Abstract
Older adults with multiple myeloma (MM) are a growing population, and personalizing treatment based on disease and health status is imperative. Similar to MM staging systems that provide disease-related prognostic information, myeloma-specific frailty tools can better identify subgroups at greatest risk for treatment-related toxicity and early treatment discontinuation, as well as predict overall survival. Several myeloma-specific validated tools are well studied. Although these fitness/frailty scores have shaped our understanding of the heterogeneity among older adults with myeloma, the application of such scores in treatment decision making (ie, transplant considerations, relapse) is an unmet need. Here we outline how to incorporate frailty assessments in the evaluation of older adults with MM in the clinical setting with consideration of other factors such as patient preferences, treatment risks/benefits, life expectancy, and disease biology.
Learning Objectives
Frailty can be objectively characterized in older adults with MM using validated scoring systems.
Disease and patient factors will influence treatment decisions (health status, biology, risks/benefits, and shared decision making).
CLINICAL CASE
A 70-year-old woman with newly diagnosed standard-risk, Revised International Staging System (R-ISS) III, immunoglobulin G κ multiple myeloma (MM) presents for consultation. Until 4 months ago, she lived independently, had an active lifestyle, exercised 3 times weekly, and served as the lead volunteer coordinator for a nonprofit organization. She was also independent in all activities of daily living (ADLs) and instrumental activities of daily living (IADLS). She now reports severe lower back pain that limits her mobility, fatigue, and shortness of breath after walking 1 block that has limited her function. She has well-controlled diabetes mellitus (glipizide) and hypertension (amlodipine, lisinopril) and reports taking several over-the-counter vitamins to maintain her vitality. She is widowed and lives alone. Her Eastern Cooperative Oncology Group (ECOG) performance status is 2. Positron emission tomo- graphy/computed tomography confirms diffuse lytic lesions in the humeri, femora, pelvis, and spine along with multiple compression fractures involving T6 to T8. Her estimated glomerular filtration rate is 55. She emphasizes that maintaining her independence and quality of life are her top priorities. She wants to know if there are tools to gauge her health and would like the best therapy for her personally.
Myeloma fitness/frailty scores can inform treatment tolerance
Predicting treatment-related toxicity is especially critical for older adults with myeloma due to the heterogeneity that exists in aging. Differences in how individuals age arise from age-associated losses in physical and cognitive function and the additive impact of medical comorbidities, which becomes more prevalent with advancing age.1,2 Previous studies have shown that performance status (Karnofsky, ECOG) is incapable of fully capturing the fitness level of an older adult.3 Furthermore, although staging systems such as the ISS and R-ISS can provide valuable disease-related prognostic information, these staging systems are incapable of fully discerning the subgroup of patients at greatest risk for treatment-related toxicity and early treatment discontinuation.4,5
To account for some of these aging-related deficits, the International Myeloma Working Group (IMWG) proposed a scoring system for myeloma patient frailty that predicts treatment-related toxicity and survival, using age, the Katz ADL, the Lawton IADL, and the Charlson Comorbidity Index (CCI)6 (Table 1). In the clinical vignette, the patient has an IMWG score of 1 = intermediate-fit. Among the intermediate-fit subgroup, the 3-year overall survival (OS) was 76% (hazard ratio [HR], 1.61; 95% CI, 1.02-2.56; P = .042), the cumulative incidence of grade 3 or higher nonhematologic adverse events at 12 months was 16.7% (HR, 1.23; 95% CI, 0.89-1.71; P = .217), and the cumulative incidence of treatment discontinuation at 12 months was 20.8% (HR, 1.41; 95% CI, 1.00-2.01; P = .052).6
Myeloma fitness/frailty risk scores
| Frailty score . | Geriatric domains . | Biologic marker . | Cytogenetics included? . | Score range . | Interpretation . |
|---|---|---|---|---|---|
| IMWG6 | ADLs IADLs CCI | None | No | 0-2 | 0 (fit) 1 (intermediate-fit) 2 (frail) |
