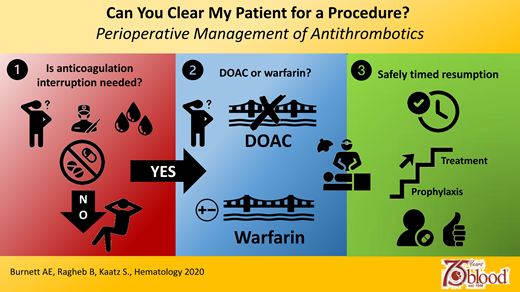
Abstract
Periprocedural management of antithrombotics is a common but challenging clinical scenario that renders patients vulnerable to potential adverse events such as bleeding and thrombosis. Over the past decade, periprocedural antithrombotic approaches have changed considerably with the advent of direct oral anticoagulants (DOACs), as well as a paradigm shift away from bridging in many warfarin patients. Successfully navigating this high-risk period relies on a number of individualized patient assessments conducted within a framework of standardized, systematic approaches. It also requires a thorough understanding of antithrombotic pharmacokinetics, multidisciplinary coordination of care, and comprehensive patient education and empowerment. In this article, we provide clinicians with a practical, stepwise approach to periprocedural management of antithrombotic agents through case-based examples of relevant clinical scenarios.
Learning Objectives
Explain significant differences between perioperative management of DOAC and warfarin, including the rationale for these differences
Determine if temporary interruption of anticoagulant therapy is needed through evaluation of factors, including bleeding and thrombotic potential of the procedure and the patient
Identify warfarin patients who do and do not warrant consideration for perioperative bridging
Develop safe, effective perioperative anticoagulation plans for DOAC and warfarin patients based on existing evidence and expert consensus
Introduction
Although exact numbers are not known, it is estimated that 6 to 8 million people in the United States are prescribed oral anticoagulation (OAC) with either warfarin or a direct oral anticoagulant (DOAC).1,2 Approximately 10% to 15% of these patients will require temporary interruption of anticoagulation for surgery or an invasive procedure, which equates to 600 000 to 1 200 000 interruptions annually.3,4
The periprocedural period is a time of significant risk for anticoagulation patients for a multitude of reasons, including but not limited to the following:
A number of complex individualized assessments must be made to estimate bleeding and thrombotic risks of both the procedure and the patient, which are largely based on expert consensus.5,6
It is estimated that surgical patients will experience up to 15 transitions of care.7 Each transition is associated with vulnerability to medication discrepancies, therapeutic overlap, failure to resume anticoagulation, communication errors, and missed coordination of follow-up.8,9
Anecdotal experience suggests providers lack familiarity with the pharmacokinetics of the DOACs, which differ dramatically from those of warfarin. It is not uncommon in clinical practice to discover DOAC patients with periprocedural plans with inappropriately prolonged hold times and/or overlapping therapy with low molecular weight heparin (LMWH) as providers try to manage these agents in a similar manner as they would warfarin.
The increased risk for adverse events in the periprocedural period has prompted a number of quality improvement and patient safety initiatives and shifts in practice.
In 2019, the Joint Commission revised its National Patient Safety Goal (NPSG) 03.05.01 pertaining to anticoagulants to include a new element of performance (EP3) calling for hospitals to use approved protocols and evidence-based practice guidelines for periprocedural management of all patients taking oral anticoagulants.10
The Centers for Medicare & Medicaid Services offers merit- based incentive payments to physicians who provide documentation of periprocedural anticoagulation management plans, including timing of interruption, management of concomitant antithrombotics, bridging (if indicated), laboratory measurements, timing of resumption, and discussion of plan with the patient.11
An increasing number of health systems are using clinical pharmacists and anticoagulation stewardship programs to optimize development and application of guideline-recommended periprocedural plans.12,13
It is important to acknowledge that perioperative consultation may be accompanied by differences of opinion and approaches between involved disciplines that requires thorough discussion and consensus building. In addition, a patient who is cleared for surgery should not be deemed “risk-free” from adverse events. Multidisciplinary collaboration before, during, and after surgery to assess for and mitigate risk as much as possible is essential for optimized patient care.
