) | Ibrutinib 560 mg/d | 58 (10) | 15.8 | 27.6 | Infections (22%), anemia (16%), atrial fibrillation (8%) |
| MAGNOLIA67 | 68 | EMZL n = 26 NMZL n = 26 SMZL n = 12 Uncertain n = 4 | 2 (1-6) | Zanubrutinib 160 mg twice daily | 68 (26) | NR (82.5 at 15 mo) | NR (93% at 12 mo) | Neutropenia (10.3%), infections (16.2%) |
| Strati et al68 | 43 | EMZL n = 19 NMZL n = 13 SMZL n = 11 | 2 (1-4) | Acalabrutinib 100 mg twice daily | 53 (13) | 27 | 75.8% (12 mo) | Neutropenia (14%), infections (7%) |
| PI3K inhibitors | ||||||||
| 101-0980 | 15 | NA | 2 (2-9) | Idelalisib 150 mg twice daily | 46.7 (6.7) | 6.6 | 18.4 | Neutropenia (27%), AST/ALT elevation (13%), diarrhea (13%), pneumonia (7%) |
| DYNAMO81 | 18 | EMZL n = 9 NMZL n = 4 SMZL n = 5 | 2 (1-8) | Duvelisib 25 mg twice daily | 39 (5.5) | 15.5 | NA | Neutropenia (28%), diarrhea (17%) |
| CHRONOS-182 | 23 | EMZL n = 4 NMZL n = 15 SMZL n = 4 | 3 (2-9) | Copanlisib 60 mg ev, days 1, 8, 15 q28 | 70 (9) | 24.1 | 17.4 | Hyperglycemia (39%), hypertension (39%), neutropenia (13%), diarrhea (13%), pneumonia (13%) |
| CHRONOS-370 (phase 3) | 95 | EMZL n = 35 NMZL n = 37 SMZL n = 23 | 2 | Copanlisib-rituximab | 86 (39) | 22.1 | 20.4 | Hyperglycemia (56%), hypertension (40%), neutropenia (16%), pneumonia (7%), diarrhea (5%) |
| UNITY-NHL71 | 69 | EMZL n = 38 NMZL n = 20 SMZL n = 11 | 2 (1-6) | Umbralisib 800 mg/d | 49.2 (15.9) | NR (50.5% at 24 mo) | NR | Diarrhea (2.9%), ALT/AST elevation (4.3%) |
| UNITY-NHL83 | 72 | EMZL n = 33 NMZL n = 31 SMZL n = 8 | 2 (1-9) | Umbralisib-ublituximab | 70 (21) | 16.6 | NR | Neutropenia (18%), diarrhea (13%), ALT/AST elevation (15%) |
| CITADEL-20472 | 100 | EMZL n = 34 NMZL n = 31 SMZL n = 35 | 2 (1-8) | Parsaclisib 20 mg/d (8 weeks), then 20 mg once weekly or 2.5 mg/d | 58 (6) | 16.5 | 12.2 | Diarrhea (12%), neutropenia (10%), pneumonia (9%) |
| Lenalidomide | ||||||||
| AUGMENT73 (phase 3) | 63 | EMZL n = 30 NMZL n = 18 SMZL n = 15 | 1 (1-12) | Lenalidomide 20 mg (days 1-21), 12 cycles + R | 65 (29) | 20.2 | 17.4 | Neutropenia (50%) |
| MAGNIFY (phase 3b) | 74 | EMZL n = 15 NMZL n = 43 SMZL n = 16 | 2 | Lenalidomide 20 mg (days 1-21), 12 cycles + R(+18 mo maint. R2 vs R) | 68 (39) | 41.2 | 38.6 | Neutropenia (37%) |
| CAR T | ||||||||
| ZUMA-575 | 24 | EMZL n = 17 NMZL n = 7 SMZL n = 0 | 3 (2-5) | Axicabtagene ciloleucel 2 × 106/kg | 83 (63) | 17.3 | NR | CRS (8%), ICANS (36%), infections (18%) |
| Study . | N pts . | MZL subtype . | N prior lines . | Agent/regimen . | ORR, % (CR) . | mPFS,mo . | mDOR,mo . | Grade 3-4 AEs . |
|---|---|---|---|---|---|---|---|---|
| BTK inhibitors | ||||||||
| Noy et al65,66 | 63 | EMZL n = 32 NMZL n = 17 SMZL n = 14 | 2 (1-9) | Ibrutinib 560 mg/d | 58 (10) | 15.8 | 27.6 | Infections (22%), anemia (16%), atrial fibrillation (8%) |
