Learning Objectives
Understand the appropriate patient populations for each of the newer sickle cell disease–modifying therapies: L-glutamine, voxelotor, and crizanlizumab
Describe the expected clinical outcomes with each medication
CLINICAL CASE
A 28-year-old man with sickle cell disease (SCD) with genotype hemoglobin (Hgb) SS with baseline hemoglobin of 8.5 presents to establish care with the adult sickle cell program. He has a history of acute chest syndrome (ACS) that started 2 years ago. He also has frequent vaso-occlusive crises (VOC), with 6 crises a year requiring evaluation and treatment in the emergency department. How would you prioritize disease-modifying therapies in this patient with SCD?
Introduction
Sickle cell disease is the most common inherited hemoglobinopathy that causes a variety of complications over the course of a patient's life span. There is much heterogeneity in phenotypes, only partially explained by the variety of genotypes that can include the sickle cell mutation coupled with hemoglobin C or β-thalassemia mutations. Regardless of the mutation, the hemoglobin polymerization leads to hemolysis and vaso-occlusion. Several pathophysiologic models, including vascular adhesion, hemolysis, inflammation, and oxidative damage for pathology in SCD, serve as targets for future intervention. Newer therapies for SCD, including L-glutamine, voxelotor, and crizanlizumab, focus on decreasing either hemolysis and the resulting anemia or VOC.
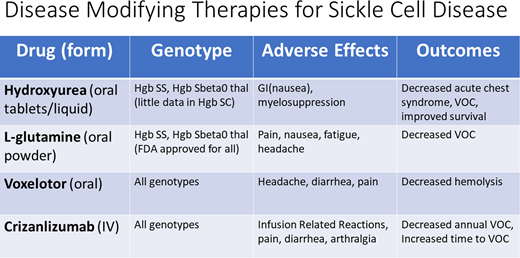
Hydroxyurea
For many years, clinicians treating patients with SCD did not have many options for disease-modifying therapy that prevented complications of SCD. Although newer treatments have emerged, hydroxyurea remains the first choice for treating those with Hgb SS or Hgb Sβ0 thalassemia (sickle cell anemia [SCA]) and has been shown to prevent acute chest syndrome (ACS), decrease VOC, and improve survival.1 There are less data evaluating the use of hydroxyurea in patients with Hgb SC or other variants. We do not review hydroxyurea in this article but instead focus on the newer therapies.
Newer therapies: L-glutamine
Although the exact mechanism of action is unclear, it is hypothesized that L-glutamine reduces oxidative stress in erythrocytes by increasing the proportion of the reduced form of nicotinamide adenine dinucleotides in sickle red cells. In turn, this decreases erythrocyte adherence to the endothelium and, in preliminary studies, resulted in decreased VOC and hospitalizations. A phase 3 study followed 230 patients aged 5 to 58 years with SCA and at least 2 VOC in the year prior in a randomized double-blind placebo-controlled study for 48 weeks (Table 1). Two-thirds of patients in each group continued on concomitant hydroxyurea. Those patients who received 0.3 g/kg/dose of L-glutamine twice daily had fewer pain crises compared with the placebo group (median, 3.0 vs 4.0; P = .005). In addition, fewer hospitalizations occurred in the L-glutamine group compared with the placebo group (2.0 vs 3.0, P = .005). Although not statistically significant, there was also a decrease in the mean number of emergency department visits (1.1 in L-glutamine vs 1.5 in placebo). The study did not find a difference in laboratory values between groups, including hemoglobin and other markers of hemolysis. The most common adverse events were musculoskeletal pain, noncardiac chest pain, nausea, fatigue, and headache; serious adverse events were more common in the placebo vs the L-glutamine group (87.1% vs 78.2%). Although reportedly unrelated to the study medication or placebo, only 68.4% of participants completed the trial, 63.8% of the L-glutamine group and 75.6% of the placebo group.2 A postapproval study of real-world use of L-glutamine revealed that after 14 months, only 19% of patients continued taking the medication while 42% discontinued the medication and 35% never filled the prescription.3 The findings in both studies raise questions about the tolerability and efficacy of L-glutamine. Although approved by the US Food and Drug Administration for all genotypes, there are little data about efficacy in patients with genotypes other than SCA.
