Abstract
The prevention of central nervous system (CNS) relapse in diffuse large B-cell lymphoma (DLBCL) continues to be one of the most contentious areas of lymphoma management. Outcomes for patients with secondary CNS lymphoma (SCNSL) have historically been very poor. However, in recent years improved responses have been reported with intensive immunochemotherapy approaches, and there is a growing interest in potential novel/cellular therapies. Traditional methods for selecting patients for CNS prophylaxis, including the CNS International Prognostic Index, are hampered by a lack of specificity, and there is accumulating evidence to question the efficacy of widely employed prophylactic interventions, including intrathecal and high-dose methotrexate (HD-MTX). Given the potential toxicity of HD-MTX in particular and the ongoing need to prioritize systemic disease control in high-risk patients, there is an urgent need to develop more robust methods for identifying patients at highest risk of CNS relapse, as well as investigating prophylactic interventions with greater efficacy. Here we review new evidence in this field from the last 5 years, focusing on the potential use of molecular diagnostics to improve the identification of high-risk patients, recent large data sets questioning the efficacy of HD-MTX, and the current approach to management of patients with SCNSL. We provide a suggested algorithm for approaching this very challenging clinical scenario.
Learning Objectives
Understand the currently available methods for identifying patients at high risk of CNS relapse and the potential for novel molecular diagnostics to improve patient selection in the future
Review recent evidence to question the efficacy of traditional methods for delivering CNS prophylaxis and to evaluate the increasing focus on alternative interventions for this important clinical problem
CLINICAL CASE
A 62-year-old man with no previous medical history presented in January 2020 with a short history of weight loss, night sweats, hip pain, and bilateral groin lymphadenopathy. His lactate dehydrogenase (LDH) was elevated (>3 times the upper limit of normal). Fluorodeoxyglucose-positron emission tomographic (FDG-PET) imaging revealed widespread hypermetabolic lymphadenopathy (largest lesion, 4 cm diameter) as well as pathological FDG uptake in multiple areas of bone (scapula, L3 vertebra, left hemipelvis) and in the left kidney. A core biopsy from a left inguinal lymph node demonstrated a diagnosis of diffuse large B-cell lymphoma (DLBCL), non-germinal center subtype (Hans algorithm), with overexpression of MYC (>90%) and BCL2 (>60%) by immunohistochemistry— that is, the double-expressor subtype. Fluorescence in situ hybridization (FISH) studies showed no evidence of MYC, BCL2, or BCL6 rearrangements. His Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 1, resulting in an international prognostic index (IPI) of 4 (age >60, stage IVB, raised LDH, ≥2 extranodal (EN) sites) and a central nervous system (CNS) IPI of 5 (aforementioned IPI factors plus renal involvement). Six cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) therapy were planned at 21-day intervals. Consideration was given as to whether CNS prophylaxis should be incorporated to reduce risk of CNS relapse.
Introduction
CNS relapse (otherwise referred to as secondary CNS lymphoma [SCNSL]) is a relatively rare but often devastating complication for patients with DLBCL. Estimates of CNS relapse incidence vary, occurring overall in approximately 5% of DLBCL patients but with subgroups in which the risk is significantly higher.1,2 Most CNS relapse events occur either during or closely following frontline immunochemotherapy, with a median time in recent prospective clinical trials of 6 to 8 months.1,3 Management of SCNSL is often challenging, with historically poor outcomes. As a result, much attention has focused on both the identification of patients at highest risk for this complication, as well as prophylactic treatments aimed at abrogating risk as much as possible. Although our understanding of which patients are at highest risk of SCNSL has improved, particularly with the introduction of the CNS-IPI and increased understanding of the molecular biology of DLBCL,4 decision-making around prophylactic interventions continues to be based either on retrospective analyses or data extrapolated from other disease subtypes, with no prospective randomized trials performed aimed at addressing CNS prophylaxis efficacy directly.
Clinicians often are faced with the dilemma of trying to prevent such a feared complication whilst ensuring that the patient is not exposed to additional therapy with associated risk of toxicity and a limited evidence-base to demonstrate its efficacy. The limitations of the evidence to inform decision-making are reflected in the variation between national guidelines on the topic (Table 1), as well as the significant disparity in practice between centers within the same health care system.