| R-MCI7 | Age Fried frailty Lung function Renal function Karnofsky performance status | None | Yes | 0-9 | 0-3 (fit) 4-6 (intermediate- fit) 7-9 (frail) |
| Facon frailty scale8 | Age CCI ECOG performance status9 | None | No | 0-1 ≥2 | 0-1 (nonfrail) ≥2 (frail) |
| Myeloma Research Alliance risk profile10 | Age WHO performance status9 | CRP ISS | No | <−0.256 −0.256-−0.0283 >−0.0283 | <−0.256 (low risk) −0.256 to −0.0283 (medium risk) >−0.0283 (high risk) |
| Mayo frailty index11 | Age WHO performance status9 | NT- proBNP | No | 0-3 | 0 (stage 1) 1 (stage 2) 2 (stage 3) 3 (stage 4) |
| Ancona vulnerability score12 | CCI WHO performance status9 | None | No | 0-2 | 0 (low) 1 (intermediate) 2 (high) |
| Frailty score . | Geriatric domains . | Biologic marker . | Cytogenetics included? . | Score range . | Interpretation . |
|---|---|---|---|---|---|
| IMWG6 | ADLs IADLs CCI | None | No | 0-2 | 0 (fit) 1 (intermediate-fit) 2 (frail) |
| R-MCI7 | Age Fried frailty Lung function Renal function Karnofsky performance status | None | Yes | 0-9 | 0-3 (fit) 4-6 (intermediate- fit) 7-9 (frail) |
| Facon frailty scale8 | Age CCI ECOG performance status9 | None | No | 0-1 ≥2 | 0-1 (nonfrail) ≥2 (frail) |
| Myeloma Research Alliance risk profile10 | Age WHO performance status9 | CRP ISS | No | <−0.256 −0.256-−0.0283 >−0.0283 | <−0.256 (low risk) −0.256 to −0.0283 (medium risk) >−0.0283 (high risk) |
| Mayo frailty index11 | Age WHO performance status9 | NT- proBNP | No | 0-3 | 0 (stage 1) 1 (stage 2) 2 (stage 3) 3 (stage 4) |
| Ancona vulnerability score12 | CCI WHO performance status9 | None | No | 0-2 | 0 (low) 1 (intermediate) 2 (high) |
CRP, C-reactive protein; NT-proBNP, N-terminal pro B-type Natriuretic Peptide; WHO, World Health Organization.
After discussing her frailty score, the patient inquires whether additional frailty scales exist and whether these apply to newly diagnosed MM
Additional frailty scores have been developed incorporating age, comorbidities, physical performance, and biomarkers (Table 1). Engelhardt et al7 found that age, myeloma cytogenetics, frailty (Fried phenotype), performance status, and pulmonary and renal function (Revised Myeloma Comorbidity Index [R-MCI]) were prognostic for OS in a prospective cohort of 801 patients with newly diagnosed MM. Of the 801 evaluable patients, 30.8% were considered fit, 55.7% intermediate-fit, and 13.5% frail.7 In the derivation cohort, 552 patients classified as frail had a near 10-fold greater risk of dying than those considered fit (HR, 9.57; 95% CI, 6.52-14.03).7 In our clinical vignette, we are not provided with data to estimate the patient's Fried frailty phenotype (requires gait speed, grip strength, weight, self-reported exhaustion, and physical activity level).13 Neither are we provided with pulmonary function test data, and as such, we cannot calculate this patient's R-MCI score. A retrospective analysis of 1618 trial participants enrolled in the FIRST trial14,15 reported on a simplified frailty score developed from age, CCI, and ECOG.8 Using this predictive score, patients considered frail (score ≥2) had inferior outcomes, especially OS. This simplified score was subsequently externally validated by Stege et al16 using data from participants enrolled in HOVON87/NMSG18. For many centers, lung function testing and formal frailty scoring (ie, Fried frailty phenotype) may be out of scope of practice. Notwithstanding, frailty scoring is typically collected by patients as self-report, and functional testing such as gait speed requires no cost; these instruments are quick, valid, and cost-effective and inform survival.
Rationale for considering nontransplant strategies in older patients with myeloma
At this time, the optimal tool for assessing frailty for treatment decisions (ie, transplant and nontransplant) has yet to be determined. Despite the widespread use of the IMWG frailty score in clinical and research settings, it has its limitations. Namely, the use of chronologic age can detract from the concept of biologic/functional age, and CCI is likely to exclude myeloma- specific comorbidities. Recommendations for longitudinal assessments of fitness and frailty have been based on consensus expert opinion.17-19 Although these fitness/frailty scores have shaped our understanding of the heterogeneity among older adults with myeloma, the application of such scores in the clinical setting requires consideration of other factors such as patient preferences, life expectancy, and disease biology.
