Abstract
Growing recognition that the ovary is an end organ in sickle cell disease (SCD), advances in SCD treatment and cure, and innovations in assisted reproductive technologies invite progressive challenges in fertility care for women with SCD. The reproductive life span of women with SCD may be reduced because ovarian reserve declines more rapidly in people with SCD compared to unaffected people. Some young women have diminished ovarian reserve, a risk factor for infertility. Referrals for fertility preservation may be offered and anticipatory guidance about when to seek infertility care provided. For a subset of people with SCD, this information is also applicable when pursuing in vitro fertilization with preimplantation genetic testing to avoid implantation of an embryo with SCD. Here we explore the dimensions of SCD-related fertility care illustrated by the case of a 28-year-old woman with hemoglobin SS disease who initially presented for a hematology consultation for preconception counseling. This case highlights the complexity of preconception SCD management and care and the need to partner with patients to help align pregnancy hopes with SCD treatment and the many associated uncertainties.
Learning Objectives
Recognize that the ovaries are end organs damaged by sickle cell disease and possibly sickle cell treatments
Understand how the regular assessment of pregnancy intention in women with sickle cell disease informs disease-specific preconception care and contraceptive guidance
Discuss how assisted reproductive technologies for fertility preservation and in vitro fertilization, with or without preimplantation genetic testing, are aspects of contemporary sickle cell disease care
CLINICAL CASE
A 28-year-old woman with hemoglobin SS presents for preconception counseling. She has had few acute sickle cell disease (SCD) complications recently and attributes this to hydroxyurea, which she started at age 15 after multiple acute chest syndrome and painful crisis events. She has chronic hip pain from bilateral femoral head avascular necrosis (AVN) that she treats with ibuprofen and acetaminophen. She takes 2000 mg of hydroxyurea with excellent hematologic response: hemoglobin, 9.8 gm/dL; mean corpuscular volume, 128.6 fL, absolute neutrophil count, 2.7 K/µL; hemoglobin F, 26.8%. She takes hormonal contraception. Her husband does not have sickle cell trait. She wants to know: How should she prepare for pregnancy?
Preconception counseling for women with SCD is complicated. Pregnancy and conception need to be addressed in all people of childbearing age at least annually.1 Table 1 provides a minimal list of topics to address. Encouraging patients to develop a (flexible) reproductive life plan can support concrete information sharing.2
Anticipatory preconception counseling includes providing basic information about the reality that SCD pregnancy is high risk and requires high-risk obstetric care.3 Women who do not wish to become pregnant and who are not using contraception can be offered information about contraception and referred for care.4,5 Screening for menstruation-associated SCD pain is also appropriate.6,7 The diagnosis of a hemoglobinopathy trait or disease in a partner sometimes changes reproductive plans, so early offers for patients' reproductive partners to obtain hemoglobin electrophoresis testing with interpretation can inform such plans (Figure 1).8-10 Genetic counseling is offered to address SCD inheritance.11 In some countries this testing and counseling is a standard premarital requirement.10
Preconception counseling for people with SCD includes ensuring that the reproductive partner can obtain hemoglobin electrophoresis testing and correct interpretation. This information may inform reproductive decisions.
Preconception counseling for people with SCD includes ensuring that the reproductive partner can obtain hemoglobin electrophoresis testing and correct interpretation. This information may inform reproductive decisions.