Summary of consensus guideline recommendations for CNS prophylaxis in DLBCL
| Guideline . | Patient selection . | Method for CNS prophylaxis suggested . |
|---|---|---|
| British Society for Haematology (2021)8 | Offer to: • High (4-6) CNS-IPI • ≥3 EN sites • High-risk EN site involvement—testicular, renal/adrenal, intravascular Consider in: • Breast involvement • Uterine involvement | • HD-MTX (≥3 g/m2 for 2-3 cycles) as early as possible as part of first-line therapy without compromising dose and time intensity of R-CHOP-like treatment • IT prophylaxis not recommended if HD-MTX successfully delivered • Consider IT as well as systemic prophylaxis in testicular DLBCL |
| NCCN (2022)48 | Consider in: • High (4-6) CNS-IPI • Double/triple-hit HGBL • High-risk EN site involvement—testicular, breast, primary cutaneous, renal/adrenal | • HD-MTX (3-3.5 g/m2 for 2-4 cycles) during or after the course of treatment and/or • IT methotrexate and/or cytarabine (4-8 doses) during or after the course of treatment |
| ESMO (2018)49 | Consider in: • High IPI • High-risk EN site involvement—testicular, renal/adrenal, breast, bone marrow, bone | • HD-MTX is “an option . . . even though the level of supporting evidence is low” • “Little or no role” for IT therapy |
| Guideline . | Patient selection . | Method for CNS prophylaxis suggested . |
|---|---|---|
| British Society for Haematology (2021)8 | Offer to: • High (4-6) CNS-IPI • ≥3 EN sites • High-risk EN site involvement—testicular, renal/adrenal, intravascular Consider in: • Breast involvement • Uterine involvement | • HD-MTX (≥3 g/m2 for 2-3 cycles) as early as possible as part of first-line therapy without compromising dose and time intensity of R-CHOP-like treatment • IT prophylaxis not recommended if HD-MTX successfully delivered • Consider IT as well as systemic prophylaxis in testicular DLBCL |
| NCCN (2022)48 | Consider in: • High (4-6) CNS-IPI • Double/triple-hit HGBL • High-risk EN site involvement—testicular, breast, primary cutaneous, renal/adrenal | • HD-MTX (3-3.5 g/m2 for 2-4 cycles) during or after the course of treatment and/or • IT methotrexate and/or cytarabine (4-8 doses) during or after the course of treatment |
| ESMO (2018)49 | Consider in: • High IPI • High-risk EN site involvement—testicular, renal/adrenal, breast, bone marrow, bone | • HD-MTX is “an option . . . even though the level of supporting evidence is low” • “Little or no role” for IT therapy |
ESMO, European Society for Medical Oncology; HGBL, high-grade B-cell lymphoma; NCCN, National Comprehensive Cancer Network.
A 2017 American Society of Hematology Educational Program review gave a comprehensive overview of the risk factors for CNS relapse and evidence to guide prophylactic interventions at that time.5 In this article we focus on updates in the field in the last 5 years, with particular attention to the advances in molecular diagnostics and implications for SCNSL, as well as new evidence to question the efficacy of high-dose methotrexate (HD-MTX).
How do we identify patients at high risk of CNS relapse?
Clinical risk factors
Numerous studies have investigated potential risk factors for CNS relapse in DLBCL.5 In 2016 the German High-Grade Non- Hodgkin Lymphoma Study Group (DSHNHL) developed a prognostic model (CNS-IPI) incorporating the 5 standard IPI factors as well as involvement of the kidneys or adrenal glands, stratifying patients into 3 risk categories (Table 2).4 Notably, patients with 5 or 6 risk factors had a much higher risk of CNS relapse of 15% and 32.5%, respectively. Although the CNS-IPI is a robust model and has been validated in subsequent studies, it lacks specificity, and half of events occur among patients with low to intermediate scores. It should also be noted that although a small number of patients in the DSHNHL trials used to formulate the CNS-IPI had Burkitt lymphoma, the final model is validated for patients with DLBCL only, and CNS prophylaxis strategies for Burkitt lymphoma should be considered separately.
