Abstract
The platelet collection and distribution system, based on volunteer nonremunerated donors, apheresis platelet collections, and primarily 1-directional distribution of platelets for up to 5-day room temperature storage at hospitals, typically performs well and provides therapeutic support for hundreds of thousands of patients annually. However, direct and indirect effects of the coronavirus disease 2019 pandemic, particularly during the Omicron wave, produced dramatic systemic failures and severe shortages. We propose 4 initiatives to reinforce the existing platelet pipeline and buffer the platelet supply against future unexpected disruptions.
Learning Objectives
Understand the current pipeline for providing platelets for transfusion and how this system can fail, leading to severe shortages
Learn about 4 different approaches to supplement the current platelet pipeline and how they can affect platelet supply
Understand gaps in knowledge that may influence the outcomes of any of these 4 approaches to supplement the platelet supply
CLINICAL CASE
A 74-year-old man presented with back pain due to a thoracoabdominal aortic aneurysm. The surgeon requested 4 apheresis platelet units for surgery. However, due to the Omicron wave, the systemwide platelet inventory was 75% below par level. After triaging platelets for other patients, only 1 unit could be committed for this surgery. The clinical team decided to monitor the aneurysm until sufficient platelets were available for surgery. Two days later, the patient's aneurysm turned into a dissection involving the ascending aorta. The platelet inventory had not increased appreciably, and only 2 platelet units could be provided for surgery. The patient died during the procedure due to uncontrolled hemorrhage, with last recorded platelet count of 27 000/µL.
Current logistics underlying the platelet transfusion pipeline
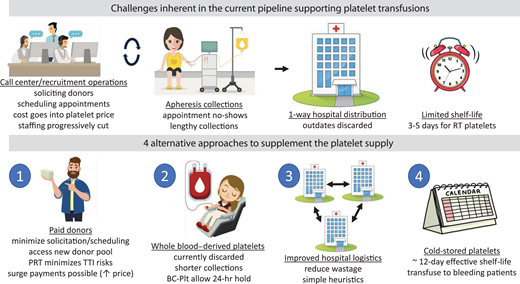
In the United States, over 2.2 million platelet transfusions are performed annually.1 Despite sophisticated systems to collect, process, and distribute platelets from apheresis donors, shortages still occur. Sometimes these shortages are transient, geographically localized, or readily mitigated. However, the coronavirus disease 2019 Omicron surge led to a protracted, widespread, and severe disruption of the platelet supply. Hospital-based transfusion services were challenged to radically adjust operations and implement enhanced blood conservation strategies to mitigate the effects of shortages.2 The impact on fixed-site apheresis platelet collections was multifactorial, with fewer volunteers willing or able to donate, and unprecedented staff call-outs; the effect was exacerbated by prior US Food and Drug Administration (FDA) requirements to mitigate risks of bacterial contamination, which decreased the yield of platelet units.
A review of platelet logistics suggests approaches to reinforce the pipeline and prevent dramatic disruptions in the future (see the visual abstract). The current system depends on recruiting new volunteer (nonremunerated) donors, converting whole-blood donors to apheresis platelet donors, and scheduling collections. These donor recruitment operations, accounting for up to two-thirds of blood pricing,3 have been affected by budget cuts and staffing loss.4 Additionally, apheresis procedures are lengthy,5 limiting daily collections. When donors fail to show up, the recruitment effort is wasted and apheresis instruments are idle. Unfortunately, financial constraints and historical customer preferences have prevented many blood centers from producing platelet-rich plasma platelets (PRP-Plt) from whole-blood donations, representing lost platelet products.
After platelets are released to hospitals, their 3- to 5-day room temperature shelf-life presents significant risks of outdating. Larger hospitals try to minimize wastage by redistributing shorter-dated units within their system. Small hospitals either accept high platelet wastage rates in order to transfuse the occasional patient or cooperate with other hospitals (or blood centers) to rotate inventory for optimal usage. Nonetheless, in a 2019 survey, almost 20% of all platelet units expired in hospitals while an additional 9% eventually expired on blood center shelves.1
Our aim is not to propose that the current system should be abandoned but rather to suggest modifications that can strengthen it against disruptions like the pandemic. As recently proposed by one of the coauthors,5 we suggest 4 specific approaches: (1) use paid donors as an additional source, (2) continue efforts toward producing buffy coat platelets (BC-Plt), (3) improve logistics to (re)distribute platelets for optimal usage, and (4) implement cold-stored platelets to extend shelf-life and better support bleeding patients. There are additional initiatives, such as reducing platelet dosage,6,7 that could also be considered in a comprehensive strategy to prevent platelet shortages.