Atrial fibrillation
CLINICAL CASE
A 65-year-old female patient is to undergo an elective right hip replacement. Her medical history includes a bioprosthetic aortic valve, nonvalvular atrial fibrillation, hypertension, and diabetes. She is referred for periprocedural DOAC recommendations. Laboratory values, including renal and liver function, are normal.
Step 1: Does anticoagulation need to be interrupted?
Bleeding risk of the procedure
The need for OAC interruption is determined primarily by the bleeding risk of the procedure. Unfortunately, a high-quality, evidence-based schema to categorize procedural bleeding risk has not been well established and has led to differences across guidelines and variations in practice.14-16 Recently, the International Society on Thrombosis and Haemostasis issued guidance on this with the intent of promoting more standardized approaches.17 In our practice, we also often refer to the very comprehensive procedural bleeding risk appendix published with the 2017 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation in Patients With Nonvalvular Atrial Fibrillation.18 In Table 1, we have provided a list (nonexhaustive) of procedures with minimal bleeding risk that likely do not require interruption that can greatly simplify management and mitigate risk for harm. For other procedures, we suggest clinicians use existing procedure categorization tools as a framework for discussion with surgeons and other interventionalists when developing a perioperative plan.
Minor surgeries or procedures that may not require interruption of OAC
| • Minor dental (eg, 1-2 tooth extraction, cleaning) |
| • Minor dermatologic or cutaneous |
| • Cataract |
| • Cardiac implantable devices (pacemakers, defibrillators) |
| • Cardiac ablations, cardioversion, electrophysiology studies |
| • Endovascular procedures (eg, angioplasty) |
| • Endoscopy without resection or biopsy |
| • Intramuscular vaccination |
| • Percutaneous coronary interventions (radial approach) |
| • Minor dental (eg, 1-2 tooth extraction, cleaning) |
| • Minor dermatologic or cutaneous |
| • Cataract |
| • Cardiac implantable devices (pacemakers, defibrillators) |
| • Cardiac ablations, cardioversion, electrophysiology studies |
| • Endovascular procedures (eg, angioplasty) |
| • Endoscopy without resection or biopsy |
| • Intramuscular vaccination |
| • Percutaneous coronary interventions (radial approach) |
Bleeding risk of the patient
The intrinsic bleeding risk of the patient should also be considered during periprocedural planning. Characteristics that we routinely consider when assessing patient bleeding risk include thrombocytopenia, renal dysfunction with uremia, significant hepatic impairment with baseline coagulopathy, history of bleeding, and concomitant medications such as antiplatelets or nonsteroidal anti-inflammatory drugs. Although many of these are not modifiable, being aware of their presence may help anticipate and address complications.
Minimal bleed risk procedures
Studies have shown that many low bleeding risk minor surgeries or procedures (which constitute up to 20% of cases) can be safely done without warfarin interruption.19 In addition, randomized controlled trials and meta-analyses have shown that warfarin interruption with or without LMWH bridging leads to more adverse events than no warfarin interruption.20-23 There is less certainty with continuing DOACs around minor procedures. However, a growing body of evidence suggests uninterrupted DOACs may be reasonable24-26 and possibly safer than uninterrupted warfarin in many procedures (Table 1).27,28
Our patient is undergoing a hip replacement that we would consider a high bleed risk procedure, and DOAC should be interrupted.
Step 2: If OAC interruption is needed, does the patient require bridging?