CNS-IPI risk categories with corresponding 2 year rates of CNS relapse and proportion of patients in each category from training (DSHNHL) and validation (BCCA) cohorts
| Risk group . | Risk factors . | DSHNHL cohort . | BCCA cohort . | ||
|---|---|---|---|---|---|
| N (%) . | 2-year risk of CNS relapse . | N (%) . | 2-year risk of CNS relapse . | ||
| Low | 0-1 | 1002 (46) | 0.6% | 463 (31) | 0.8% |
| Intermediate | 2-3 | 896 (41) | 3.4% | 694 (46) | 3.9% |
| High* | 4 | 188 (9) | 7.4% | 344 (23) | 12% |
| High | 5 | 62 (3) | 15% | ||
| High | 6 | 13 (1) | 32.5% | ||
| Risk group . | Risk factors . | DSHNHL cohort . | BCCA cohort . | ||
|---|---|---|---|---|---|
| N (%) . | 2-year risk of CNS relapse . | N (%) . | 2-year risk of CNS relapse . | ||
| Low | 0-1 | 1002 (46) | 0.6% | 463 (31) | 0.8% |
| Intermediate | 2-3 | 896 (41) | 3.4% | 694 (46) | 3.9% |
| High* | 4 | 188 (9) | 7.4% | 344 (23) | 12% |
| High | 5 | 62 (3) | 15% | ||
| High | 6 | 13 (1) | 32.5% | ||
One point is scored for any of the following: age >60 years, LDH > normal, ECOG performances status >1, stage III/IV disease, extranodal involvement ≥2 sites, kidney and/or adrenal involvement.
BCCA, British Colombia Cancer Agency.
High risk group (4-6 factors) overall 2-year risk of CNS relapse of 10.2% in DSHNHL cohort
Certain EN sites have been associated with a higher risk of CNS recurrence, with kidney/adrenal involvement included in the CNS-IPI model and intravascular lymphoma a distinct entity with a well-established risk of CNS involvement at baseline or at relapse. Testicular involvement has long been recognized as a risk factor, in the context of both limited and advanced stage, with a 10-year CNS relapse risk of 10% to 25% (see section Testicular DLBCL).6 Breast involvement has been associated with a higher risk of CNS relapse (~15%) in retrospective series,7 whereas other EN sites such as the uterus, blood, bone marrow, or epidural area showed more inconsistent results and are unlikely to be independently predictive of CNS relapse.8 Finally, a large retrospective study reported that the involvement of 3 or more EN sites as determined by PET-computed tomography conferred a 3-year cumulative risk of CNS relapse of 15%.9
Biological risk factors
The dual overexpression of MYC and BCL2, determined by immunohistochemistry (double-expressor DLBCL), has not been consistently associated with a high risk of CNS relapse.3,10 However, most double-expressor cases are classified as the activated B-cell (ABC) subtype, which, when determined by gene expression profiling, has been associated with a CNS relapse risk of 7% to 9% and 15% when combined with a high CNS-IPI.3,10
Recently, multiplatform analysis defined new molecular subgroups, or clusters.11,12 The MCD and C5 clusters, characterized by a high frequency of MYD88L265P and CD79 mutations, occur almost exclusively in the ABC subtype. Genetic alterations defining these subtypes are also recurrently mutated in primary EN lymphomas originating in the CNS, testes, breasts, skin, and intravascular spaces. Interestingly, a recent series of SCNSL (n = 13) confirmed a higher prevalence of the MCD subtype than a reference cohort of relapsed DLBCL with no CNS involvement (38% vs 8%; P = .003).13 Furthermore, the hcMCD subtype defined by MYD88L265P mutation or more than 3 mutations in CD79, PIM1, ETV6, BTG1, PRDM1, or PBL1XR1 constituted almost half of the patients with CNS recurrence (46%). The remaining cases were either double-hit lymphoma (DHL) or associated with TP53 mutations. Although these data need to be validated, there is clear potential for next generation sequencing analysis to help identify patients at risk of CNS relapse.
High-grade B-cell lymphomas harboring MYC translocation along with BCL2 and/or BCL6 translocation (DHL or triple-hit lymphomas] have historically been associated with a high risk of CNS involvement). However, there is accumulating evidence to suggest that early data overestimated this risk, as FISH was not performed consistently,10 and such patients often meet other clinical criteria.