Supplementing the volunteer blood donor system with paid donations
The recruitment of volunteer nonremunerated donors combined with extensive predonation medical histories, sophisticated infectious disease testing, and pathogen reduction treatments (PRTs) has made blood transfusion one of the safest of all medical therapies. Nonetheless, there are challenges, including the relative inelasticity of supply. Although donation lulls during holidays can be addressed through public appeals, and local weather events can be compensated by shipments from unaffected regions, when large swaths of the country are affected, such as during the coronavirus disease 2019 pandemic, these approaches have limited buffering capacity, and platelet inventories can plummet to critically low levels.
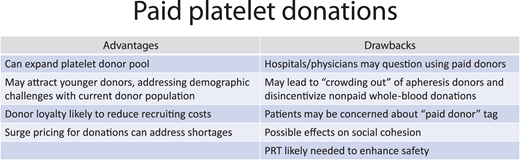
Among approaches to mitigate such events, the most direct is to pay donors, based on several factors (Figure 1). Remuneration for source plasma donations has been highly successful in the United States, which supplies ~70% of source plasma worldwide.3 Additionally, this approach is elastic. Like surge pricing brings more Lyft drivers into areas with waiting customers, increased remunerations can motivate donations to address blood deficits. Furthermore, paid donations are not new. Blood centers have incentivized “volunteer” donors for years, in accordance with FDA guidance, through gift cards (currently, up to $25) or branded apparel identifying people as donors. By comparison, larger payments ($40-$50 gift cards, similar to source plasma donations) are not particularly remarkable and are legal per the US Code of Federal Regulations, provided that labeling reflects donor compensation.5
Not surprisingly, donor payment is an area of controversy, partly because remuneration is poorly defined.3 For example, according to Dr. Peter Marks (Center for Biological Evaluation and Research, FDA), if a donor receives any amount of cash, their unit is considered a paid donation.3 In contrast, if compensation is received as a gift card or time off work, it is not a paid donation.3 The World Health Organization (WHO) takes a more hardline stance, advocating for only voluntary nonremunerated blood donations, including source plasma.8 WHO also broadly defines remuneration as receipt of payment “either in the form of cash, or in kind which could be considered a substitute for money. This would include time off work other than reasonably needed for the donation and travel.”8 Thus, blood centers that provide donors with gift cards, plasma collectors that pay donors, and organizations or countries that provide excessive time off work run afoul of WHO guidance.
There are 2 main reasons why WHO promotes voluntary nonremunerated blood donations: the assertion that paid blood donations threaten blood safety and that they “erode community solidarity and social cohesion.”8 The first reason does not seem applicable to developed countries, where viral nucleic acid testing combined with PRT should mitigate any increased risk of TTI from paid platelet donors. For example, a review of over 2 million (nonremunerated) platelet donations transfused between 2010 and 2018 showed no transfusion-transmitted infections (TTI) from 40 000 PRT apheresis platelets.9 It should be noted that other components in short supply during the pandemic, such as RBCs, cannot currently undergo PRT and would likely require an alternative approach to boost supply. A more thorough discussion of the impact of PRT on platelet safety can be found in the accompanying article from Dr. Szczepiorkowski.10
The second reason includes both concerns for the vulnerability of disadvantaged populations with ready access to plasma collection centers,11 as well as the risks that paid donations could “crowd out” or reduce voluntary donations. The latter possibility has not been well studied and limited data are conflicting.3 Records from St. Louis, Missouri, which experienced a large increase in paid plasma donations, showed no impact on nonremunerated blood donation volumes in that region (Figure 3 in Stubbs et al5 ). In contrast, participants at a recent Association for Advancement of Blood and Biotherapeutics summit shared anecdotal evidence of crowding out in their regions.3 As remuneration is likely to appeal to some donors but alienate others,9,12 further investigations of the net impact of donor payments on the blood supply are warranted. For example, would paid platelet- only collectors reduce platelet revenues of other blood centers, negatively affecting their operating margin and their ability to provide RBCs and other blood products?
Early adoption of platelet donor remuneration is unlikely to be led by large blood organizations, which must maintain the support of their volunteer donor base and clients. For example, in an American Red Cross (ARC) survey of 8000 current and potential platelet donors, only 15% to 18% said incentives would motivate them to donate platelets.13 Additionally, in parallel surveys of health care decision makers, only 21% indicated they would unconditionally accept these products.13 Furthermore, paid donation is unlikely to directly affect staffing shortages, although for-profit blood centers may have the financial flexibility to pay higher wages to retain and recruit sufficient staff.