Preconception care for women with sickle cell disease
| 1. If pregnancy not desired, address contraception approach |
| 2. Hemoglobin electrophoresis for reproductive partners with interpretation |
| 3. Genetic counseling |
| 4. Changes to SCD treatment during conception, pregnancy |
| 5. Changes to usual medicines (ie, discontinue NSAIDs, chelators, ACE-I/ARB; start prenatal vitamin) |
| 6. Review historic complications that can affect pregnancy or worsen during pregnancy (ie, AVN, renal disease, cardiopulmonary disease, acute chest, pain, retinopathy) |
| 7. Plan for multidisciplinary high-risk obstetrics and SCD care |
| These are the main themes of preconception counseling for women with SCD. Results from the first 2 steps may direct care in additional directions, such as for gynecology or reproductive endocrinology/infertility care. |
| ACE-I, angiotensin-converting enzyme inhibitors, ARB, angiotensin receptor blocker, NSAIDs, nonsteroidal anti-inflammatory drugs. |
| 1. If pregnancy not desired, address contraception approach |
| 2. Hemoglobin electrophoresis for reproductive partners with interpretation |
| 3. Genetic counseling |
| 4. Changes to SCD treatment during conception, pregnancy |
| 5. Changes to usual medicines (ie, discontinue NSAIDs, chelators, ACE-I/ARB; start prenatal vitamin) |
| 6. Review historic complications that can affect pregnancy or worsen during pregnancy (ie, AVN, renal disease, cardiopulmonary disease, acute chest, pain, retinopathy) |
| 7. Plan for multidisciplinary high-risk obstetrics and SCD care |
| These are the main themes of preconception counseling for women with SCD. Results from the first 2 steps may direct care in additional directions, such as for gynecology or reproductive endocrinology/infertility care. |
| ACE-I, angiotensin-converting enzyme inhibitors, ARB, angiotensin receptor blocker, NSAIDs, nonsteroidal anti-inflammatory drugs. |
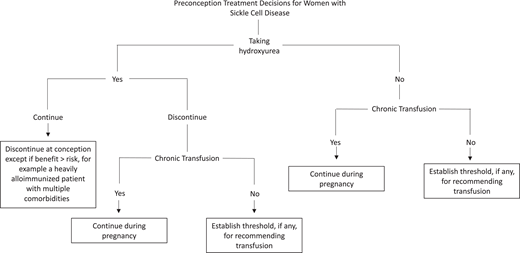
Preconception changes to SCD treatment regimens are challenging (Figure 2). The patient in our clinical case takes hydroxyurea, which is contraindicated at conception and during pregnancy due to an understudied concern for teratogenicity and embryo development.1,12 Discontinuing effective treatment introduces the possibility of potentially severe SCD complications. The only definitive therapy available is red cell transfusion.13 Many clinicians recommend chronic transfusions in this setting, but barriers include logistical difficulties, treatment refusal, a lack of established treatment indications, and, most dreadfully, alloimmunization.14 In low-resource settings, transfusions may be altogether impossible due to blood supply limitations. There are limitations to each approach, and some patients elect to continue hydroxyurea, as there are reports that women conceive while on therapy. Unfortunately, little is definitively known about those who do not conceive or report early pregnancy losses on therapy.12,15 Insufficient evidence exists to continue other SCD therapies.
Preconception SCD treatment decision tree for clinicians and patients highlights complex considerations and the need for transparent, shared decision-making. These choices are not equally available to all people: for people without expert SCD care and/or where resources are limited, access to blood transfusions may be sorely limited. In cases of severe SCD and in settings where red cell transfusion is unavailable or impossible, hydroxyurea during pregnancy might need to be considered even without strong evidence to address safety.
Preconception SCD treatment decision tree for clinicians and patients highlights complex considerations and the need for transparent, shared decision-making. These choices are not equally available to all people: for people without expert SCD care and/or where resources are limited, access to blood transfusions may be sorely limited. In cases of severe SCD and in settings where red cell transfusion is unavailable or impossible, hydroxyurea during pregnancy might need to be considered even without strong evidence to address safety.
