Abstract
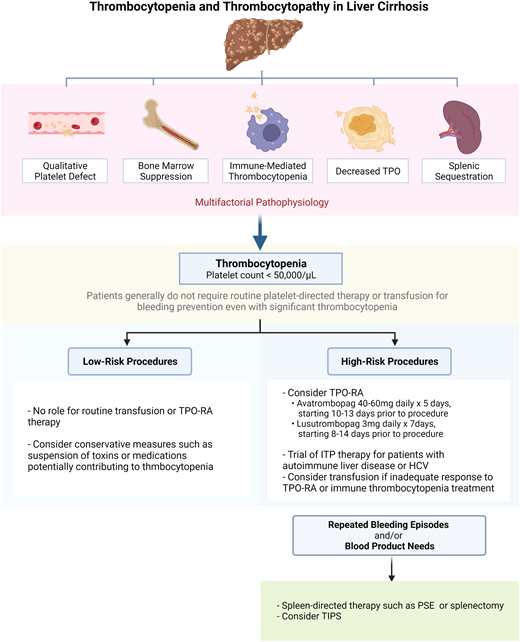
Abnormal bleeding in patients with liver disease may result from elevated portal pressure and varix formation, reduced hepatic synthesis of coagulation proteins, qualitative platelet dysfunction, and/or thrombocytopenia. Major mechanisms of thrombocytopenia in liver disease include splenic sequestration and impaired platelet production due to reduced thrombopoietin production. Alcohol and certain viruses may induce marrow suppression. Immune thrombocytopenia (ITP) may co-occur in patients with liver disease, particularly those with autoimmune liver disease or chronic hepatitis C. Drugs used for the treatment of liver disease or its complications, such as interferon, immunosuppressants, and antibiotics, may cause thrombocytopenia. Periprocedural management of thrombocytopenia of liver disease depends on both individual patient characteristics and the bleeding risk of the procedure. Patients with a platelet count higher than or equal to 50 000/µL and those requiring low-risk procedures rarely require platelet-directed therapy. For those with a platelet count below 50 000/µL who require a high-risk procedure, platelet-directed therapy should be considered, especially if the patient has other risk factors for bleeding, such as abnormal bleeding with past hemostatic challenges. We often target a platelet count higher than or equal to 50 000/µL in such patients. If the procedure is elective, we prefer treatment with a thrombopoietin receptor agonist; if it is urgent, we use platelet transfusion. In high-risk patients who have an inadequate response to or are otherwise unable to receive these therapies, other strategies may be considered, such as a trial of empiric ITP therapy, spleen-directed therapy, or transjugular intrahepatic portosystemic shunt placement.
Learning Objectives
Describe the multiple mechanisms that contribute to thrombocytopenia in patients with liver disease
Stratify the periprocedural management of thrombocytopenia of liver disease based on the platelet count and bleeding risk of the procedure
CLINICAL CASE 1
A 54-year-old woman with decompensated but stable alcoholic cirrhosis and a baseline platelet count of 25 000/µL requires endoscopy with possible variceal banding. How should she be managed for the procedure?
Introduction
Patients with cirrhosis may be prone to bleeding due to elevated portal pressure with formation of varices, decreased hepatic synthesis of procoagulant proteins, thrombocytopenia, and platelet dysfunction.1-3 Bleeding risk evaluation and thrombocytopenia management in patients with liver disease require consideration of measures beyond simple blood product transfusion, owing to a poor correlation between platelet count and bleeding outcomes as well as a concurrent risk of thrombosis, which may be exacerbated by platelet transfusion.4-6 Here we review the mechanisms of thrombocytopenia and platelet dysfunction in cirrhosis and discuss management strategies (Visual Abstract).