DOACs
There are significant pharmacokinetic differences between DOACs and warfarin, and thus their perioperative management requires different approaches.26,29 In patients with normal renal function, DOACs have a much shorter half-life than warfarin (approximately 12 hours vs approximately 40 hours, respectively) and much faster offset. Thus, withholding DOACs for a prolonged period of time (eg, 5 days as is routinely done with warfarin) potentially places the patient at risk for a thrombotic event. Also, given the rapid offset of DOACs, it is not necessary to interrupt them preprocedurally and replace with LMWH. In the postprocedure setting, it is imperative to recognize the faster onset of DOAC anticoagulant action (approximately 3 hours) compared with warfarin (approximately 4-5 days) and the need for carefully timed resumption to mitigate bleeding risk. Very importantly, DOACs should never be overlapped with parenteral anticoagulants, such as LMWH, as this is not necessary due to their rapid onset and studies showing significantly increased bleeding.3,4,25
The Perioperative Anticoagulation Use for Surgery Evaluation (PAUSE) cohort study30 used a simple DOAC interruption and resumption protocol without any bridging in patients with atrial fibrillation undergoing surgical procedures. This was based in part on successful use of a similar approach in an earlier prospective multicenter trial evaluating perioperative management of dabigatran.31 Timing of interruption and resumption was based on DOAC pharmacokinetic properties, procedural bleed risk, and renal function. The investigators hoped to show a major bleeding rate of less than 2.0% and a stroke or transient ischemic attack (TIA) rate of less than 1.5% with 95% certainty. Of note, this study used number of days and not hours for interruption and resumption timing, as shown in Table 2. Because dabigatran is primarily eliminated through the kidneys (80%), creatinine clearance was estimated (using the Cockcroft-Gault equation and actual body weight), and hold times were doubled in dabigatran patients with an estimated clearance of less than 50 mL/min. Results showed with 95% confidence that bleeding rates were not greater than 2.00%, 1.73%, and 2.65% with apixaban, dabigatran, and rivaroxaban, respectively, and arterial thromboembolism was not greater than 1.5% with any DOAC. In addition, it was shown that more than 90% of DOAC patients collectively had little to no residual anticoagulant effect of more than 30 ng/mL (which has been suggested as an acceptable preoperative plasma concentration32 ), precluding the need for routine preoperative laboratory assessment when the PAUSE protocol is followed. The Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis suggests that if a preoperative quantitative DOAC level is 30 ng/mL or less, it is reasonable to proceed with an invasive procedure.33 To our knowledge, there are no data to suggest enhanced utility of measuring DOAC concentrations before elective procedures compared with a simple standardized, pharmacokinetic-based protocol as was used in the PAUSE trial. Urgent or emergent procedures pose a challenge, and there may be a role for DOAC measurement in select situations. However, it is critical to bear in mind that these quantitative DOAC assays are not available in many hospitals, and if they are, turnaround times may preclude any utility.
Periprocedural DOAC interruption protocol from PAUSE study
| Characteristic . | Apixaban . | Rivaroxaban . | Dabigatran CrCl ≥50 . | Dabigatran CrCl <50 . | Edoxaban . |
|---|---|---|---|---|---|
| Preprocedural interruption | Days | Days | Days | Days | Not studied |
| Low bleeding risk procedure | 1 | 1 | 1 | 2 | |
| High bleeding risk procedure | 2 | 2 | 2 | 4 | |
| Postprocedural resumption | |||||
| Low bleeding risk procedure | 1 | 1 | 1 | 1 | |
| High bleeding risk procedure | 2-3 | 2-3 | 2-3 | 2-3 |
| Characteristic . | Apixaban . | Rivaroxaban . | Dabigatran CrCl ≥50 . | Dabigatran CrCl <50 . | Edoxaban . |
|---|---|---|---|---|---|
| Preprocedural interruption | Days | Days | Days | Days | Not studied |
| Low bleeding risk procedure | 1 | 1 | 1 | 2 | |
| High bleeding risk procedure | 2 | 2 | 2 | 4 | |
| Postprocedural resumption | |||||
| Low bleeding risk procedure | 1 | 1 | 1 | 1 | |
| High bleeding risk procedure | 2-3 | 2-3 | 2-3 | 2-3 |
An unanswered question is what to do in patients with a history of venous thromboembolism (VTE), and we extrapolate the PAUSE study timing of interruption and resumption to these patients with confidence in the major bleeding rates but uncertainty in VTE recurrence.
An additional point is the PAUSE study differs slightly from the recommendations by the American Society of Regional Anesthesia, which recommend holding DOACs for 72 hours prior to neuraxial anesthesia and longer for dabigatran if the creatinine clearance is low.34
We would hold the DOAC for 2 days before surgery and restart the DOAC on postoperative day 2 or 3.
In the postprocedural interim until therapeutic anticoagulation is resumed, we would ensure adequate prophylaxis (eg, enoxaparin 40 mg subcutaneously [SQ] once daily) to prevent postoperative VTE.