Additional layers of anticipatory guidance are needed and are summarized here and discussed extensively elsewhere.1,3 These include providing information regarding the physical demands of normal pregnancy, as the expected weight gain, lordotic posture, and a growing uterus are uncomfortable for most women and can lead to SCD complications. End-organ function, especially the lungs, heart, and kidneys, before pregnancy requires considered evaluation. Some medications are discontinued (nonsteroidal anti-inflammatories, chelators), and analgesic choices are restricted to acetaminophen and opioids. This patient has bilateral AVN of the hips, which can compromise vaginal delivery.3 The fetus is also at risk for adverse outcomes, including fetal demise and intrauterine growth restriction.3 Negative effects on children's development are theoretically possible since, in general, antenatal anemia and opioid exposure are associated with these outcomes, but children born to women with SCD are little studied outside the immediate postpartum setting.16
Finally, there are established uncertainties and biased perceptions about fertility in women with SCD.12 In patients pursuing unassisted conception, addressing infertility concerns may help. Infertility is a clinical diagnosis sometimes supported by laboratory or imaging findings. In the general population, infertility in women under 35 years old is defined as the failure to conceive after 12 months of unprotected intercourse and in women over 35 years old as the failure to conceive after 6 months.17 In general, the shorter allowed duration of attempting to conceive in older women is because after age 35 women have poorer egg quality, and ovarian reserve declines more precipitously. Age-based thresholds for infertility diagnosis may not apply to women with SCD, who have a sharper trajectory of decline in ovarian reserve.18-22 At Johns Hopkins, we offer reproductive endocrinology and infertility (REI) specialist consultation to women who have attempted to conceive for 6 months or longer. Helping patients know when to seek care can help with timely REI referral; recognized barriers to infertility care make attention to referral timing especially important.23
The complexities of SCD preconception care make obvious why ideally all women with SCD should have access to comprehensive SCD care with colocalized nurse coordinators and subspecialists, including genetic counselors, gynecologists, maternal fetal medicine specialists, and reproductive endocrinologists in support of patient informational, care, and decision-making needs.24
CLINICAL CASE (Continued)
The patient returns with her husband's hemoglobinopathy test results. She was told that the test confirms that her husband does not have sickle cell trait. The reviewed hemoglobin electrophoresis shows hemoglobin AC. Her husband has hemoglobin C trait, and there is a 50% chance that their child will have hemoglobin SC disease. She wants to know: Now what?
People with the genetic potential to have a child with SCD need accurate information about reproductive options (Figure 1).10,11 These include unassisted pregnancy with or without fetal diagnostic testing and the choice of elective termination and in vitro fertilization (IVF) with preimplantation genetic testing for monogenetic disorders (PGT-M). IVF+PGT-M targets monogenic disorders and must be distinguished from PGT to screen embryos for aneuploidy (PGT-A). Families of children with SCD and adults with SCD indicate interest in IVF+PGT-M, even when not pursuing this reproductive choice.11 Patient education material developed with SCD clinician, advocate, and patient input meeting health literacy recommendations of the Centers for Disease Control and Prevention may help inform care.11 As families weigh options, some value meeting a pediatric hematologist, speaking to parents of children with SCD, or speaking to an affected adult with the specific genotype in question. Reproductive choices respect patient preferences and values. In many countries, including the US, legal, logistical, and financial barriers to the entire spectrum of reproductive health care infringe upon the human right to reproductive autonomy. Unfortunately, surmounting legal, logistical, and financial barriers to desired care can be difficult, and these barriers are compounded by racial and regional disparities in reproductive health care.23
The possibility of having a child with SCD can be emotionally complex for some people with SCD and their families.8,11 Some, but not all, women with SCD express anguish about how to proceed. Stigmatized reproductive choices alongside medical and emotional vulnerability may compromise close and supportive relationships. Disclosing reproductive decision-making to usually supportive friends or family members can be uncomfortable and even relationship jeopardizing. The clinician offers nonjudgmental support for patients, serving as a safe harbor as patients navigate existential and practical concerns.