Pathophysiology
Qualitative platelet defects in liver disease
The multiphasic platelet-vessel interaction is altered in cirrhosis and worsens with increasing severity of the disease as classified by the Child-Pugh score.7 Cirrhotic platelets exhibit impaired adhesion to the subendothelium under flow conditions in vitro, possibly owing to defects in platelet glycoprotein 1b.8 This defect may be partially offset by an elevation in circulating von Willebrand factor in patients with cirrhosis.9 An acquired glycoprotein VI signaling defect from excess soluble fibrin and fibrin degradation products has also been described in cirrhosis and may contribute to platelet dysfunction.10,11
Historically, platelet aggregation in response to various stimuli appeared to be blunted in patients with cirrhosis as measured by conventional light transmission aggregometry, possibly through reduced transmembrane signaling.12,13 However, a recent whole-blood aggregometry study suggested increased aggregation in cirrhotics after adjusting for thrombocytopenia.14 Enhanced platelet activation under certain conditions may contribute to thrombosis or fibrosis.15,16
Thrombocytopenia in liver disease
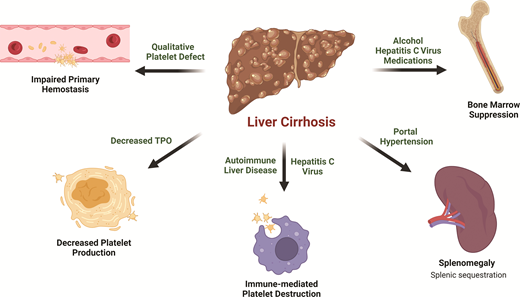
Thrombocytopenia is the most common hematologic abnormality seen in liver disease and is multifactorial (Figure 1). The most notable contributor is portal hypertension, which leads to congestive splenomegaly and the sequestration of platelets in the spleen.17 An inverse relationship between spleen size and platelet count has been observed.18,19 In cases of massive splenomegaly, which is uncommon in patients with liver disease, up to 90% of the total platelet mass may be distributed in the spleen.
Inappropriately low thrombopoietin (TPO) also plays a significant role in thrombocytopenia of liver disease. TPO, which is primarily produced by the liver, promotes megakaryopoiesis and thrombopoiesis by binding to the c-MPL receptor on platelet progenitors. Circulating TPO levels are regulated through the binding of TPO to mature platelets and their subsequent clearance. The expression of TPO mRNA in healthy liver is uninfluenced by thrombocytopenia.20 Rather, circulating TPO levels rise because there are fewer platelets for TPO to bind, therefore encouraging thrombopoiesis.21 In patients with cirrhosis, TPO production is reduced due to impaired hepatic synthetic function.22 In addition, TPO clearance may be accelerated by binding to platelets sequestered in the spleen.23 The net result is low circulating TPO levels.24,25 The key role of TPO in the thrombocytopenia of liver disease is underscored by the characteristic rise in TPO level accompanied by platelet count recovery following liver transplant.26,27
Bone marrow suppression in certain causes of cirrhosis, such as viral or alcoholic hepatitis, can also lead to the inadequate production of platelets.28 Hepatitis viruses have been shown to directly inhibit bone marrow progenitor cell proliferation and differentiation in vitro,29 and a wide range of bone marrow abnormalities have been observed in patients with hepatitis C–associated cytopenias.30 Alcohol reduces the platelet life span and causes ineffective megakaryopoeisis.31,32 The platelet count normalizes after alcohol cessation in patients without significant liver fibrosis.33
Treatment of underlying etiologies of cirrhosis can be complicated by significant medication-related bone marrow suppression that results in thrombocytopenia. Interferon (IFN)-based therapies were once the standard of care for hepatitis C virus, but significant thrombocytopenia often leads to treatment interruption or discontinuation, limiting effective therapy.34 IFN inhibits the cytoplasmic maturation of megakaryocytes and platelet production through transcription factor modulation.35 Furthermore, IFN blunts the TPO response to thrombocytopenia by the downregulation of protein synthesis or secretion without affecting mRNA expression.36 Azathioprine, a common treatment for autoimmune hepatitis and other autoimmune diseases, blocks purine synthesis and suppresses bone marrow proliferation in a dose-dependent manner.37 Only a small subset of patients on azathioprine develop thrombocytopenia, but it may exacerbate baseline thrombocytopenia in advanced liver disease patients. Exposure to other common medications, such as β-lactam and fluoroquinolone antibiotics, frequently used to treat infectious complications such as subacute bacterial peritonitis, may be another potential cause of thrombocytopenia due to bone marrow suppression or drug-induced immune thrombocytopenia.38,39
Finally, immune-mediated thrombocytopenia (ITP) may be seen in patients with autoimmune liver diseases, such as autoimmune hepatitis and primary biliary cirrhosis.40-43 Chronic hepatitis C with or without cirrhosis is also a well-known predisposing condition associated with secondary ITP,44 which frequently improves with viral eradication and also responds to conventional ITP therapy.45-47
Management
Low-bleeding-risk procedures
It is vital to consider the inherent procedural risk when assessing bleeding risk in patients with cirrhosis. Examples of low- and high-bleeding-risk procedures are summarized in Table 1.