For patients in whom candidacy of transplant is a concern due to frailty, there are a number of options. The results from a single-center, single-arm phase 2 study enrolling transplant- ineligible adults with newly diagnosed MM found the triplet regimen of reduced-intensity lenalidomide, subcutaneous bortezomib, and dexamethasone to be tolerable and effective.20 Despite 62% of participants experiencing low-grade sensory neuropathy, only 1 patient experienced grade 3 or higher neuropathy symptoms. After a 60-month follow-up, the median progression-free survival (PFS) was 41.9 months, and the median OS has still not been reached. Based on this study's findings, reduced-intensity lenalidomide, subcutaneous bortezomib, and dexamethasone could be considered for select transplant-ineligible older adults who may be at higher risk for treatment-related neuropathy. The excellent outcomes achieved with the upfront use of daratumumab, lenalidomide, and dexamethasone (DRd) in this patient population rival those achieved by induction, transplant, and maintenance. This recommendation is supported by findings from the MAIA trial,21 a phase 3 multicenter study in which 44% of enrolled participants were 75 years or older and demonstrated a longer median PFS favoring the daratumumab-containing arm. Among those treated with lenalidomide and dexamethasone alone, the median PFS was 34.4 months vs not reached for those who received daratumumab, lenalidomide, and dexamethasone (HR, 0.53; P < .0001). The latest update of the MAIA study reported an estimated 48-month PFS of 60%.22 There are numerous nontransplant regimens19,23,24 ; other alternatives could include daratumumab, bortezomib, melphalan, prednisone (D-VMP); borezomib, lenalidomide, dexamethasone (VRD); cyclophosphadmide, bortezomib, dexamethasone (CyborD). The most common induction strategy for those aged less than 65 to 74 years, in the United States, is VRD frontline therapy.25 Studies are currently under way evaluating whether fitness-based or risk-adapted therapy assignments in myeloma improve disease- and patient-related outcomes26-30 and to understand whether myeloma therapies result in longitudinal changes in an individual's fitness/frailty status.
Rationale for considering transplant in the older patients with myeloma
Autologous stem cell transplant (ASCT), even in the era of novel agents, continues to be a part of the therapeutic paradigm with clear improvements in PFS when incorporated in the upfront setting.31 Historically, the bulk of evidence to support the ongoing use of high-dose melphalan was generated from trials enrolling younger patients, typically 65 years or younger, which excludes almost two-thirds of patients with newly diagnosed myeloma.32 Although European guidelines continue to recommend limiting ASCT to patients younger than 70 years, there is increasing evidence to support the safe use of ASCT in older patients, with emphasis placed on biological age rather than chronological age.33,34
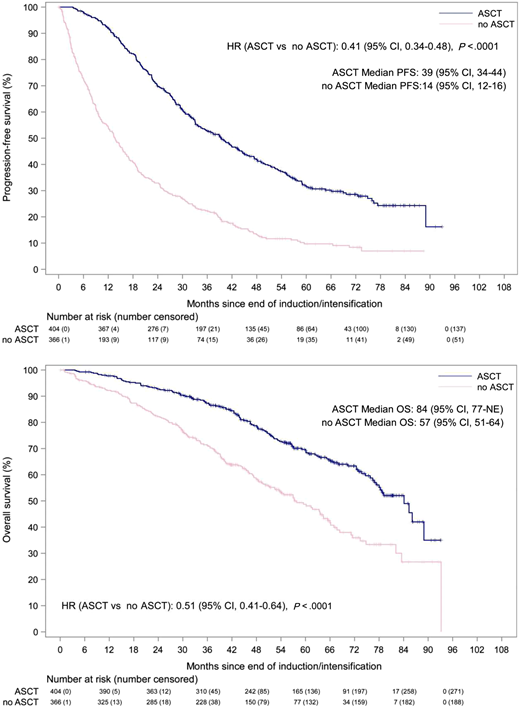
A meta-analysis attempting to quantify the benefit of upfront ASCT in older patients showed a significant improvement in OS (HR, 0.44; 95% CI, 0.34-0.58; P < .001), but authors commented on the paucity of evidence directly comparing ASCT with non-ASCT approaches in this age group.35 Another subanalysis of the large phase 3 myeloma XI trial attempted to use an overlapping cohort of age-matched patients (64-70 years) to compare outcomes and toxicity for those enrolled in the ASCT and non-ASCT arms.36 Importantly, the decision to enroll in the transplant arm was not randomized but based on investigator discretion and is a central limitation of this study. The study yielded a significant advantage in favor of upfront ASCT with improvements in PFS (HR, 