Baseline screening
Baseline screening with brain imaging and lumbar puncture/cerebrospinal fluid (CSF) analysis is increasingly used to identify high-risk patients with CNS involvement who may benefit from CNS-directed therapies. Several studies have shown that CSF analysis with flow cytometry is more sensitive than cytology for the detection of occult CNS involvement.14 However, a proportion of patients with a negative flow cytometry result relapse in the CNS shortly after treatment, suggesting the need for more sensitive techniques. Cell-free circulating tumor DNA (ctDNA) has recently appeared as a prognostic biomarker in patients with CNS lymphoma, with good correlation between ctDNA levels (MYD88L265P mutation) and treatment response and outcomes.15-17 Two studies have assessed the role of CSF ctDNA analysis in patients with high-risk B-cell lymphoma.17,18 The first analyzed specific tumor-derived mutations in sequential CSF samples from 12 patients receiving frontline treatment, and CSF ctDNA was detected 3 months before CNS relapse in 1 of 2 patients in whom this occurred.17 More recently, Olszewski et al analyzed CSF from 22 patients with aggressive B-cell lymphoma using a next generation sequencing-minimal residual assay.18 At diagnosis, CSF ctDNA was identified in 8 patients, 2 of whom relapsed in the CNS, with a 12-month cumulative risk of CNS recurrence of 29% in patients with a positive analysis vs a 0% risk for patients with negative CSF.18 Taken together, acknowledging the limitation of the small number of patients, these results suggest the potential utility of CSF ctDNA to identify patients with a higher risk of CNS relapse. Further studies are ongoing to validate these findings before the technology can be incorporated into routine clinical practice.
How do we manage patients with CNS relapse/SCNSL?
Historically, SCNSL has been associated with a dismal prognosis and median overall survival (OS) of approximately 6 months.19 Identifying effective therapeutic approaches remains challenging, and the majority of patients still progress or relapse shortly after treatment.
In recent years, combinations of intensive chemotherapies including HD-MTX followed by autologous stem cell transplantation (ASCT) have been adopted, with 2-year OS of 25% to 68% reported.20,21 Recently, the MARIETTA phase 2 study examined the efficacy of 3 courses of MATRix (rituximab, MTX, cytarabine, thiotepa) plus 3 courses of RICE (rituximab, ifosfamide, etoposide, carboplatin) followed by carmustine and thiotepa-conditioned ASCT in 75 patients with CNS involvement at diagnosis or relapse. Two-year OS for the intention-to-treat population was 46%,22 while those undergoing ASCT (37/75 patients) had 2-year OS of 83%. Two-year progression-free survival (PFS) of 71% was reported in patients with SCNSL at initial diagnosis, but PFS was only 28% in patients previously treated with R-CHOP. Although this study was restricted to patients under the age of 70, an ECOG PS of 3 or lower, and adequate organ function, thiotepa-based ASCT is increasingly utilized in older patients.23 However, induction chemotherapy regimens are intense and less well tolerated in older patients in the real-world setting.24
Chimeric antigen receptor (CAR) T-cell therapy has shown promising results in relapsed/refractory DLBCL, including in older and unfit patients. There is accumulating data demonstrating the efficacy and safety of CAR T cells in CNS lymphoma.25 In SCNSL, the TRANSCEND study demonstrated complete remission (CR) in 3 of 6 patients, with grade 3 neurological events in 2 cases (27).26 Similarly, small (≤8 patients) series of patients with highly refractory SCNSL treated with commercial agents have been reported as having CR rates of approximately 50% with no significant toxicity.27,28 These findings suggest that CAR T cells may be a viable salvage treatment for this challenging population, especially for patients not fit enough to receive intensive immunochemotherapy. Furthermore, a number of phase 1/2 studies evaluating CAR T cells in CNS lymphoma are currently ongoing (NCT04608487, NCT04464200, NCT03484702).
Methods for delivery of CNS prophylaxis
Intrathecal chemotherapy
For many years, intrathecal (IT) cytotoxic chemotherapy was used as CNS prophylaxis in DLBCL, with supporting evidence derived mainly from nonrandomized, retrospective analyses as well as data extrapolated from other B-cell malignancies. More recently, a systematic review of stand-alone IT prophylaxis analyzed a total of 7357 patients treated with anti-CD20 monoclonal antibody–based immunochemotherapy, incorporating 3 post hoc trial analyses and 10 retrospective studies.29 Overall, IT prophylaxis was not found to be associated with a reduction in CNS relapse rate on univariable or multivariable analyses. The delivery of IT therapy can be challenging and uncomfortable for the patient, with some evidence to suggest an association with infection-related hospitalization in older patients.30 With an increasing recognition that the majority of CNS relapses in DLBCL involve the brain parenchyma, an area not penetrated by IT therapy alone, IT use has diminished in this setting, with a move toward systemic antimetabolite therapy instead. An exception to this is in testicular DLBCL, where IT therapy may continue to have a role based on data from prospective IELSG trials (see below).