CLINICAL CASE (Continued)
The patient does well, and a year later, she is going to have her other hip replaced. Unfortunately, her insurance has changed, and she can no longer afford a DOAC and is taking warfarin. Her medical history is unchanged, and her laboratory tests remain normal.
Warfarin
Due to the long offset of warfarin (approximately 5 days), clinicians must determine timing of interruption to provide the desired residual anticoagulant effect, as well as any role for bridging with a parenteral anticoagulant. For clarity, when we use the term bridging, we mean therapeutic-intensity anticoagulation, such as enoxaparin 1 mg/kg twice daily. The decision to bridge or not is based on the patient's indication for anticoagulation, underlying thrombotic risk, and the thrombotic risk of the procedure. Although bridging of warfarin was once routinely employed, retrospective observational evidence published over the past decade has consistently shown bridging to be associated with net harm and suggests it should be avoided in most cases.6,35-37
The randomized controlled Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial sought to answer whether forgoing LMWH bridging in atrial fibrillation patients requiring warfarin interruption for a procedure would result in less major bleeding without an increase in arterial thromboembolism. Per standardized protocol, warfarin was held for 5 days preprocedurally, and patients were randomized to placebo or LMWH (dalteparin) with 30-day primary outcomes of major bleeding (superiority) and arterial thromboembolic event (noninferiority). There was no difference in arterial thromboembolism 0.4% vs 0.3%, and major bleeding was reduced with no bridging (1.3%) vs LMWH bridging (3.2%) (P < .005).37 This trial prompted a paradigm shift away from bridging in most patients with atrial fibrillation.
In the BRIDGE trial, warfarin was resumed the night of the procedure at the patient's usual home dose. The mean time to reestablish a therapeutic international normalized ratio (INR) was 8 days. To minimize this period of subtherapeutic anticoagulation, it is reasonable to consider a boosted dose of warfarin for 1 to 2 days after the procedure in the absence of high bleeding risk.38
Thromboembolic risk stratification
We use a thromboembolic risk stratification approach based on available evidence and expert consensus recommendations from multiple organizations to guide decisions on bridging warfarin patients who require temporary interruption for a procedure (Table 3).18,19,39,40
Thromboembolic risk stratification and bridging considerations
| Indication . | Mechanical heart valve . | Atrial fibrillation . | VTE . | ||||
|---|---|---|---|---|---|---|---|
| Guideline(s) . | ACCP 2012, ACC/AHA 2020 . | ACCP 2012, ACC 2017 . | ACCP 2012, ASH 2018 . | ||||
| Thrombotic risk . | Criteria . | Recommendation . | Criteria* . | Recommendation . | Criteria . | Recommendation . | |
| High | • All mitral valve • Caged-ball and tilting disc • Stroke or TIA in past 6 months | Suggest bridging/reasonable to bridge | CHADS2 >4 | CHA2DS2-VASc >7 | Suggest bridging | • Within 3 months • Severe thrombophilia | CHEST 2012: Suggest bridging ASH 2018: Not addressed |
| Moderate | Bileaflet aortic valve + risk factors • Atrial fibrillation • Prior stroke or TIA • Hypertension • Diabetes • Congestive heart failure • Age >75 years | Individualized decision based on patient and procedural nuances | CHADS2 3-4 | CHA2DS2-VASc 5 or 6 | Individualized decision based on patient and procedural nuances | • Past 3-12 months • Recurrent VTE • Active cancer • Nonsevere thrombophilia | CHEST 2012: Individualized decision based on patient and procedural nuances ASH 2018: Do not bridge |
| Low | Bileaflet aortic valve without risk factors | Do not bridge | CHADS2 ≤2 | CHA2DS2-VASc <4 | Do not bridge | More than 12 months ago | CHEST 2012: Do not bridge ASH 2018: Do not bridge |
| Indication . | Mechanical heart valve . | Atrial fibrillation . | VTE . | ||||
|---|---|---|---|---|---|---|---|
| Guideline(s) . | ACCP 2012, ACC/AHA 2020 . | ACCP 2012, ACC 2017 . | ACCP 2012, ASH 2018 . | ||||