CLINICAL CASE (Continued)
The patient returns. She and her husband are pursuing IVF+ PGT-M, which is covered by her private health insurance. She had initial ovarian reserve testing performed by an outside REI. Her anti-Mullerian hormone (AMH) level is 0.82 ng/ml, and her menstrual cycle day 3 follicle-stimulating hormone is 12 IU. Transvaginal ultrasound measured an antral follicle count of 7. Her REI says that she has diminished ovarian reserve (DOR), a risk factor for poor IVF outcomes, including canceled cycles and a low number of eggs retrieved. She wants to know: “Should I have had this testing sooner? Does DOR have to do with sickle cell or my 13 years of hydroxyurea use?”
The ovaries are SCD end organs vulnerable to inflammation and hypoxic ischemic damage.12,20 SCD and some treatments may damage ovarian follicles, depleting the finite egg supply.25 This helps explain why age-associated decline in ovarian reserve appears to be accelerated in women with SCD (Figure 2).18-21 In a small study of women with sickle cell anemia (SCA), menopause occurred earlier than the general population.26 This might be expected in a disease associated with accelerated aging and supports the hypothesis that women with SCD have a narrower reproductive window than unaffected women.22 As infertility risks may accrue at a younger age in women with SCD than in unaffected women, tests to measure ovarian reserve may help direct care (Table 2).25
Common hormone labs sent in support of ovarian reserve testing and reproductive endocrinology/infertility evaluations
| . | Clinical significance . | Limitations . |
|---|---|---|
| Follicle-stimulating hormone | - Pituitary hormone that binds to ovarian granulosa cells where androgens are converted to estrogens - Stimulates folliculogenesis - Ovarian reserve marker - ≥25-40 IU × 2, premature ovarian insufficiency | Fluctuates with menstrual cycle |
| Luteinizing hormone | - Pituitary hormone that stimulates progesterone and androgen production within ovarian theca cells - Oocyte maturation—progresses from arrested prophase I to metaphase II (state required for fertilization) | Fluctuates with menstrual cycle |
| Estradiol | - Produced by testosterone via aromatase in granulosa cells - Breast and uterine development during puberty - Prepares endometrium for embryo implantation - Maintains bone mineral density - Measure of ovarian function | Fluctuates with menstrual cycle |
| Progesterone | - Stabilizes and maintains uterine lining for pregnancy - Decline induces menses | Fluctuates with menstrual cycle |
| AMH | - Ovarian reserve marker - <1-1.1 ng/mL consistent with diminished ovarian reserve (assay dependent) - Helps predict ovarian response to IVF medications | Does not fluctuate with menstrual cycle Varies with age Wide range of normal Reflects growing follicle pool May not reflect dormant primordial follicle pool |
| Antral follicle count | - Ovarian reserve measure - Helps predict ovarian response to IVF medications and pregnancy rate | Inter- and intracycle variation Prone to observer bias |
| . | Clinical significance . | Limitations . |
|---|---|---|
| Follicle-stimulating hormone | - Pituitary hormone that binds to ovarian granulosa cells where androgens are converted to estrogens - Stimulates folliculogenesis - Ovarian reserve marker - ≥25-40 IU × 2, premature ovarian insufficiency | Fluctuates with menstrual cycle |
| Luteinizing hormone | - Pituitary hormone that stimulates progesterone and androgen production within ovarian theca cells - Oocyte maturation—progresses from arrested prophase I to metaphase II (state required for fertilization) | Fluctuates with menstrual cycle |
| Estradiol | - Produced by testosterone via aromatase in granulosa cells - Breast and uterine development during puberty - Prepares endometrium for embryo implantation - Maintains bone mineral density - Measure of ovarian function | Fluctuates with menstrual cycle |
| Progesterone | - Stabilizes and maintains uterine lining for pregnancy - Decline induces menses | Fluctuates with menstrual cycle |
| AMH | - Ovarian reserve marker - <1-1.1 ng/mL consistent with diminished ovarian reserve (assay dependent) - Helps predict ovarian response to IVF medications | Does not fluctuate with menstrual cycle Varies with age Wide range of normal Reflects growing follicle pool May not reflect dormant primordial follicle pool |