HD-MTX
Approximately 70% to 80% of CNS relapses in DLBCL involve the brain parenchyma,31 and therefore there is a rationale for prophylactic therapies that cross the blood-brain barrier and penetrate all CNS compartments. Intravenous HD-MTX has increasingly been used over the last 10 years as CNS prophylaxis in DLBCL, with initial supporting evidence mainly derived from its efficacy in primary CNS lymphoma. Over the years several studies that have the common theme of being nonrandomized, retrospective analyses have addressed this area, but they have been of variable size and have produced discrepant results (Table 3).32-40 While there has been widespread incorporation of HD-MTX as prophylaxis for high-risk patients, disagreement has arisen about the safest and most effective way to incorporate it into frontline therapy. The practice of “intercalated” HD-MTX was first described in a single-center retrospective study of 65 patients in which HD-MTX was delivered at days 10 to 15 in between cycles of R-CHOP, resulting in a CNS relapse rate of 3%.41 While this approach delivers early CNS-directed therapy, potentially advantageous given the often early onset of CNS relapse, it introduces potential toxicity and delays to systemic R-CHOP therapy, with many choosing to wait and deliver HD-MTX after R-CHOP completion instead.
Summary of recent studies evaluating use of HD-MTX in DLBCL
| Study (year) . | n . | Design . | Risk factors . | Systemic treatment . | CNS Prophylaxis . | CNS relapse . | Comments . |
|---|---|---|---|---|---|---|---|
| Lewis et al32 (2022) | 2300 | Multicenter, retrospective | CNS-IPI ≥4 Testicular, breast involvement DHL | R-CHOP (94%) R-EPOCH (6%) | 1. HD-MTX (18%) 2. No HD-MTX (82%) | 1. 9.2% (5 y) 2. 8.1% (5 y) | No benefit HD-MTX |
| Wilson et al33 (2022) | 1384 | Multicenter, retrospective | High-risk EN sites CNS-IPI ≥4 ≥2 EN and LDH ↑ | R-CHOP | 1. HD-MTX (all, intercalated, or EOT) | 1. 5.7% (3 y) 2. 5.8% (3 y) | No difference between EOT and intercalated HD-MTX |
| Orellana-Noia et al34 (2022) | 1030 | Multicenter, retrospective | Not described | R-CHOP (48%) R-EPOCH (45%) Other (7%) | 1. HD-MTX (20%) 2. IT (77%) | 1. 6.8% 2. 5.4% | No benefit HD-MTX vs IT |
| Puckrin et al35 (2021) | 326 | Multicenter, retrospective | CNS-IPI ≥4 Testicular DHL LDH ↑ + ECOG >1 + >1 EN | R-CHOP (85%) Intensive chemotherapy (15%) | 1. HD-MTX (35%) 2. No HD-MTX (65%) | 1. 12.2% 2. 11.2% | No benefit HD-MTX |
| Bobillo et al36 (2021) | 585 | Single-center, retrospective | CNS-IPI ≥4 High-risk EN sites DHL | R-CHOP (68%) R-EPOCH (15%) Other (17%) | 1. HD-MTX (7%) 2. IT MTX (43%) 3. None (50%) | 1. 7.5% (5 y) 2. 5.5% (3 y) 3. 5% | No benefit (IT or HD-MTX) |
| Ong et al37 (2021) | 226 | Multicenter, retrospective | High-risk EN sites CNS-IPI ≥4 | R-CHOP | 1. HD-MTX (29%) 2. No HD-MTX (71%) | 1. 3.1% (3 y, isolated) 2. 14.6% (3 y, isolated) | HD-MTX significantly reduced risk of isolated CNS relapse |
| Wilson et al38 (2020) | 334 | Multicenter, retrospective | CNS-IPI ≥4 High-risk EN sites ≥2 EN sites and LDH ↑ | R-CHOP | 1. HD-MTX (all, intercalated, or EOT) | 1. 6.8% (3 y) 2. 4.7% (3 y) | No difference between EOT and intercalated HD-MTX |
| Lee et al39 (2019) | 130 | Single-center, retrospective | CNS-IPI ≥4 High-risk EN sites ≥2 EN and LDH ↑ | R-CHOP | 1. HD-MTX (49%) 2. None (51%) | 1. 6.9% (2 y) 2. 8.1% (2 y) | No benefit HD-MTX |
| Goldschmidt et al40 (2019) | 480 | Multicenter, retrospective | High-risk EN sites Stage IV, LDH ↑, ≥1 EN | CHOP +/−R (80%) | 1. HD-MTX (27%) 2. None (73%) | 1. 6.9% 2. 6.3% | No benefit HD-MTX |