0.41; P < .0001) and OS (HR, 0.51; P < .0001), after adjusting for potential baseline covariates (frailty, response to induction) (Figure 1). Interestingly, no differences in toxicity were observed compared with the cohort younger than 65 years who also underwent ASCT. These results echo those reported by a large analysis of 2092 patients 70 years or older from the Center for International Blood and Marrow Transplant Research database, showing identical outcomes for patients who were able to tolerate a 200-mg/m2 dose compared with younger patients.34 Again, no differences were seen in nonrelapse mortality, but patients who received dose reductions (140 mg/m2) did have inferior survival estimates, thought secondary to comorbidities such as renal failure or frailty. Although the evidence is not ideal, an open discussion about the pros and cons of ASCT is warranted, taking into account the patient's own preferences and goals for treatment. Patients with myeloma in general do tend to prioritize PFS but also place high value on quality of life, the side effects of treatment, and potential impact that treatments have on their caregivers.37,38
PFS and OS in matched population aged 64 to 70 years treated with or without ASCT, from myeloma XI subanalysis (with permission).36
PFS and OS in matched population aged 64 to 70 years treated with or without ASCT, from myeloma XI subanalysis (with permission).36
Establishing the risk/benefit ratio for an older patient with myeloma
For the treating clinician, the challenge is how best to identify older patients who will derive the greatest benefit from upfront ASCT. As discussed, available frailty scores can assist with decision making, providing an objective assessment of the initial and changing “fitness” of patients with myeloma undergoing therapy.17 Although considered a standard, it is important to remember that the IMWG Frailty Score was derived from a cohort of patients deemed to be transplant ineligible.6 Similarly, the recently published simplified frailty score8 and UK Myeloma Research Alliance Risk Profile were also derived and validated in a cohort of transplant-ineligible patients.10 The R-MCI was derived from a cohort including 343 patients 65 years or older and 383 who underwent ASCT, but the exact overlap between these 2 groups is unclear.7 Nonetheless, given that those designated as “frail” with these measures are more likely to experience significant toxicities to induction therapy, the additional toxicity of an ASCT in those identified as such would likely outweigh the potential benefit.6
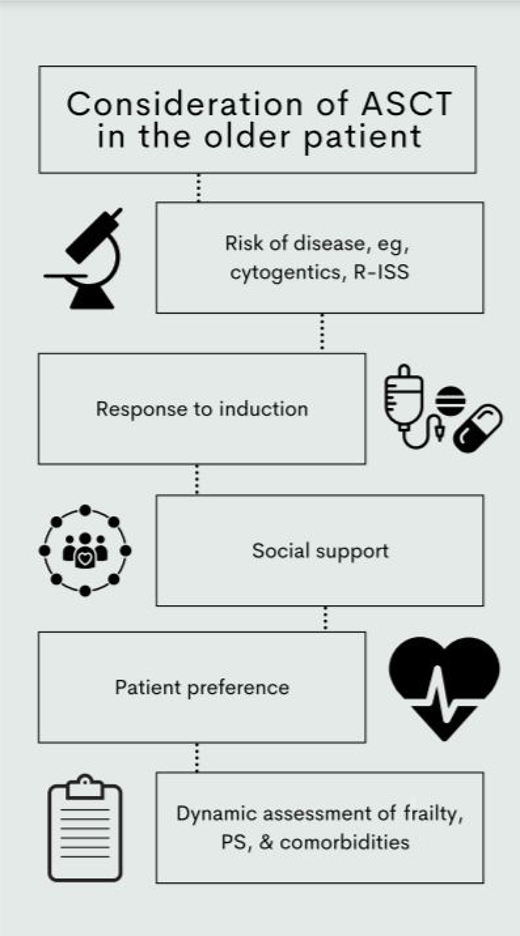
The hematopoietic cell transplantation specific comorbidity index (HCT-CI) was originally developed in the setting of allotransplant but subsequently evaluated in a cohort of 1156 patients with myeloma and found to be predictive of outcome.39 Patients with a score of 1 to 2 and more than 2 (compared with HCT-CI of 0) or a Karnofsky performance status (KPS) less than 90 had inferior OS. It is noteworthy that treatment-related mortality was low at 2% and equivalent for patients with an HCT-CI score of 0 or more than 2. The main cause of treatment-related mortality was disease relapse and progression. Again, only 23% of this cohort was 65 years or older, and even fewer were 70 years or older (n = 72, 6%). Regardless, in the absence of a better alternative, it remains a recommended method of evaluating patients pretransplant, with suggestion to consider alternative approaches in those with an HCT-CI more than 2 or KPS less than 90.40,41 Other factors that will also influence the discussion around incorporation of ASCT include the biology of the disease, prognostic risk, response to induction, and the wishes of the patient and their social situation (Figure 2).