| MAGNOLIA67 | 68 | EMZL n = 26 NMZL n = 26 SMZL n = 12 Uncertain n = 4 | 2 (1-6) | Zanubrutinib 160 mg twice daily | 68 (26) | NR (82.5 at 15 mo) | NR (93% at 12 mo) | Neutropenia (10.3%), infections (16.2%) |
| Strati et al68 | 43 | EMZL n = 19 NMZL n = 13 SMZL n = 11 | 2 (1-4) | Acalabrutinib 100 mg twice daily | 53 (13) | 27 | 75.8% (12 mo) | Neutropenia (14%), infections (7%) |
| PI3K inhibitors | ||||||||
| 101-0980 | 15 | NA | 2 (2-9) | Idelalisib 150 mg twice daily | 46.7 (6.7) | 6.6 | 18.4 | Neutropenia (27%), AST/ALT elevation (13%), diarrhea (13%), pneumonia (7%) |
| DYNAMO81 | 18 | EMZL n = 9 NMZL n = 4 SMZL n = 5 | 2 (1-8) | Duvelisib 25 mg twice daily | 39 (5.5) | 15.5 | NA | Neutropenia (28%), diarrhea (17%) |
| CHRONOS-182 | 23 | EMZL n = 4 NMZL n = 15 SMZL n = 4 | 3 (2-9) | Copanlisib 60 mg ev, days 1, 8, 15 q28 | 70 (9) | 24.1 | 17.4 | Hyperglycemia (39%), hypertension (39%), neutropenia (13%), diarrhea (13%), pneumonia (13%) |
| CHRONOS-370 (phase 3) | 95 | EMZL n = 35 NMZL n = 37 SMZL n = 23 | 2 | Copanlisib-rituximab | 86 (39) | 22.1 | 20.4 | Hyperglycemia (56%), hypertension (40%), neutropenia (16%), pneumonia (7%), diarrhea (5%) |
| UNITY-NHL71 | 69 | EMZL n = 38 NMZL n = 20 SMZL n = 11 | 2 (1-6) | Umbralisib 800 mg/d | 49.2 (15.9) | NR (50.5% at 24 mo) | NR | Diarrhea (2.9%), ALT/AST elevation (4.3%) |
| UNITY-NHL83 | 72 | EMZL n = 33 NMZL n = 31 SMZL n = 8 | 2 (1-9) | Umbralisib-ublituximab | 70 (21) | 16.6 | NR | Neutropenia (18%), diarrhea (13%), ALT/AST elevation (15%) |
| CITADEL-20472 | 100 | EMZL n = 34 NMZL n = 31 SMZL n = 35 | 2 (1-8) | Parsaclisib 20 mg/d (8 weeks), then 20 mg once weekly or 2.5 mg/d | 58 (6) | 16.5 | 12.2 | Diarrhea (12%), neutropenia (10%), pneumonia (9%) |
| Lenalidomide | ||||||||
| AUGMENT73 (phase 3) | 63 | EMZL n = 30 NMZL n = 18 SMZL n = 15 | 1 (1-12) | Lenalidomide 20 mg (days 1-21), 12 cycles + R | 65 (29) | 20.2 | 17.4 | Neutropenia (50%) |
| MAGNIFY (phase 3b) | 74 | EMZL n = 15 NMZL n = 43 SMZL n = 16 | 2 | Lenalidomide 20 mg (days 1-21), 12 cycles + R(+18 mo maint. R2 vs R) | 68 (39) | 41.2 | 38.6 | Neutropenia (37%) |
| CAR T | ||||||||
| ZUMA-575 | 24 | EMZL n = 17 NMZL n = 7 SMZL n = 0 | 3 (2-5) | Axicabtagene ciloleucel 2 × 106/kg | 83 (63) | 17.3 | NR | CRS (8%), ICANS (36%), infections (18%) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CR, complete response; CRS, cytokine release syndrome; ev, endovenous; FFP, freedom from progression; ICANS, immune effector cell–associated neurotoxicity syndrome; NA, not available; NR, not reached; R, rituximab; R2 , rituximab-lenalidomide; WW, watch and wait.