| Study (year) . | n . | Design . | Risk factors . | Systemic treatment . | CNS Prophylaxis . | CNS relapse . | Comments . |
|---|---|---|---|---|---|---|---|
| Lewis et al32 (2022) | 2300 | Multicenter, retrospective | CNS-IPI ≥4 Testicular, breast involvement DHL | R-CHOP (94%) R-EPOCH (6%) | 1. HD-MTX (18%) 2. No HD-MTX (82%) | 1. 9.2% (5 y) 2. 8.1% (5 y) | No benefit HD-MTX |
| Wilson et al33 (2022) | 1384 | Multicenter, retrospective | High-risk EN sites CNS-IPI ≥4 ≥2 EN and LDH ↑ | R-CHOP | 1. HD-MTX (all, intercalated, or EOT) | 1. 5.7% (3 y) 2. 5.8% (3 y) | No difference between EOT and intercalated HD-MTX |
| Orellana-Noia et al34 (2022) | 1030 | Multicenter, retrospective | Not described | R-CHOP (48%) R-EPOCH (45%) Other (7%) | 1. HD-MTX (20%) 2. IT (77%) | 1. 6.8% 2. 5.4% | No benefit HD-MTX vs IT |
| Puckrin et al35 (2021) | 326 | Multicenter, retrospective | CNS-IPI ≥4 Testicular DHL LDH ↑ + ECOG >1 + >1 EN | R-CHOP (85%) Intensive chemotherapy (15%) | 1. HD-MTX (35%) 2. No HD-MTX (65%) | 1. 12.2% 2. 11.2% | No benefit HD-MTX |
| Bobillo et al36 (2021) | 585 | Single-center, retrospective | CNS-IPI ≥4 High-risk EN sites DHL | R-CHOP (68%) R-EPOCH (15%) Other (17%) | 1. HD-MTX (7%) 2. IT MTX (43%) 3. None (50%) | 1. 7.5% (5 y) 2. 5.5% (3 y) 3. 5% | No benefit (IT or HD-MTX) |
| Ong et al37 (2021) | 226 | Multicenter, retrospective | High-risk EN sites CNS-IPI ≥4 | R-CHOP | 1. HD-MTX (29%) 2. No HD-MTX (71%) | 1. 3.1% (3 y, isolated) 2. 14.6% (3 y, isolated) | HD-MTX significantly reduced risk of isolated CNS relapse |
| Wilson et al38 (2020) | 334 | Multicenter, retrospective | CNS-IPI ≥4 High-risk EN sites ≥2 EN sites and LDH ↑ | R-CHOP | 1. HD-MTX (all, intercalated, or EOT) | 1. 6.8% (3 y) 2. 4.7% (3 y) | No difference between EOT and intercalated HD-MTX |
| Lee et al39 (2019) | 130 | Single-center, retrospective | CNS-IPI ≥4 High-risk EN sites ≥2 EN and LDH ↑ | R-CHOP | 1. HD-MTX (49%) 2. None (51%) | 1. 6.9% (2 y) 2. 8.1% (2 y) | No benefit HD-MTX |
| Goldschmidt et al40 (2019) | 480 | Multicenter, retrospective | High-risk EN sites Stage IV, LDH ↑, ≥1 EN | CHOP +/−R (80%) | 1. HD-MTX (27%) 2. None (73%) | 1. 6.9% 2. 6.3% | No benefit HD-MTX |
In the last year, a number of studies in this area have been published to date, arguably providing the most robust data to inform our practice in the absence of prospective clinical trials. These studies have addressed 2 separate questions: (1) Is HD-MTX effective?, and (2) How should it be incorporated into frontline (R-CHOP/R-CHOP-like) therapy? Lewis et al carried out an international multicenter analysis of 2300 patients deemed at high risk for SCNSL on the basis of high CNS-IPI, the presence of double-hit FISH abnormalities, or the involvement of high risk sites (breast or testicular).32 Patients received either HD-MTX (n = 410) with or without concurrent IT therapy, IT therapy alone, or no CNS prophylaxis. There was no significant difference in the 5-year cumulative incidence of CNS relapse between the HD-MTX and no–HD-MTX arms (9.1% vs 8.4%, respectively), with results unchanged when analyses were restricted to patients achieving CR at the end of systemic treatment (5.0% vs 6.0%) and in subanalyses of patients with “ultra”–high-risk characteristics. These findings are consistent with those of another large retrospective study by Orellana-Noia et al, in which no reduction in CNS relapse was seen in patients receiving HD-MTX (n = 236) compared to those receiving IT prophylaxis alone (n = 894).