| Thrombotic risk . | Criteria . | Recommendation . | Criteria* . | Recommendation . | Criteria . | Recommendation . | |
| High | • All mitral valve • Caged-ball and tilting disc • Stroke or TIA in past 6 months | Suggest bridging/reasonable to bridge | CHADS2 >4 | CHA2DS2-VASc >7 | Suggest bridging | • Within 3 months • Severe thrombophilia | CHEST 2012: Suggest bridging ASH 2018: Not addressed |
| Moderate | Bileaflet aortic valve + risk factors • Atrial fibrillation • Prior stroke or TIA • Hypertension • Diabetes • Congestive heart failure • Age >75 years | Individualized decision based on patient and procedural nuances | CHADS2 3-4 | CHA2DS2-VASc 5 or 6 | Individualized decision based on patient and procedural nuances | • Past 3-12 months • Recurrent VTE • Active cancer • Nonsevere thrombophilia | CHEST 2012: Individualized decision based on patient and procedural nuances ASH 2018: Do not bridge |
| Low | Bileaflet aortic valve without risk factors | Do not bridge | CHADS2 ≤2 | CHA2DS2-VASc <4 | Do not bridge | More than 12 months ago | CHEST 2012: Do not bridge ASH 2018: Do not bridge |
ACCP 2012 based on CHADS2; 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation based on CHA2DS2-VASc.
ACC, American College of Cardiology; ACCP, American College of Chest Physicians; AHA, American Heart Association.
We would not use bridging anticoagulation in our atrial fibrillation patient because her CHA2DS2-VASc (Congestive heart failure = 1, Hypertension = 1, age >/= 75 years = 2, Diabetes = 1, Stroke/TIA = 2, Vascular disease = 1, Age 65-74 = 1, Sex category = 1) score is 4.
We would resume her warfarin the night of the procedure.
While the patient is still in the hospital, we would ensure adequate prophylaxis with low-dose anticoagulation (eg, enoxaparin 40 mg SQ once daily) to prevent postoperative VTE until the INR is 2 or more.
Mechanical heart valve
CLINICAL CASE (Continued)
The patient did well after her second hip surgery and has been doing so much walking that her left knee now needs replacement. Unfortunately, her bioprosthetic aortic valve gave out in the interim, and she now has a bileaflet mechanical aortic valve. Her other medical history is unchanged, and laboratory values remain normal. She presents for perioperative warfarin management again.
The American College of Chest Physicians (ACCP) 2012 guidelines and the American College of Cardiology (ACC)/American Heart Association 2020 guidelines are concordant in their classification of mechanical heart valves as (1) high risk if any mitral, caged-ball, or tilting disk valves or recent (within 6 months) stroke or TIA; (2) moderate risk with bileaflet aortic valve and any other risk factor, which includes atrial fibrillation, prior stroke or TIA, hypertension, diabetes, congestive heart failure, or older than 75 years; and (3) low risk with bileaflet aortic valves with no other risk factors (Table 3).19,39
The only randomized trial for LMWH bridging for warfarin interruption in patients with mechanical heart valves that we are aware of is the recently published Postoperative low molecular weight heparin bridging treatment for patients at high risk of arterial thromboembolism (PERIOP 2) trial.41 Patients with atrial fibrillation or non-high-risk mechanical valves who required warfarin interruption for a procedure (n = 1471) were randomized to LMWH bridging or placebo and followed for 90 days. LMWH bridging consisted of therapeutic dose dalteparin 200 IU/kg subcutaneously on days −3 and −2 before procedure and half the therapeutic dose of 100 IU/kg subcutaneously the day before the procedure. Postprocedurally (same day or next day), patients in the intervention arm at high bleed risk were given fixed-dose prophylactic dalteparin 5000 IU, and those at low bleed risk were given dalteparin 200 IU/kg subcutaneously until the INR was more than 1.9.
In the subgroup of 304 patients with non-high-risk mechanical valves, no bridging vs bridging major thromboembolism event rates were 0% vs 0.7% (P = .49), and major bleeding occurred in 2% vs 0.7% (P = .62) respectively. The investigators concluded there is no benefit from postprocedure LMWH bridging in patients with non-high-risk mechanical valves.