Comparing study populations and outcomes
| Study . | Drug/dose . | Genotype . | Other therapies . | Outcome . |
|---|---|---|---|---|
| Niihara et al (2018)2 : placebo-controlled RCT (230 patients, 5-58 y) | L-glutamine 0.3 mg/kg/dose BID for 48 weeks | Hgb SS/Hgb Sβ0 thalassemia | Excluded patients on transfusions Two-thirds on HU in each group | Median VOC: 3 (L-glutamine), 4 (placebo) Hospitalizations: 2 (L-glutamine), 3 (placebo) |
| HOPE trial (2019)4 : placebo-controlled RCT (274 patients, 12-65 y) | 1500 mg voxelotor (vox), 900 mg vox, placebo daily for up to 72 weeks | Hgb SS, Hgb SC, Hgb Sβ0, Hgb SB+, other variants | Excluded patients on transfusions Two-thirds on HU | Hgb increase >1 g/dL from baseline: 51% (1500 mg vox) vs 7% (placebo) Decreased indirect bilirubin: –29.3% (1500 mg vox) vs −3.2% (placebo) |
| Zaidi et al (2020)5 : real-world use of vox retrospective (1275 patients, 6-month follow-up) | 17 patients on chronic transfusions | After vox initiation: 55% patients had Hgb increase >1 g/dL Annualized VOC rate decreased 3.86 to 3.64 | ||
| SUSTAIN trial (2017)6 : placebo-controlled RCT (198 patients, 16-63 y) | 2.5 mg/kg crizanlizumab (criz), 5 mg/kg criz, placebo, 14 doses over 52 weeks | Hgb SS, Hgb SC, Hgb Sβ0, Hgb SB+, other variants | Excluded patients on chronic transfusions Two-thirds on HU in each group | Median annual VOC: 1.63 (high-dose criz) vs 2.98 (placebo) Median time to first VOC: 4.07 months (high-dose criz) vs 1.38 months (placebo) |
| Kutlar et al (2019)7 : SUSTAIN subgroup analysis | 5 mg/kg criz, placebo | Hgb SS vs other genotypes | Two-thirds on HU | In patients with 5-10 VOCs in prior year, concomitant HU use, OR more clinically severe HbSS genotype: criz increased likelihood of being VOC free vs placebo |
| Kanter et al (2021)8 : real-world use of criz retrospective (238 patients with >1 dose) | 5 mg/kg criz | Hgb SS, Hgb SC, Hgb Sβ0, Hgb SB+, other variants | 72 patients (32%) discontinued criz due to perceived lack of improvement, infusion-related reactions, logistics 32 patients (55%) with ≥12 infusions had significant decrease in ED use and hospitalizations |
| Study . | Drug/dose . | Genotype . | Other therapies . | Outcome . |
|---|---|---|---|---|
| Niihara et al (2018)2 : placebo-controlled RCT (230 patients, 5-58 y) | L-glutamine 0.3 mg/kg/dose BID for 48 weeks | Hgb SS/Hgb Sβ0 thalassemia | Excluded patients on transfusions Two-thirds on HU in each group | Median VOC: 3 (L-glutamine), 4 (placebo) Hospitalizations: 2 (L-glutamine), 3 (placebo) |
| HOPE trial (2019)4 : placebo-controlled RCT (274 patients, 12-65 y) | 1500 mg voxelotor (vox), 900 mg vox, placebo daily for up to 72 weeks | Hgb SS, Hgb SC, Hgb Sβ0, Hgb SB+, other variants | Excluded patients on transfusions Two-thirds on HU | Hgb increase >1 g/dL from baseline: 51% (1500 mg vox) vs 7% (placebo) Decreased indirect bilirubin: –29.3% (1500 mg vox) vs −3.2% (placebo) |
| Zaidi et al (2020)5 : real-world use of vox retrospective (1275 patients, 6-month follow-up) | 17 patients on chronic transfusions | After vox initiation: 55% patients had Hgb increase >1 g/dL Annualized VOC rate decreased 3.86 to 3.64 | ||
| SUSTAIN trial (2017)6 : placebo-controlled RCT (198 patients, 16-63 y) | 2.5 mg/kg crizanlizumab (criz), 5 mg/kg criz, placebo, 14 doses over 52 weeks | Hgb SS, Hgb SC, Hgb Sβ0, Hgb SB+, other variants | Excluded patients on chronic transfusions Two-thirds on HU in each group | Median annual VOC: 1.63 (high-dose criz) vs 2.98 (placebo) Median time to first VOC: 4.07 months (high-dose criz) vs 1.38 months (placebo) |
| Kutlar et al (2019)7 : SUSTAIN subgroup analysis | 5 mg/kg criz, placebo | Hgb SS vs other genotypes | Two-thirds on HU | In patients with 5-10 VOCs in prior year, concomitant HU use, OR more clinically severe HbSS genotype: criz increased likelihood of being VOC free vs placebo |
| Kanter et al (2021)8 : real-world use of criz retrospective (238 patients with >1 dose) | 5 mg/kg criz | Hgb SS, Hgb SC, Hgb Sβ0, Hgb SB+, other variants | 72 patients (32%) discontinued criz due to perceived lack of improvement, infusion-related reactions, logistics 32 patients (55%) with ≥12 infusions had significant decrease in ED use and hospitalizations |
BID, twice a day; ED, emergency department; HU, hydroxyurea; RCD, randomized controlled trial.