Wilson et al reported an international multicenter analysis of 1384 patients, all of whom received HD-MTX CNS prophylaxis delivered either intercalated (n = 749) or at the end of R-CHOP therapy (n = 635).33 There was no difference in CNS relapse between the 2 delivery approaches (3-year rate of 5.7% vs 5.8%, respectively), with intercalated delivery causing significantly higher rates of R-CHOP delay. Notably, in analyses restricted to patients with high CNS-IPI (n = 600), the 3-year rate of CNS relapse was 9.1%, very similar to the rates reported in the original CNS-IPI study in which minimal CNS prophylaxis was used.4
Both these studies carry inherent caveats associated with retrospective data collection. Notably, the Lewis et al study had a relatively low number of patients in the HD-MTX arm, with potential for a signal toward benefit in very high-risk patients being missed as a result. The Wilson et al study had wide variation in the criteria used for selection for CNS prophylaxis, and both data sets contained significant numbers of patients receiving concurrent IT prophylaxis. However, both studies add compelling data to the argument that HD-MTX may not significantly reduce rates of CNS relapse for the majority of patients deemed to be “high risk” by traditional criteria. If the absolute risk reduction of 1% with HD-MTX from the Lewis et al study is accurate, 100 high-risk patients would need to be treated to avoid 1 CNS relapse. HD-MTX carries a significant risk of toxicity, including acute kidney injury, mucositis, and hepatotoxicity.38 Considering that systemic treatment failure is a greater risk than CNS relapse, it appears likely that the balance of risks for the vast majority of patients favors prioritizing systemic therapy and forgoing HD-MTX altogether or, at the very least, delivering at end of treatment (EOT). An alternative approach for some very high-risk patients may be to use intensified systemic regimens that incorporate HD-MTX—for example, R-CODOX-M/IVAC, which has promising data in a phase 2 trial but has not been demonstrated to be superior to R-CHOP in a randomized trial and is associated with significant toxicity.42
Testicular DLBCL
Testicular lymphoma has been associated with a high risk of long-term CNS relapse, with a 5-year risk of 10% and 25% for limited (primary testicular lymphoma [PTL]) and advanced disease in the rituximab era, respectively.6 Biologically, over 75% of testicular lymphomas resemble the ABC subtype and are enriched for the somatic mutations commonly seen in CNS lymphoma, such as MYD88L265P and CD79, which are present in up to 70% of cases.
Two prospective studies explored the role of CNS prophylaxis in the rituximab era. The IELSG-10 phase 2 study demonstrated that patients with PTL treated with R-CHOP and contralateral radiation therapy plus 4 doses of IT MTX had a lower risk of CNS relapse compared to historical series (5-year cumulative risk of 6% vs 20%).43 More recently, the IELSG-30 trial included a total of 54 patients with PTL receiving R-CHOP, contralateral radiation therapy, and 2 courses of HD-MTX (dose, 1.5 g/m2), along with 4 doses of IT liposomal cytarabine. Preliminary results showed no CNS relapses after a median follow-up of 5 years.44 According to these studies, patients with testicular lymphoma may benefit from CNS prophylaxis incorporating HD-MTX and/or IT chemotherapy.
CLINICAL CASE (Continued)
Due to the presence of high-risk features for SCNSL, the patient had a baseline MRI head/spine and lumbar puncture with CSF analysis (flow cytometry) with no CNS disease evident. He went on to receive 6 cycles of R-CHOP-21, with intercalated HD-MTX (3 g/m2) planned on day 8 of R-CHOP cycle 2 and cycle 4. No IT prophylaxis was administered. Following the first HD-MTX treatment after R-CHOP cycle 2, the patient experienced a 7-day hospital admission with grade 2 renal toxicity and line infection. As a result, cycle 3 R-CHOP was delayed by 10 days. No further HD-MTX was given, and he received the remaining cycles of R-CHOP on schedule, with end-of-treatment PET-computed tomography demonstrating a complete metabolic response. At 12 months' follow-up, he remains well with no evidence of systemic or CNS disease relapse.