In a systemic review of 5 studies of unfractioned heparin or LMWH bridging in patients with mechanical heart valves, major bleeding rates ranged from 4.39% to 10.07%. Bleeding definitions varied, precluding the pooling of results. Although observational data, this analysis suggests that bleeding risk associated with bridging in valve patients is not negligible and underscores the need for limiting to patients at increased thrombotic risk.42
Some observational data suggest that prophylactic dose anticoagulation may be a viable perioperative approach for select mechanical valve patients requiring temporary interruption in warfarin, as well as those with newly implanted valves as a bridge to a therapeutic INR of 2 or more.43,44 This may mitigate bleeding risk but also provides important postoperative VTE prophylaxis. However, it cannot be recommended as a routine approach for all patients until better data are available.
We would check her INR about 7 to 10 days prior to surgery and, if in the therapeutic range, would hold warfarin 5 days preoperatively to allow nadir of anticoagulant effect at the time of the procedure.
We would have careful shared decision making with the patient and her orthopedic surgeon regarding the potential benefits and harms of bridging with LMWH because her thromboembolic risk is moderate, and unlike warfarin interruption in atrial fibrillation, there is no strong evidence to guide us.
After much discussion, it is ultimately decided she does not require bridging therapy based on her moderate thromboembolic risk from her bileaflet mechanical aortic valve.
VTE
CLINICAL CASE (Continued)
A year later, the patient is now going to have her other (right) knee replaced (fourth major orthopedic surgery), and this consultation is more complex.
After her last knee surgery, the decision to not use bridging with therapeutic dose LMWH was interpreted as to not use any LMWH, and the patient never received any venous thromboembolism prophylaxis postoperatively while warfarin rose to the appropriate INR target.
She developed a deep vein thrombosis, which was treated in the usual manner, and she remains on warfarin for her mechanical heart valve and atrial fibrillation.
The ACCP classifies VTE as (1) high, VTE within 3 months or severe thrombophilia; (2) moderate, VTE within 3 to 12 months, nonsevere thrombophilia (eg, heterozygous factor V Leiden or prothrombin gene mutation), recurrent VTE, or active cancer within 6 months; and (3) low, VTE more than 12 months ago and no other risk factors. They suggest bridging for high risk, no bridging for low risk, and individualized decision making for moderate risk.19 The American Society of Hematology (ASH) 2018 guidelines on optimal management of anticoagulant therapy, which use the same VTE risk stratification as the ACCP, differ slightly, in that they suggest no bridging in patients with VTE at moderate risk (Table 3).40
There are no randomized trials for bridging in patients with VTE who require periprocedural warfarin interruption that we are aware of. A systematic review of 28 observational studies comparing bridging vs no bridging in 6915 procedures showed no difference in VTE rates (0.7% vs 0.5%), an increase in major bleeding (1.8% vs 0.4%), and an increase in all bleeding (3.9% vs 0.4%), respectively. The 95% confidence intervals overlapped for VTE and major bleeding but did not for all bleeding.35
There is no clear answer for this patient. She is at low risk from a purely VTE standpoint based on ACCP and ASH guidelines.
However, we would likely bridge just on practicality of her collective risk factors.
We would initiate perioperative bridging with therapeutic dose LMWH and hold for 24 hours prior to the procedure.
We would resume therapeutic dose LMWH 2 to 3 days postoperatively because knee surgery is a high bleeding risk surgery.
This time, however, prophylactic dose LMWH is to be given on postoperative days 1 and 2 for VTE prevention until therapeutic dose LMWH is started for the moderate-risk mechanical valve (and will also “cover” the low-risk atrial fibrillation and prior VTE).
Postprocedure VTE prophylaxis
As highlighted in each of the case vignettes above, most if not all surgical patients warrant postoperative VTE prophylaxis in the interim until they can be safely increased to therapeutic anticoagulation at the appropriate time (ie, 24 hours for low bleed risk procedures, 48-72 hours for high bleed risk procedures). This is termed a “step-up” approach, and the most commonly used prophylactic agents are LMWH and heparin. Although DOACs could be considered a means of VTE prophylaxis in the step-up period, patients may not immediately be able to tolerate oral medications. In addition, some patients may have altered absorption, including those with postoperative ileus or patients who have undergone bariatric surgery. For these patients, use of an anticoagulant that does not require oral intake or absorption would be preferred.