| Antral follicle count | - Ovarian reserve measure - Helps predict ovarian response to IVF medications and pregnancy rate | Inter- and intracycle variation Prone to observer bias |
When normal, ovarian reserve measures do not predict fertility—that is, the ability to become pregnant. However, these results may change management. In oncology patients who require gonadotoxic treatment, ovarian reserve testing informs counseling about risks for premature ovarian insufficiency before and after treatment and fertility preservation decisions.21,27 This approach may be warranted in SCA as some young women with SCA have DOR.20,21 In general, women found to be at risk for developing ovarian insufficiency or severe DOR are referred to REI to discuss fertility preservation. This forms the basis for the recent conclusion that fertility-preserving interventions should be offered to girls and women with SCA.21,27 Not all patients want ovarian reserve testing, and so, until further evidence is generated, testing may be considered during reproductive life- planning discussions, in alignment with goals for SCD treatment or cure, and with shared decision-making.12
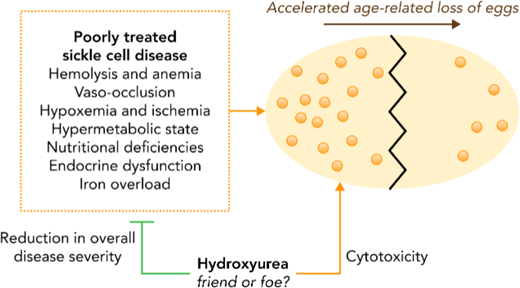
This patient's AMH is in the first quartile of values for age as measured in Multi-Center Study of Hydroxyurea participants, most of whom were hydroxyurea exposed (Figure 3).20 When this patient started treatment in the early 2000s, little evidence addressed hydroxyurea's effects on ovarian reserve or fertility, infants did not routinely start treatment, and fertility-preserving interventions were less advanced. Now there are standard fertility-preserving interventions for prepubescent and postpubescent females, and studies of AMH in young women identify both SCA and hydroxyurea use as DOR risk factors.20,21,28 Hydroxyurea's association with DOR may be a causal relationship but might alternatively or concomitantly reflect that hydroxyurea is a marker of disease severity (Figure 4).27 Since the patient has DOR and is pursuing IVF+PGT-M, she chose to stop hydroxyurea and started chronic transfusions. This decision is more difficult when transfusions are not an option.12
AMH levels in women with hemoglobin SS who participated in the follow-up to the Multi-Center Study of Hydroxyurea. Age-associated decline occurs, and median levels in study subjects (dark gray bar) are lower than median age-matched assay values. The median AMH in women aged 26 to 30 is consistent with diminished ovarian reserve, an infertility risk factor. Almost all subjects had some hydroxyurea exposure.20 Reproduced with permission from British Journal of Haematology.20
AMH levels in women with hemoglobin SS who participated in the follow-up to the Multi-Center Study of Hydroxyurea. Age-associated decline occurs, and median levels in study subjects (dark gray bar) are lower than median age-matched assay values. The median AMH in women aged 26 to 30 is consistent with diminished ovarian reserve, an infertility risk factor. Almost all subjects had some hydroxyurea exposure.20 Reproduced with permission from British Journal of Haematology.20
The mechanism of accelerated decline in ovarian reserve in women with SCD is not yet established. Many possible contributors may be differentially impactful at different ages or with different SCD and treatment exposures. Reproduced with permission from Blood.27
The mechanism of accelerated decline in ovarian reserve in women with SCD is not yet established. Many possible contributors may be differentially impactful at different ages or with different SCD and treatment exposures. Reproduced with permission from Blood.27
CLINICAL CASE (Continued)
The patient initiates chronic transfusions targeting hemoglobin of 10 gm/dL and hemoglobin S of 30%. She wants to know if she needs to do anything else before she begins controlled ovarian hyperstimulation (COH) for oocyte harvest to start an IVF+PGT-M cycle
The process of IVF for women with SCD is described.11,25 Egg harvesting involves COH and transvaginal ultrasound-guided egg retrieval, a short, outpatient surgical procedure performed under sedation.