Considerations when evaluating older patients for consideration of ASCT. PS, performance status.
Considerations when evaluating older patients for consideration of ASCT. PS, performance status.
CLINICAL CASE (induction strategies)
This patient had a normal echo and pulmonary function test, achieved a very good partial response with 3 cycles of induction with VRD, and had no high-risk cytogenetics. Her ECOG was 1 and KPS 80% at her pretransplant appointment. Although her renal function improved, she still scored as intermediate-fit according to the R-MCI based on a glomerular filtration rate less than 60, but HCT-CI was low risk based on the higher threshold for renal function (score 1). She had a supportive caregiver in good health who had a solid understanding of the risks and benefits. She was offered upfront ASCT given the low potential risks of toxicity and potential benefits as highlighted above. Nonetheless, after a long discussion about the relative pros and cons, on the basis of personal preference, she opted for a non-ASCT strategy.
For fit older patients who decline upfront ASCT, there are a number of options. The excellent outcomes achieved with the upfront use of DRd in this patient population rival those achieved by induction, transplant, and maintenance. The latest update of the MAIA study reported an estimated 48-month PFS of 60%.22 Other alternatives could include D-VMP, VRD, or CyborD. The most common induction strategy for those aged less than 65 to 74 years, in the United States, is VRD frontline therapy and was subsequently recommended for this patient.25
CLINICAL CASE (relapsed MM)
The patient tolerated VRD induction therapy and was in a very good partial response and continued on lenalidomide maintenance. She returned to volunteering and remained independent. After 3 years on maintenance therapy, at age 73 years, the patient developed acommunity-acquired pneumonia and was hospitalized for 1 week. She then reported progressing back pain and was identified to have a new vertebral compression deformity. Serologic testing revealed a rise in her κ light chains and immunoglobulin G κ immunoglobulin protein.
Relapsed MM
When MM relapses, this is a challenging time for any patient and is further confounded if the patient's health status is frail, clinical health is tenuous due to illness, or there is impairment in end- organ function. Importantly, frailty is a dynamic state, and a reassessment can further define next treatment decisions. Most studies suggest that health-related quality of life tracks with disease control; therefore, addressing next steps for relapsed disease would also improve health-related quality of life. For relapsed MM, most phase 3 randomized controlled trials have compared the effectiveness and safety of 3-drug regimens to 2-drug regimens, generally depicting PFS benefits of 3-drug combinations over 2-drug combinations. For fit patients, a 3-drug regimen has become the standard of care for relapsed disease. For patients who are frail according to standardized criteria (eg, IMWG), data are quite limited on tolerance of relapsed therapies. Age-stratified subgroup analysis is not an indicator of health status and should not be used as a surrogate of tolerance. In this patient scenario, a thoughtful exploration of options to personalize therapy is indicated.