Commonly performed procedures in patients with cirrhosis classified by bleeding risk
| Procedure type . | Low bleeding risk . | High bleeding risk . |
|---|---|---|
| Endoscopic | Diagnostic procedures Endoscopic variceal ligation Transesophageal echocardiogram | Bronchoscopy with biopsy Colonoscopy with polypectomy Endoscopic retrograde cholangiopancreatography with sphincterotomy |
| Percutaneous | Paracentesis Thoracocentesis | Percutaneous liver biopsy Tunneled ascitic/pleural drain placement Cranial/spinal surgery |
| Vascular | Peripheral/central venous catheterization Transjugular liver biopsy | Transjugular intrahepatic portosystemic shunt Transcatheter arterial chemoembolization |
| Other | Dental procedures including extractions Skin biopsy | Intraocular procedures |
| Procedure type . | Low bleeding risk . | High bleeding risk . |
|---|---|---|
| Endoscopic | Diagnostic procedures Endoscopic variceal ligation Transesophageal echocardiogram | Bronchoscopy with biopsy Colonoscopy with polypectomy Endoscopic retrograde cholangiopancreatography with sphincterotomy |
| Percutaneous | Paracentesis Thoracocentesis | Percutaneous liver biopsy Tunneled ascitic/pleural drain placement Cranial/spinal surgery |
| Vascular | Peripheral/central venous catheterization Transjugular liver biopsy | Transjugular intrahepatic portosystemic shunt Transcatheter arterial chemoembolization |
| Other | Dental procedures including extractions Skin biopsy | Intraocular procedures |
Adapted with permission from the International Society on Thrombosis and Haemostasis.52
In patients with stable cirrhosis in need of a low-bleeding-risk procedure, platelet-directed therapy or transfusion is generally unnecessary, even when the platelet count is less than or equal to 50 000/µL. The platelet count decreases with worsening liver disease, but the absolute number correlates poorly with bleeding outcomes.4 In a prospective study of patients with decompensated cirrhosis undergoing an invasive procedure, a platelet count less than or equal to 50 000/µL was not predictive of bleeding complications.5
Due to the weak association between platelet count, coagulation tests, and procedural bleeding risk,48-50 guidelines recommend against the prophylactic transfusion of blood products for low-risk procedures in stable cirrhosis patients. Not only has platelet transfusion not been shown to reduce bleeding outcomes in this context, but it may also be associated with harm. Standard risks include alloimmunization and hypersensitivity,51 which may limit the ability to treat future bleeding episodes. Experimental evidence has demonstrated increased platelet activation and thrombin/antithrombin complex generation after the administration of platelets in cirrhotic patients, suggesting a potentially prothrombotic state after platelet transfusion.6 If any additional factors that exacerbate thrombocytopenia, such as alcohol consumption or immunosuppressive medications, are identified, temporary suspension in the periprocedural setting may be beneficial.
CLINICAL CASE 1 (Continued)
The patient tolerated endoscopy and variceal banding without the use of platelet-targeted therapy or platelet transfusion. On routine imaging, she was found to have a pancreatic cyst. An endoscopic ultrasound with fine needle aspiration was recommended. Her platelet count remains approximately 25 000/µL.
High-bleeding-risk procedures
Even though stable cirrhosis patients do not require routine blood-product use prior to low-risk procedures, platelet-targeted therapy or transfusion may be indicated for high-risk procedures,52 though there are a lack of high-quality data to support this practice. Table 2 summarizes recommendations from major society guidelines regarding platelet and coagulation parameter thresholds for high-risk procedures.53-57 There is no consensus among these guidelines on the role of prophylactic platelet transfusion or a specific platelet count threshold, though targeting a platelet count above or equal to 50 000/µL may be useful for high-risk procedures, especially in patients judged to be at high bleeding risk.58 An in vitro study suggested that a platelet count around 55 000/µL was adequate for platelet procoagulant function,59 but this study did not evaluate the primary hemostatic function of platelets. Optimization of other aspects of coagulopathy (eg, prothrombin time, international normalized ratio [INR], fibrinogen) may also be considered for patients undergoing high-risk procedures to further minimize bleeding risk, though this, too, is controversial (Table 2).