Women with SCD have unique risks for COH complications attributable to patient and stimulation regimen factors. SCD is a thrombophilia risk factor and some degree of renal injury occurs in most affected people. COH causes a hyperestrogenic state and is therefore a thrombophilia risk.29 COH can also cause ovarian hyperstimulation syndrome (OHSS), a capillary leak syndrome that occurs on a spectrum of severity ranging from mild volume overload to acute kidney injury requiring dialysis.29 Contemporary rates of severe OHSS are low, as increasingly sophisticated stimulation protocols are tailored to individual risk.25,29 However, SCD complications such as pain and acute chest syndrome are also described.29 These complications can be life-threatening and, less dramatically, interrupt stimulation cycles. A published clinical protocol addresses considerations in caring for people with SCD undergoing COH.29
COH and oocyte retrieval require collaborative care to mitigate procedural risks.29 All patients need support to adhere to the time-limited, high-stakes COH medication regimens that require daily injectable medication for 8 to 12 days, usually with the assistance of a care partner.28 To reduce SCD complications and reduce a painful crisis with OHSS, which can be uncomfortable, exchange transfusion can be offered before stimulation (approximately 1 to 2 weeks before egg retrieval). On a case-by-case basis, anticoagulation is recommended. At egg retrieval, dexamethasone is avoided due to SCD-specific risks.30 We ask patients to measure daily weights for a week after egg harvest to enhance the early detection of OHSS. Fertility specialists who infrequently care for SCD patients should be advised of the potential for severe complications in SCD patients. They may elect to be more conservative with dosing ovarian stimulation medications and use protocols that reduce OHSS risk.
CLINICAL CASE (Continued)
The patient’s first cycle of COH and oocyte retrieval is uncomplicated and yields 4 oocytes and 1 euploid embryo. PGT identifies the embryo as chromosomally normal (euploid) but positive for hemoglobin SC disease. A second cycle yields 5 oocytes and 2 euploid embryos. One is positive for hemoglobin SC. The second has hemoglobin AS, is transferred, and implants. Then at 8 weeks the patient miscarries. She calls to share this news and asks, “Is this a typical IVF+PGT experience for women with SCD?”
Women with SCD face a generally increased risk of miscarriage compared to unaffected women.3 In women with infertility using IVF, embryos screened with PGT-A have reduced miscarriage rates because aneuploidies are the most common reason for early pregnancy loss.31 Cytogenetic testing on the products of conception for pregnancy losses that occur with a PGT-tested embryo is usually recommended. In this case the spontaneous miscarriage was managed at home without an opportunity to send testing. Although the PGT process excludes common embryonic causes of miscarriage, both maternal factors and/or unmeasured embryo factors may contribute to miscarriage.
This patient had a very low egg yield. This is reported in SCD and is possibly attributed to a low baseline ovarian reserve for age.25,29 Counseling people pursuing IVF+PGT-M in general is challenging because existing data mostly address IVF outcomes in couples whose indication for care is infertility. However, in a case study of 60 couples in the UK pursuing IVF+PGT-M for preimplantation SCD diagnosis, outcomes were promising.32 Sixty- three percent of women had a live birth, and couples who did not become pregnant stopped trying before using all available IVF cycles covered by the National Health Service. This hopeful study included 3 women with SCD aged 31, 33, and 35, each of whom had a live birth. Two required 1 cycle of COH, and the third required 3 cycles. SCD genotype and treatment information was not included.
CLINICAL CASE (Continued)
Almost 3 years later, the patient anticipates a third IVF+PGT-M cycle. She changes health insurance. Her new center lacks SCD expertise, and her insurance company denies her appeal to receive care at a specialty SCD center. Exchange transfusions are logistically difficult and further complicated when she becomes alloimmunized. She is twice hospitalized with pain. Her AMH level continues to decline. A new obstetrician reviews her case and stresses that she could die in pregnancy. She calls to ask, “Is that true?”