Voxelotor
Voxelotor is a daily oral medication that inhibits Hgb S polymerization to stabilize hemoglobin in the oxygenated state. This then reduces red blood cell (RBC) sickling, extends RBC half-life, and can improve disease outcomes with limited toxicity, as studied in the 2019 HOPE trial. This trial followed 274 participants with any SCD genotype between 12 and 65 years of age in a double-blind, placebo-controlled, randomized trial comparing placebo to high- and low-dose voxelotor (1500 vs 900 mg daily). Study participants, of whom more than 90% had SCA, had baseline hemoglobin levels between 5.5 and 10.5 g/dL and reported 1 to 10 VOC in the 12 months prior to enrollment. Again, two-thirds of patients in each group remained on concomitant hydroxyurea, and those on chronic RBC transfusions were excluded. A higher percentage of those patients taking 1500 mg daily voxelotor had an increase in their hemoglobin level compared with the placebo group (59% vs 7%). In addition, the 1500-mg voxelotor group had significantly greater reductions in other markers of hemolysis, including indirect bilirubin and percentage of reticulocytes compared with the placebo group. Occurrence of adverse events of at least grade 3 was similar across treatment groups and primarily included headache, diarrhea, and pain. Most of these events were deemed not related to the trial drug or placebo according to the investigators. Notably, the trial did not show a significant difference in the incidence of VOC across the trial groups. Dropout rates were consistent across trial groups: 27% in the high-dose group and 21% in the low-dose and placebo groups.4
A real-world effectiveness study of voxelotor evaluated claims data for 1275 patients and replicated initial trial results showing an average increase in hemoglobin of 1 g/dL. For those patients who were transfused at least once in the year prior to the initiation of voxelotor, there was a significant decrease in the annual rate of transfusion (3.39 vs 1.75). This study also demonstrated a favorable but not significant decrease in annualized rates of VOC (3.86 to 3.64) after voxelotor initiation.5
Although consistent hemoglobin increases have been demonstrated and studies included patients with genotypes other than SCA, there has not yet been evidence to show improvement in clinical outcomes, like ACS or VOC.
Crizanlizumab
A monthly intravenous infusion, crizanlizumab interrupts the initiation of the inflammatory cascade by P-selectin. Preclinical data demonstrate that P-selectin inhibition can reduce the risk of inflammation, vaso-occlusion, and resulting VOC. The SUSTAIN trial, a phase 2 double-blind, randomized, placebo-controlled trial, included 198 patients who received placebo, low-dose crizanlizumab (2.5 mg/kg/dose), or high-dose crizanlizumab (5 mg/kg/dose) over 52 weeks. Patients were 16 to 65 years old, could have any genotype SCD, reported 2 to 10 VOCs in the 12 months prior, but could not receive routine RBC transfusions. Two-thirds of each treatment group were concomitantly taking hydroxyurea. Those who received high-dose crizanlizumab reported a 45.3% reduction in the median rate of crises per year vs placebo (1.63 vs 2.98, P = .01). In addition, the median time to first crisis was significantly longer in high-dose crizanlizumab compared with placebo (4.07 vs 1.38 months, P = .02). There was no noted difference in laboratory markers of hemolysis. Adverse events that occurred more frequently in crizanlizumab groups at rates higher than in the placebo group included arthralgia, diarrhea, pruritis, vomiting, and chest pain. Early discontinuation was consistent across all treatment groups, ranging from 32% in the low-dose group, 36% in the high-dose group, and 37% in the placebo group.6
A subgroup analysis of patients from this trial demonstrated benefit from crizanlizumab treatment in patients with a high number of prior VOCs, those on concomitant hydroxyurea therapy, and/or those with the Hgb SS genotype.7 What has emerged, however, in reports of postapproval real-world use is a notable number of infusion-related reactions, including severe VOCs that can lead to prolonged hospitalizations. One study reported 72 life span patients (32%) discontinued crizanlizumab for various reasons, including perceived lack of benefit and infusion-related reactions, indicating that more research is needed into prevention and management of these complications.8
Conclusions
After decades without new disease-modifying therapies for SCD, it is certainly exciting to have more treatment options. What is not yet clear is how to prioritize use, particularly in patients with milder genotypes, and the ways in which these therapies can be most effectively combined. The studies described in this review usher in a new era of treating SCD but also illustrate a pressing need for future research directions. We must continue to evaluate the effect of each therapy on additional drivers of morbidity and mortality in SCD like ACS and stroke, with a particular focus on preventing complications in pediatric patients. Clinicians treating patients with SCD must also collect and disseminate real-world data to best understand how these therapies work in combination with each other.
Recommendations
Hydroxyurea remains the first choice for disease-modifying therapy in patients with SCA, particularly for those with a history of ACS. (Grade 1A)
Crizanlizumab should be considered for patients with frequent VOCs, those with genotype Hgb SC disease, and/or those not able to tolerate a daily medication. (Grade 2B)
Voxelotor should be considered for patients with low baseline hemoglobin and sequelae of chronic anemia due to SCD. (Grade 2B)
Conflict-of-interest disclosure
Stephanie Guarino: Novartis: consultancy.
Sophie Lanzkron: Shire: research funding; Pfizer: current holder of individual stocks in a privately held company; Bluebird Bio: consultancy; Teva: current holder of individual stocks in a privately held company; Novo Nordisk: consultancy; GBT: research funding; Imara: research funding; CSL Behring: research funding; Novartis: research funding.
Off-label drug use
Stephanie Guarino: nothing to disclose.
Sophie Lanzkron: nothing to disclose.