Special situations
Some additional clinical situations warrant discussion but are beyond the scope this article. We have summarized these in Tables 4 and 5.45-51
Special situations that may influence perioperative antithrombotic management
| Situation or issue . | Comments . | Suggested actions . |
|---|---|---|
| Urgent/emergent procedures | • If there is not adequate time to allow natural normalization of the patient's coagulation status before surgery, use of reversal agents, prohemostatic agents, or specific antidotes may be indicated and should be done judiciously and thoughtfully. • Rapidly returning a patient to their native, prothrombotic state along with any intrinsic risk of thrombosis posed by the reversal agents or antidotes themselves may increase the risk for adverse events. | • Shared decision making with the patient, multidisciplinary discussion, and consultation with a thrombosis specialist • Clinicians are referred to existing guidance on reversal of anticoagulation.40,45,46 |
| Patients on concomitant antiplatelet therapies | • This is an opportune time to evaluate the overall clinical necessity of concomitant antiplatelet therapy. If not indicated, clinicians should discuss permanent discontinuation with the patient and prescriber. • Whether to temporarily interrupt concomitant antiplatelet therapies is a complex decision that is based on several factors, including indication, recency of events, bleeding, and thrombotic risks of the procedure and patient. • Perioperative antiplatelet strategies should be individually tailored based on multidisciplinary input. | • Shared decision making with the patient, multidisciplinary discussion, and consultation with prescriber of antiplatelet therapy (eg, cardiologist, neurologist) and thrombosis specialist • Clinicians are referred to existing guidance on perioperative antiplatelet management.47,48 |
| History of heparin-induced thrombocytopenia (HIT) | • Patients with a history of HIT should not receive any heparin or LMWH products, including small doses such as flushes or VTE prophylaxis. | • Use an alternative, nonheparin anticoagulant such as bivalirudin, fondaparinux, or a DOAC as appropriate based on patient's clinical status and clinical situation. • Clinicians are referred to existing guidance on HIT.49 |
| Inferior vena cava (IVC) filters | • The estimated incidence of VTE recurrence in the first month after an acute event off of anticoagulant therapy is estimated to be 40%.50 • If possible, delay nonurgent/emergent procedures to allow at least 3 months of anticoagulation therapy following an acute VTE. • If the procedure cannot be delayed and the VTE occurred in the previous 30 days, a retrievable IVC filter may be considered. | • If the patient is anticipated to be off anticoagulation for <48 hours, aggressive pharmacologic prophylaxis with expedient escalation to therapeutic dosing is preferred. • If a retrievable filter is considered, a plan for timely removal should be clearly delineated prior to placement. • Clinicians are referred to existing guidance on IVC filters.51 |
| Severe renal impairment | • Warfarin patients with severe renal impairment or on hemodialysis who have a clear indication for bridging cannot be managed with LMWH. | • These patients may need to have their warfarin held at a prespecified time in the outpatient setting and then be admitted for bridging therapy with intravenous heparin. |
| Situation or issue . | Comments . | Suggested actions . |
|---|---|---|
| Urgent/emergent procedures | • If there is not adequate time to allow natural normalization of the patient's coagulation status before surgery, use of reversal agents, prohemostatic agents, or specific antidotes may be indicated and should be done judiciously and thoughtfully. • Rapidly returning a patient to their native, prothrombotic state along with any intrinsic risk of thrombosis posed by the reversal agents or antidotes themselves may increase the risk for adverse events. | • Shared decision making with the patient, multidisciplinary discussion, and consultation with a thrombosis specialist • Clinicians are referred to existing guidance on reversal of anticoagulation.40,45,46 |