Treatment options for relapsed disease can be tailored based on disease biology, tempo of disease relapse, tolerance of prior therapies, health status, and shared decision making.42 Drug classes include immunomodulatory drugs (lenalidomide, pomalidomide), proteasome inhibitors (bortezomib, carfilizomib, ixazomib), monoclonal antibodies (daratumumab, isatuximab, elotuzumab), B cell maturation antigen targets (balantamab-mafodotin), novel therapy (histone deacetylase inhibitors, selective inhibitors of nuclear export), or alkylator therapy (cyclophosphamide, melphalan, melphalan flufenamide [peptide conjugate]) with steroids (dexamethasone, prednisone) in various combinations (Table 2). Class switching from first-line treatment to second-line therapy is recommended using a different mechanism of action not previously used. Among frail patients, if 2-drug therapy was offered at induction, such as lenalidomide and dexamethasone or bortezomib and dexamethasone, if frailty remains or intolerance develops, the patients could readily switch to the alternate 2-drug class. Consistently across studies, dexamethasone is dose attenuated by advancing age and is recommended for better tolerance. Steroids are a backbone of myeloma therapy but are associated with many multiorgan side effects that can affect quality of life and adherence, particularly for older adults.44 Steroid dose attenuations are well characterized in MM, and low-dose dexamethasone is recommended due to inferior survival with higher-dose dexamethasone.45 More recent literature examined lower dosages of lenalidomide with early discontinuation of dexamethasone that was proven safe and resulted in similar outcomes to patients on continuous lenalidomide-dexamethasone.46 Unlike many frontline studies with steroid attenuations, limited prospective data are available for dose attenuations of standard relapsed therapies for frail patients.
Relapsed multiple myeloma: early and late relapse studies
| VPd (OPTIMISMM) . | Anti-CD38 . | Anti-SLAMF7 . | Sel/Dex (STORM) . | Melflufen/Dex (HORIZON OP-106) . | Belantamab-mafadotin (DREAMM-2) . | Idecabtagene vicleucel . |
|---|---|---|---|---|---|---|
| KRd (ASPIRE) | DVd (CASTOR) | ERd (ELOQUENT-2) | Sel/Pom/Dex (STOMP arm1) | Melflufen/Bort/Dex (ANCHOR OP-104) | Belamaf/Pom/Dex (ALGONQUIN) | Ciltacabtagene autoleucel |
| IRd (TOURMALINE-MM1) | DRd (POLLUX) | EPd (ELOQUENT-3) | Sel/Carf/Dex (STOMP arm6) | Melflufen/Dara/Dex (ANCHOR OP-104) | Belamaf/Bort/Dex (DREAMM-6armB) | Orvacabtagene autoleucel |
| SVd (BOSTON) | DKd (CANDOR) | Sel/Dara/Dex (STOMP arm5) | P-BCMA-101 | |||
| DPd (APOLLO) | ||||||
| Isa-Pd (ICARIA) | ||||||
| Isa-Kd (IKEMA) |
| VPd (OPTIMISMM) . | Anti-CD38 . | Anti-SLAMF7 . | Sel/Dex (STORM) . | Melflufen/Dex (HORIZON OP-106) . | Belantamab-mafadotin (DREAMM-2) . | Idecabtagene vicleucel . |
|---|---|---|---|---|---|---|
| KRd (ASPIRE) | DVd (CASTOR) | ERd (ELOQUENT-2) | Sel/Pom/Dex (STOMP arm1) | Melflufen/Bort/Dex (ANCHOR OP-104) | Belamaf/Pom/Dex (ALGONQUIN) | Ciltacabtagene autoleucel |
| IRd (TOURMALINE-MM1) | DRd (POLLUX) | EPd (ELOQUENT-3) | Sel/Carf/Dex (STOMP arm6) | Melflufen/Dara/Dex (ANCHOR OP-104) | Belamaf/Bort/Dex (DREAMM-6armB) | Orvacabtagene autoleucel |
| SVd (BOSTON) | DKd (CANDOR) | Sel/Dara/Dex (STOMP arm5) | P-BCMA-101 | |||
| DPd (APOLLO) | ||||||
| Isa-Pd (ICARIA) | ||||||
| Isa-Kd (IKEMA) |
Table modified from Tables 2 to 5 in Nathwani et al.43
Carf, carfilzomib; Dara, daratumumab; Dex, dexamethasone; DKd, daratumumab, carfilzomib, dexamethasone; DPd, daratumumab, pomalidomide, dexamethasone; DVd, daratumumab, bortezomib, dexamethasone; EPd, elotuzumab, pomalidomide, dexamethasone; ERd, elotuzumab, lenalidomide, dexamethasone; IRd, ixazomib, lenalidomide, dexamethasone; Isa, isatuximab; Pom, pomalidomide; Sel, selinexor; SVd, selinexor, bortezomib, dexamethasone; VPd, bortezomib, pomalidomide, dexamethasone.