Coagulation parameter and platelet count thresholds prior to high-bleeding-risk procedures in patients with cirrhosis
| . | EASL 202253 . | ISTH 202152 . | AASLD 202151 . | AGA 202150 . | ACG 202049 . | SIR 201948 . |
|---|---|---|---|---|---|---|
| INR | Do not correct | Do not evaluate routinely | Do not correct | Do not evaluate routinely | Do not correct | INR ≥2.5 |
| Platelet count | Do not correct | Do not correct | Do not correct | Do not evaluate routinely | ≥50 000/µL | ≥30 000/µL |
| Fibrinogen | Do not correct | Do not evaluate routinely | Do not correct | Do not evaluate routinely | No specific recommendation | ≥1 g/L |
| Viscoelastic assay | Do not use routinely | Do not use routinely | Do not use routinely | No recommendation | May be useful | No specific recommendation |
| . | EASL 202253 . | ISTH 202152 . | AASLD 202151 . | AGA 202150 . | ACG 202049 . | SIR 201948 . |
|---|---|---|---|---|---|---|
| INR | Do not correct | Do not evaluate routinely | Do not correct | Do not evaluate routinely | Do not correct | INR ≥2.5 |
| Platelet count | Do not correct | Do not correct | Do not correct | Do not evaluate routinely | ≥50 000/µL | ≥30 000/µL |
| Fibrinogen | Do not correct | Do not evaluate routinely | Do not correct | Do not evaluate routinely | No specific recommendation | ≥1 g/L |
| Viscoelastic assay | Do not use routinely | Do not use routinely | Do not use routinely | No recommendation | May be useful | No specific recommendation |
AASLD, American Association for the Study of Liver Diseases; ACG, American College of Gastroenterology; AGA, American Gastroenterological Association; EASL, European Association for the Study of the Liver; ISTH, International Society on Thrombosis and Haemostasis; SIR, Society of Interventional Radiology.
Historically, platelet transfusion was the only means to achieve a rapid platelet count increase in patients with liver disease. More recently, the elucidation of reduced TPO levels and thrombopoiesis in cirrhosis has paved the way for the use of TPO receptor agonists (RAs).60 Two second-generation oral agents, avatrombopag and lusutrombopag, are now US Food and Drug Administration approved for use in patients with thrombocytopenia related to liver disease who are undergoing an elective procedure.
Avatrombopag was studied in two randomized placebo- controlled trials, ADAPT-1 and ADAPT-2.61 These trials collectively enrolled 435 patients with liver disease and severe thrombocytopenia who required an elective procedure. Compared with placebo, avatrombopag was associated with a significant increase in platelet count and a reduction in the need for platelet transfusion. In subjects with a baseline platelet count of 40 000 to 50 000/µL, avatrombopag increased the platelet count by 37 000 to 45 000/µL, and 87% of the patients achieved the primary end point of a platelet count above 50 000/µL. Avatrombopag should be started 10 to 13 days before the procedure and taken for 5 days. The dose is 40 mg/d for patients with a platelet count of 40 000 to 50 000/µL and 60 mg/d for patients with a platelet count less than 40 000/µL. The platelet count typically begins to rise by day 4, peaks between days 10 and 13, and gradually returns to baseline over the next month.
Lusutrombopag was approved based on the L-PLUS-1 and L-PLUS-2 trials.62,63 These trials randomized 311 patients with liver disease and a platelet count less than 50 000/µL who were in need of an invasive procedure to lusutrombopag at a dose of 3 mg/d or placebo for up to 7 days. The percentage of patients who received platelet transfusion was significantly reduced with lusutrombopag in both trials (22%-35% vs 71%-87%).
There was initial concern regarding thrombotic risk with TPO receptor agonists (TPO-RAs) in liver disease based on reports of portal vein thrombosis in patients treated with romiplostim and eltrombopag.64,65 Reassuringly, there was no difference in thromboembolic outcomes between TPO-RA and placebo in the avatrombopag and lusutrombopag trials, perhaps in part because patients with prior thrombosis were excluded. Based on the ADAPT and L-PLUS trials, avatrombopag and lusutrombopag were approved by the US Food and Drug Administration to augment the platelet count prior to elective procedures in patients with chronic liver disease–associated thrombocytopenia.66
CLINICAL CASE 1 (Continued)
The patient was treated with avatrombopag at a dose of 60 mg/d for 5 days, but her platelet count did not rise above 50 000/µL. She was transfused a single unit of platelets before the biopsy and tolerated the procedure well without bleeding or thrombotic complications.
A year later, her cirrhosis has worsened, and her baseline platelet count is now 15 000/µL. She is being considered for a liver transplant. Her spleen size is 21 cm. As part of her pretransplant evaluation, she undergoes dental extraction. She experiences prolonged bleeding, requiring admission for transfusion and further management. She still has a few more teeth that need extracting. The transplant team wants to limit further transfusion. She receives the maximum dose of avatrombopag at 60 mg/d for 5 days but fails to achieve an adequate response.