These data, however, are unlikely to deter current and future entrants into the emerging paid platelet industry. For example, the ARC survey, as pointed out by Taylor,14 only included persons who previously voluntarily donated blood. This is a subset of those qualified to donate, and it is the remainder of this group, current nondonors, who are the focus for paid donation efforts. Newly converted remunerated donors are also likely to be younger individuals,15 a valuable demographic missing from the aging donor pool.5 Whether recruitment of younger donors to paid-platelet collection will negatively affect collections of other blood products deserves further study. Additionally, as health care leaders are open to paid-donor platelets under some circumstances (eg, 53% would use them as “a last option”), their comfort level may increase with time.13
BC-Plt as an attractive approach to supplement the US apheresis platelet supply
Ongoing and often unpredictable, platelet shortages could be abrogated by increased production of whole blood–derived platelets (Figure 2). Whereas PRP-Plt represented one-third of platelets transfused in 2001, usage declined to <6% in 201916 and has continued to decrease further. Unfavorable logistics played a significant role in decreased production. Additionally, in the United States, blood centers balance their budgets by offsetting competition-driven negative RBC margins through higher margins for apheresis platelets (Aph-Plt). For many years, Aph-Plt were believed to have clinical advantages, justifying their increased price and supporting their growing dominance of the market. These included lower rates of bacterial contamination, other TTIs, RhD alloimmunization, and transfusion reactions. The first 2 potential advantages have been negated by PRT,17,18 while a 2016 review suggests that the other differences are also negligible.16 Nonetheless, PRP-Plt are not likely be resurrected in the United States owing to a number of logistical challenges, including a brief 6-hour window for delivery to manufacturing sites so that PRP-Plt can reach the rotator by 8 hours after collection.
For an alternative approach to capture platelets from whole-blood collections, we should look outside the United States. In 2004, Canadian Blood Services implemented BC-Plt production and now produces >60% of its platelet doses as BC-Plt pools, reserving Aph-Plt for matched platelet needs.19 In the Netherlands, >90% of platelet transfusions are BC-Plt.19 In contrast to PRP-Plt, BC-Plt can be produced up to 24 hours after collection, turning an 8-hour barrier into a 24-hour opportunity that provides sufficient time to ship units between distant collection and manufacturing sites and mitigates other logistic (and costly) challenges such as staffing 11 pm to 6 am work shifts. The availability of automated BC-Plt manufacturing instrumentation should further reduce staffing requirements. Additionally, a recent development may serve as the trigger to implement BC-Plt in the United States. European Union legislation taking effect in 2025 will ban di(2-ethylhexyl)phthalate, with worldwide implications for manufacturers of blood collection kits and their customers.20 As the FDA will require manufacturers to fund approval studies for non–di(2-ethylhexyl)phthalate kits, including those for Aph-Plt production, some may choose to pursue regulatory approval of BC-Plt kits instead. After investment costs for automation and collection sets are recouped, implementation of BC-Plt is likely to be financially beneficial for blood collectors and hospitals alike, as well as providing a needed boost to the platelet supply.
Improved distribution logistics: system-based platelet management
While initiatives to increase the platelet supply such as paid platelet donors and BC-Plt depend on demonstrating fiscal benefits to blood centers, hospitals can independently increase their inventory through improved distribution systems. Prior to 2013, each hospital within the Emory Healthcare system independently ordered platelets from ARC. There was minimal effort to redistribute inventory between hospitals, and accordingly, outdate rates often exceeded 20%. To address this problem, we implemented a pilot project between our 2 largest hospitals: Emory University Hospital (EUH) and Emory University Hospital–Midtown (EUHM). The former has a substantial hematology/oncology service, including an ambulatory infusion clinic; the latter has a large cardiovascular surgery practice. EUHM stocked platelets based on the cardiovascular surgery schedule; if cases did not require platelets, many units were outdated. In contrast, EUH had more predictable platelet demands, driven by hematology/oncology patients, and low outdate rates. Initially, we implemented a simple system (version 1) where platelets within 24 hours of outdate at EUHM were shipped to EUH and added to their platelet inventory, and units closest to outdate were prioritized for transfusion. EUHM continued to order large quantities of platelets based on the surgery schedule.
To optimize this platelet transfer protocol and extend it to all 8 hospitals in the Emory system, we engaged a Systems Engineering group from Georgia Tech to perform a more detailed analysis. This was a nontrivial problem: the inventory is perishable, platelet units have a range of remaining shelf-lives, and there are 8 hospital “nodes” in the system, each with a different asymmetric usage structure. The investigators developed a Markov decision process to model the problem, which subsequently produced several significant findings21 : (1) the direction of platelet shipment can be quickly approximated by the age of the oldest product at each site after daily orders are met; (2) the optimized sharing algorithm instructs EUH (the “hub” hospital) to carry a larger inventory (to meet unexpected demands locally and at other hospitals), although all hospital sites can carry more platelets while also reducing overall wastage; and (3) this algorithm reduced platelet outdates to below 4% systemwide, even after incorporating all 8 hospitals.