The first novel agent selectively evaluated in patients with relapsed or refractory (R/R) MZL was the covalent Bruton's tyrosine kinase inhibitor (BTKi) ibrutinib. At an updated median follow-up of 33 months, the ORR was 58% (CR, 10%), with a median PFS of 15.7 months in 60 evaluable patients. Toxicity was consistent with previous studies in other histologies (atrial fibrillation, 8%; infection ≥ grade 3, 22%).65,66 A phase 2 trial (MAGNOLIA) evaluated the second-generation BTKi zanubrutinib in 68 patients with R/R MZL, showing even more favorable results (at 15 months: ORR, 68.2%; CR, 25.8%; PFS, 82.5%). Zanubrutinib was confirmed to be better tolerated (only 2 cases of atrial fibrillation; infection ≥ grade 3, 16%).67 The preliminary results of a similar phase 2 study with acalabrutinib were recently reported and confirmed the overall efficacy of this class of targeted agents in R/R MZL.68
The second class of novel agents extensively evaluated in R/R MZL is represented by phosphatidylinositol 3-kinase inhibitors (PI3Ki's). Overall, PI3Ki's demonstrated substantial activity in R/R MZL, but their class-specific immune-related toxicity, namely diarrhea/colitis, aspartate aminotransferase/alanine aminotransferase increase, and pneumonitis, hampered a broader use and the development of combinations. However, newer agents with improved target selectivity for δ subunit or intermittent schedules allowing regulatory T cells restoration may potentially result in a major role in R/R MZL in the next future.69
For instance, copanlisib, an intravenous PI3Kαδ inhibitor, was successfully combined with rituximab in a phase 3 placebo-controlled trial (CHRONOS-3) that included 95 patients with R/R MZL and showed significantly improved results when compared with the placebo arm (ORR, 86%; CR, 39%; median PFS, 22.1 months). Toxicity was peculiarly related to α subunit inhibition (≥grade 3 hyperglycemia, 56%, and hypertension, 40%) and required specific medications.70
Umbralisib, a highly selective PI3Kδ inhibitor, was evaluated in the phase 2 UNITY-NHL trial in 69 patients with R/R MZL. The ORR was 49.1%, and the CR was 15.9%, while PFS was 50.5% at 24 months. Notably, the reported rates of immune-related toxicities were the fewest within the class.71 These results led to FDA approval; however, in June 2022 the agency withdrew the approval due to safety concerns in a trial for chronic lymphocytic leukemia patients. Parsaclisib, another selective PI3Kδ inhibitor, demonstrated favorable preliminary results (ORR, 58%; median PFS, 16.5 months) with acceptable toxicity in 100 patients with R/R MZL (CITADEL-204 study).72 However, parsaclisib submission to the FDA was withdrawn by the company in early 2022, shortly after the first-generation PI3Ki's idelalisib and duvelisib were voluntarily removed from the US market for the FL-approved indication.
Finally, the selective PI3Kδ inhibitor zandelisib is currently being explored in R/R MZL with an intermittent dosing schedule in the phase 3 COASTAL study (NCT04745832).
Lenalidomide plus rituximab (R2) is an active combination in patients with R/R MZL, as reported in the phase 3 AUGMENT study (ORR, 65%; CR, 29%), although the improvement in PFS with respect to single-agent rituximab (hazard ratio, 0.46) did not reach statistical significance in the small subset of patients with MZL.73 The preliminary results of the subsequent phase 3 study MAGNIFY substantially confirmed the favorable results of R2 induction.74
The chimeric antigen receptor T-cell (CAR T) product axicabtagene ciloleucel has been tested in a small subset of patients with R/R MZL (n = 24) in the ZUMA-5 study. Although response rates were very high (ORR, 83%; CR, 63%), the median PFS (17.3 months) results were inferior compared to FL patients, and toxicity was substantial (neurological events ≥ grade 3, 36%).75 However, the role of CAR T needs to be further explored in larger cohorts of MZL patients, along with regularly updated follow-up.
Finally, only a few patients with MZL have been treated with the novel CD3/CD20 bispecific antibodies (mosunetuzumab, epcoritamab) within phase 1 studies; however, the results of the expansion MZL cohorts are eagerly awaited. Ongoing trials in R/R MZL are summarized in Table 5.
In conclusion, based on recently reported studies and those currently underway, the treatment landscape of relapsed MZL is expected to evolve rapidly in the next few years in the direction of multiple chemo-free options.
Acknowledgments
This work was partly supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), under the IG 2017-ID. 20767 project (Luca Arcaini) and Ricerca Corrente Fondazione IRCCS Policlinico San Matteo (Luca Arcaini).
The authors thank Prof. Stefano Luminari and Prof. Andrea Filippi for helpful comments.
Conflict-of-interest disclosure
Michele Merli: no competing financial interests to declare.
Luca Arcaini: consultancy, advisory role: Roche, Janssen-Cilag, Verastem, Incyte, EUSA Pharma, Celgene/Bristol Myers Squibb, Kite/Gilead, ADC Therapeutics; speakers' bureau: EUSA Pharma, Novartis; research funding: Gilead Sciences.
Off-label drug use
Michele Merli: nothing to disclose.
Luca Arcaini: nothing to disclose.