Spleen-directed therapies
When TPO-RA therapy fails to achieve an adequate platelet response and transfusion is not an option, methods to mitigate splenic pooling, such as partial splenic embolization (PSE) or laparoscopic splenectomy, may have a role.67,68 Splenectomy is an effective treatment for raising the platelet count but carries a significant risk of major complications such as portal vein thrombosis and sepsis.69 Total splenic artery embolization was explored as a less invasive measure, but a high rate of complications such as abscess, rupture, sepsis, and death were reported.70 PSE with antibiotic prophylaxis, on the other hand, is associated with a more favorable risk/benefit profile.71 In a 20-patient series, PSE was well tolerated and was associated with a significant rise in the platelet count. Although platelet values trended downward over the ensuing months,72 a transient increase in the platelet count may facilitate the safe completion of an essential procedure.
CLINICAL CASE 2
A 57-year-old woman with compensated cirrhosis due to autoimmune hepatitis presents for bleeding risk evaluation prior to a lung biopsy. Her platelet count is 20 000/µL, and her spleen size is 13 cm. Six months earlier, the patient was treated with a course of corticosteroids for a flare in her autoimmune hepatitis with a concomitant rise in her platelet count.
Immune thrombocytopenia
As noted above, ITP may accompany autoimmune liver diseases as well as chronic hepatitis C.40-45 When thrombocytopenia seems out of proportion to the degree of liver disease and other causes of thrombocytopenia have been excluded, concomitant ITP should be considered. If the patient has received immunosuppressive therapy in the past, close examination of the platelet count response during such therapy may provide an important clue. Acute treatment for suspected ITP may include corticosteroids or intravenous immunoglobulin (IVIG).73,74 Caution should be exercised when administering high-dose corticosteroids, which can increase the risk of sepsis, induce viral reactivation (in patients with a history of viral hepatitis), or worsen fatty liver disease and glucose dysregulation in patients with cirrhosis.75
CLINICAL CASE 2 (Continued)
The patient is treated with IVIG at 1 g/kg, and her platelet count increases to 60 000/µL. She undergoes an uncomplicated lung biopsy, which shows fibrotic tissue without malignancy. Two years later, her cirrhosis has worsened. She has developed ascites and encephalopathy. The placement of a transjugular intrahepatic portosystemic shunt (TIPS) is recommended. Her platelet count is 12 000/µL, and her spleen size is 17 cm.
TIPS and thrombocytopenia
TIPS is a therapeutic intervention to lower portal hypertension. The main goal is to treat symptoms related to portal hypertension, such as variceal bleeding, ascites, and encephalopathy.76,77 By relieving portal pressure, TIPS may also improve the platelet count, particularly in patients with a platelet count less than 100 000/µL.78 There is no consensus target platelet count prior to TIPS, and there are no randomized studies available to guide the management of thrombocytopenia in this context. Some guidelines suggest a threshold of 50 000/µL, similar to other high-bleeding-risk procedures.15
CLINICAL CASE 2 (Continued)
The patient is treated with IVIG at a dose of 1 g/kg. Her platelet count rises to 45 000/µL. She undergoes an uncomplicated TIPS placement. Following the procedure, her baseline platelet count modestly increases to 20 000/µL.
Conclusion
Liver disease is associated with both quantitative and qualitative platelet defects (Figure 1). Periprocedural management in patients with thrombocytopenia of liver disease depends on individual patient characteristics and the bleeding risk of the procedure itself. Patients with a platelet count higher than or equal to 50 000/µL and those requiring low-risk procedures (Table 1) rarely require platelet-directed therapy. For those with a platelet count below 50 000/µL who require a high-risk procedure, platelet-directed therapy should be considered, especially if the patient has other risk factors for bleeding, such as bleeding with past hemostatic challenges. Although guidelines differ (Table 2), we often target a platelet count above or equal to 50 000/µL in such patients. If the procedure is elective, we prefer a TPO-RA; if it is urgent, we use platelet transfusion. Other strategies may be considered in special situations, including a trial of ITP therapy, spleen-directed therapy, or TIPS.
Conflict-of-interest disclosure
Hana I. Lim: no competing financial interests to declare.
Adam Cuker: consultancy: New York Blood Center, Synergy; authorship royalties: UpToDate.
Off-label drug use
Hana I. Lim: nothing to disclose.
Adam Cuker: nothing to disclose.