How do we approach CNS prophylaxis in 2022?
The above case, treated prior to the publication of the most recent large HD-MTX analyses,32,33 demonstrates the difficulties in decision-making in this area. The patient had a CNS-IPI score of 5, corresponding to a 2-year risk of CNS relapse of 15% according to the trial data sets used in the formulation of the score. Had his ECOG performance status been 2 instead of 1, the CNS-IPI score would have been 6, conferring an estimated risk of 33%, although it should be emphasized that only 13 patients were in this category in the CNS-IPI trial data set. However, the patient had an inherently greater risk of systemic treatment failure. He experienced significant toxicity following the first intercalated HD-MTX, resulting in delayed R-CHOP therapy that could potentially have had detrimental effect on systemic disease control.
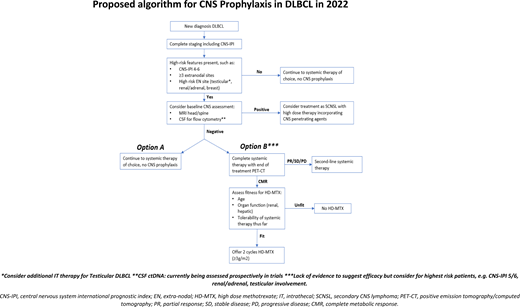
If this patient were to present now for treatment, suggested approaches based on recent data are outlined in Figure 1. The decision-making involved essentially places greater emphasis on baseline screening for occult CNS involvement in high-risk patients, as well as more judicious use of HD-MTX.
Proposed algorithm for CNS prophylaxis in DLBCL in 2022. CMR, complete metabolic response; CT, computed tomography; PD, progressive disease; PR, partial response; SD, stable disease.
Proposed algorithm for CNS prophylaxis in DLBCL in 2022. CMR, complete metabolic response; CT, computed tomography; PD, progressive disease; PR, partial response; SD, stable disease.
Future directions and conclusions
Although we have seen advances in this extremely contentious area of DLBCL management in the last 5 years, it is clear that we need to continue to develop more specific methods of identifying patients at highest risk of CNS relapse and to investigate more effective prophylactic interventions for those at highest risk. As outlined above, the use of ctDNA as a baseline screening tool carries much potential for improving patient selection for prophylaxis. The incorporation of novel agents able to cross the blood-brain barrier is likely to be an area of ongoing research. Although trials thus far have not demonstrated an overall benefit with the addition of ibrutinib or lenalidomide to R-CHOP,45,46 studies such as REMoDL-A (NCT04546620) investigating the addition of acalabrutinib are ongoing, and results with regard to CNS relapse rates will be of interest. Improving systemic disease control may be an effective way to reduce CNS relapses, particularly those that occur concurrent with systemic relapse. The recent POLARIX trial demonstrated an additional agent (polatuzumab vedotin) that can improve PFS over R-CHOP alone for the first time, raising the question of whether broad adoption of such a frontline regimen could have an impact on CNS events over time.47 Until then, we must use currently available risk-stratification models to carefully select patients for more stringent baseline screening for CNS disease and exercise greater caution in the use of prophylactic HD-MTX in light of recently published data.
Conflict-of-interest disclosure
Matthew R. Wilson: speakers’ bureau: Kite/Gilead, Janssen; consultancy/advisor: Veriton; conference/travel support: Takeda, Janssen, Kite/Gilead; research funding: Abbvie.
Sabela Bobillo: speakers’ bureau: Janssen, Roche, Gilead; travel support: Gilead.
Kate Cwynarski: consultancy/advisor: Roche, Takeda, Celgene, Atara, Gilead, KITE, Janssen, Incyte; speakers' bureau: Roche, Takeda, KITE, Gilead, Incyte; research funding: Roche, Takeda, KITE, Janssen, Bristol Myers Squibb.
Off-label drug use
Matthew R. Wilson: nothing to disclose.
Sabela Bobillo: nothing to disclose.
Kate Cwynarski: nothing to disclose.