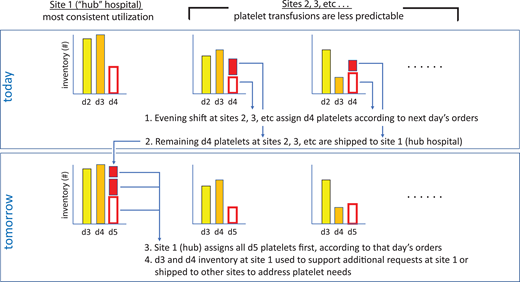
While there is a large body of work on blood inventory management dating back to the 1970s, the results of most studies typically only apply to single hospital locations22 or are difficult to implement.23 In contrast, our refined algorithm allows near-optimal platelet availability and usage yet is easily implementable throughout a large system based on simple heuristics (Figure 3). First, in the evening, nonhub hospitals assign day 4 platelets for the following day's requests. Second, remaining day 4 platelets are shipped from those sites to the hub (hospital with most consistent demand). Third, the hub hospital, typically early the following morning, assigns the shipped platelets (now day 5) and their remaining day 5 platelets. Fourth, the day 3 and day 4 inventory at the hub is used to support unfilled requests at that hospital or shipped to other hospitals to address local shortages. Note that, in contrast to the initial version, the operations analysis showed that more platelets should be ordered and stored at the hub site, which is reflected in Figure 3, in order to function as a buffer for other hospitals should their inventories become depleted. Larger hospital networks may be able to implement such a system effectively and economically, filling a need when their blood supplier(s) does not manage platelet redistribution within the system.
Platelet inventory sharing between networked hospitals based on simple heuristics.
Platelet inventory sharing between networked hospitals based on simple heuristics.
Cold-stored platelets
Recently, there has been a renewed interest in pretransfusion storage of platelets at 4°C.5 Cold-stored platelets (CS-Plt) have several potential advantages, including reduced bacterial growth, improved hemostatic function for use in bleeding patients, and no requirement for agitation.24 Additionally, they can be stored for 14 days before outdating, which improves inventory management and reduces wastage, although so far, relatively few centers have received FDA approval for prolonged storage of CS-Plt, and only when room temperature platelets (RT-Plt) are unavailable.25 While most in vitro studies suggest that CS-Plt are equivalent or superior to RT-Plt for hemostasis, other investigations raised questions about their efficacy.24 There is also some concern whether PRT can be used on CS-Plt without negatively affecting their therapeutic effectiveness.24 Clearly, clinical trials are needed, although relatively few have been completed to date. One study performed at Mayo included 40 patients, most undergoing cardiac surgery, and encouragingly showed that those transfused with CS-Plt had comparable hemostasis to those transfused with RT-Plt.26 A potential concern with stocking CS-Plts in hospital blood banks is the logistical challenge of maintaining dual inventories. However, given FDA approval, recent studies suggest that RT-Plt nearing their expiration date could be converted to CS-Plt (assuming bacterial growth during RT storage can be prevented or detected), likely simplifying inventory management.26,27 While there are still more unknowns associated with CS-Plt than with the other 3 initiatives discussed above, the potential payoff of stocking 14-day platelets (with ~12 days of effective shelf-life) for bleeding patients is significant and merits more study. For example, CS-Plt could have been ideal for treating the aneurysm patient described in the clinical case. Please see the accompanying article from Dr. Devine for further discussion of CS-Plt.28
Summary
In this article, we discuss 4 initiatives to strengthen the platelet supply against disruptions. Platelets from paid donors are already being collected at a small level. Further investigations are needed to determine the impact of paid donations on the platelet supply and whether attracting young donors to paid-platelet collection will negatively affect collections of other blood products. Introduction of BC-Plt into the US market could significantly supplement the platelet supply with a component that is functionally equivalent to Aph-Plt, which currently make up >95% of the US platelet supply. Improved (re)distribution logistics within hospital systems can be readily undertaken locally, and each platelet rescued from wastage is another patient transfusion, which has a significant impact during times of shortage. Finally, while clinical trials are still ongoing, introduction of CS-Plt into the blood bank inventory could result in a highly effective hemostatic treatment with negligible wastage rates given their 14-day expiration.
Conflict-of-interest disclosure
James R. Stubbs: no competing financial interests to declare.
Beth H. Shaz is a member of the Product Development and Scientific Program Advisory Boards of Secure Transfusion Solutions (STS), a for-profit blood collection organization that remunerates donors.
Ralph R. Vassallo is a scientific advisory board member for Fresenius Kabi, a DSMB member for the Cerus Corp, and on the speaker's bureau for Terumo BCT.
John D. Roback is a consultant to STS and a member of their Advisory Board, a former consultant to CSL Plasma, and a cofounder and consultant to Cambium Medical Technologies, LLC.
Off-label drug use
James R. Stubbs: nothing to disclose.
Beth H. Shaz: nothing to disclose.
Ralph R. Vassallo: nothing to disclose.
John D. Roback: nothing to disclose.