There are significant limits to predicting pregnancy outcomes in SCD, and all too often these kinds of “death threats” reach people with SCD, often along with misinformation about SCD pregnancy.5,13,14,33 Women deserve realistic support as they pursue meaningful family-building goals. Both structural and biological factors affect SCD-associated maternal morbidity and mortality.13,34,35 Complications can have long-term repercussions.36 At centers with integrated, multidisciplinary SCD and obstetric expertise, pregnancy-related mortality in SCD is reduced in both high- and low-income settings.13,34 Our Clinical Case patient, however, is still not pregnant; perhaps the third cycle of IVF will result in a live birth.32
The roads not taken by this couple highlight additional dimensions of SCD fertility care. This patient could have pursued unassisted conception. Whether DOR or hydroxyurea use would have affected this outcome is unknown. More evidence is needed to establish infertility risks in women with SCD, especially in those with long-standing hydroxyurea exposure or iron overload.12 The literature overwhelmingly reports pregnancy outcomes rather than systematically appraising infertility, including recurrent miscarriage in SCD; these are more difficult end points to assess.37 Possibly, the patient's DOR and poor COH response are unrelated to SCD or SCD treatment and are instead caused by unmeasured patient or partner factors, or the COH approach. Further, earlier fertility preservation may have improved egg harvest yield and made higher-quality eggs available for IVF. Young people may now benefit from this approach.27 Answers to outstanding questions about the effects of SCA and SCA therapies on ovarian reserve, ovarian response to COH, egg quality, and embryo development will help inform fertility care in SCD.21
This couple had many reproductive choices as they sought care in 1 of the fewer than 20 states in the US with at least some mandated coverage for assisted reproductive technology use; most of these states also protect the right to abortion. However, the majority of families affected by SCD have fewer choices.38 Around the globe, in high-, middle-, and low-income settings, people with SCD lack access to expert SCD care. Early mortality and severe SCD morbidity are infertility risk factors inasmuch as they may preclude future reproductive opportunities. Further, many women face physical and social consequences when they experience infertility, pursue abortion, or have a child with SCD. Despite the presence of sometimes severe clinical constraints, working to ensure that patients receive compassionate, noncoercive, and evidence-based reproductive health care is an obligation of high-quality SCD care.
This case illustrates the need for early preconception counseling for all people with SCD and exemplifies the fertility challenges faced by women with SCD and their families, some of whom receive improper testing or interpretation of hemoglobinopathy testing while others receive no testing at all. Concerns about hydroxyurea's effect on this young woman's ovarian reserve are raised, but the protective effects of treatment for over a decade are also evident. Alloimmunization and hospitalizations for pain are serious morbidities to accrue during the pursuit of pregnancy.39 There is no crystal stair for people with SCD pursuing biological parenthood, but many keep climbing.40 The question is not why people keep trying but how we can help them succeed.
Acknowledgments
Thank you to the people living with sickle cell disease and their families, especially people pursuing pregnancy, whose trust and teaching shaped this manuscript.
The title references Langston Hughes' poem “Mother to Son” (https://www.poetryfoundation.org/poems/47559/mother-to-son).
Thanks also to Lauren Anthony at Johns Hopkins University for support with tables and figures.
Conflict-of-interest disclosure
Lydia H. Pecker: consultancy: Global Blood Therapeutics; research funding: National Institutes of Health/Heart, Lung, and Blood Institute (grant K23HL146841), American Society of Hematology Clinician Scholars Award, Mellon Foundation, Doris Duke Foundation; cofounder: Sickle Cell Reproductive Health Education Directive.
Alecia Nero: no competing financial interests to declare.
Mindy Christianson: no competing financial interests to declare.
Off-label drug use
Lydia H. Pecker: hydroxyurea in pregnancy is discussed.
Alecia Nero: hydroxyurea in pregnancy is discussed.
Mindy Christianson: hydroxyurea in pregnancy is discussed.