| Patients on concomitant antiplatelet therapies | • This is an opportune time to evaluate the overall clinical necessity of concomitant antiplatelet therapy. If not indicated, clinicians should discuss permanent discontinuation with the patient and prescriber. • Whether to temporarily interrupt concomitant antiplatelet therapies is a complex decision that is based on several factors, including indication, recency of events, bleeding, and thrombotic risks of the procedure and patient. • Perioperative antiplatelet strategies should be individually tailored based on multidisciplinary input. | • Shared decision making with the patient, multidisciplinary discussion, and consultation with prescriber of antiplatelet therapy (eg, cardiologist, neurologist) and thrombosis specialist • Clinicians are referred to existing guidance on perioperative antiplatelet management.47,48 |
| History of heparin-induced thrombocytopenia (HIT) | • Patients with a history of HIT should not receive any heparin or LMWH products, including small doses such as flushes or VTE prophylaxis. | • Use an alternative, nonheparin anticoagulant such as bivalirudin, fondaparinux, or a DOAC as appropriate based on patient's clinical status and clinical situation. • Clinicians are referred to existing guidance on HIT.49 |
| Inferior vena cava (IVC) filters | • The estimated incidence of VTE recurrence in the first month after an acute event off of anticoagulant therapy is estimated to be 40%.50 • If possible, delay nonurgent/emergent procedures to allow at least 3 months of anticoagulation therapy following an acute VTE. • If the procedure cannot be delayed and the VTE occurred in the previous 30 days, a retrievable IVC filter may be considered. | • If the patient is anticipated to be off anticoagulation for <48 hours, aggressive pharmacologic prophylaxis with expedient escalation to therapeutic dosing is preferred. • If a retrievable filter is considered, a plan for timely removal should be clearly delineated prior to placement. • Clinicians are referred to existing guidance on IVC filters.51 |
| Severe renal impairment | • Warfarin patients with severe renal impairment or on hemodialysis who have a clear indication for bridging cannot be managed with LMWH. | • These patients may need to have their warfarin held at a prespecified time in the outpatient setting and then be admitted for bridging therapy with intravenous heparin. |
Additional clinical resources for periprocedural tools and guidance
| Resource . | Location . |
|---|---|
| Anticoagulation Forum Centers of Excellence | https://acforum-excellence.org |
| Managing Anticoagulation in the Perioperative Period (MAPP) | http://mappp.ipro.org |
| Michigan Anticoagulation Quality Improvement Initiative (MAQI2) | http://www.maqi2.org |
| Thrombosis Canada | https://thrombosiscanada.ca |
| Resource . | Location . |
|---|---|
| Anticoagulation Forum Centers of Excellence | https://acforum-excellence.org |
| Managing Anticoagulation in the Perioperative Period (MAPP) | http://mappp.ipro.org |
| Michigan Anticoagulation Quality Improvement Initiative (MAQI2) | http://www.maqi2.org |
| Thrombosis Canada | https://thrombosiscanada.ca |
Summary
Each year, a large number of patients taking OACs undergo an invasive procedure, with many requiring temporary interruption of therapy. This is a high-risk period for patients that requires thoughtful and methodical approaches using the best available evidence and expert consensus to balance bleeding and thrombotic risks. It is important for clinicians to be familiar with procedures where OAC does not require interruption, as this will greatly simplify management and likely minimize adverse events. For situations when interruption is indicated, it is imperative for clinicians to be familiar with key differences in pharmacokinetic properties between DOACs and warfarin, as these lead to significantly different perioperative management approaches. A stepwise system-level process, multidisciplinary collaboration, shared decision making with patients, and clear communication and documentation of the plan are all key elements of antithrombosis stewardship necessary for successful navigation and implementation of perioperative plans.
Conflict-of-interest disclosure
Allison Elaine Burnett: no relevant conflicts of interest.
Bishoy Ragheb: no relevant conflicts of interest.
Scott Kaatz: research funding: Janssen, BMS, Osmosis Research, National Institutes of Health; consultancy: Janssen, BMS, Alexion/Portola, Novartis, CSL Behring, Gilead.
Off-label drug use
Allison Elaine Burnett: There are currently no anticoagulants approved specifically for bridging therapy in warfarin patients, thus any discussion on this is off-label.
Bishoy Ragheb: There are currently no anticoagulants approved specifically for bridging therapy in warfarin patients, thus any discussion on this is off-label.
Scott Kaatz: There are currently no anticoagulants approved specifically for bridging therapy in warfarin patients, thus any discussion on this is off-label.