Most patients benefit from a monoclonal antibody, in combination, at first relapse. Daratumaumb, an anti-CD38 monoclonal antibody, can be offered in combination with a PI or IMiD at first relapse or as monotherapy among disease that is double- refractory or following third line of treatment. Triple-drug therapy for frail patients at relapse may require dose modifications. As 1 example, in the ICARIA study,47 evaluating isatuximab, pomalidomide, and dexamethasone vs pomalidomide and dexamethasone, 43% of patients required dose reductions of pomalidomide and 33% of patients required dose reductions in dexamethasone with triple therapy. However, patients randomized to the isatuximab, pomalidomide, and dexamethasone arm had a substantially longer duration of therapy compared with pomalidomide and dexamethasone (41 vs 24 weeks). In general, no age- or frailty-based dose attenuations are recommended for monoclonal antibody therapy.48 In terms of underlying health status, particularly relevant are peripheral neuropathy, cardiovascular health, thrombosis, recurrent infections, bone marrow reserve, and pulmonary health. Cardiovascular events are of significant interest in aging adults, particularly as this applies to carfilizomib-based strategies. In a post hoc analysis,49 evaluating 3 carfilizomib-based trials (ASPIRE,50 ENDEAVOR,51 ARROW52 ), 25% to 35% of patients across studies were categorized as frail using IMWG criteria. OS was reduced, and treatment-emergent adverse events were more common among frail patients (Table 3), including increased cardiac failure events (Table 4).49 In a prospective study evaluating 95 patients receiving a PI (bortezomib or carfilizomib) in relapsed disease, more than half of patients experienced grade 3 cardiac events, and most events occurred within the first 3 months of therapy.54 Moreover, patients who experienced a cardiovascular event had inferior PFS and worse OS.54 A global assessment of health and reassessment of frailty can further define next treatment options.
Carfilzomib-based studies: comparison of PFS and OS by age and fitness49
| . | ASPIRE50 . | ENDEAVOR51 . | ARROW52 . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFS (median, months) | <70 | KRd | 28.6 | Fit | KRd | 31.4 | <65 | Kd | NE* | Fit | Kd | NE* | <65 | 1 × Kd | 12.2 | Fit | 1 × Kd | 15.7 |
| Rd | 17.6 | Rd | 18.9 | Vd | 9.5 | Vd | 12.1 | 2 × Kd | 5.6 | 2 × Kd | 5.7 | |||||||
| ≥70 | KRd | 23.8 | Frail | KRd | 24.1 | 65-74 | Kd | 15.6 | Frail | Kd | 18.7 | 65-74 | 1 × Kd | 9.2 | Frail | 1 × Kd | 10.3 | |
| Rd | 15.9 | Vd | 9.5 | Vd | 6.6 | 2 × Kd | 8.4 | 2 × Kd | 6.6 | |||||||||
| Rd | 16.0 | ≥75 | Kd | 18.7 | ≥75 | 1 × Kd | 12.2 | |||||||||||
| Vd | 8.9 | 2 × Kd | 9.5 | |||||||||||||||
| OS (median, months) | <70 | Not given | Fit | KRd | 55.6 | <65 | Not given | Fit | Kd | NE* | <65 | Not given | Fit | Not given | ||||
| Rd | 43.3 | Vd | 42.2 | |||||||||||||||
| ≥70 | Frail | KRd | 36.4 | 65-74 | Frail | Kd | 33.6 | 65-74 | Frail | |||||||||
| Rd | 26.2 | ≥75 | Vd | 21.8 | ≥75 | |||||||||||||
| . | ASPIRE50 . | ENDEAVOR51 . | ARROW52 . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFS (median, months) | <70 | KRd | 28.6 | Fit | KRd | 31.4 | <65 | Kd | NE* | Fit | Kd | NE* | <65 | 1 × Kd | 12.2 | Fit | 1 × Kd | 15.7 |
| Rd | 17.6 | Rd | 18.9 | Vd | 9.5 | Vd | 12.1 | 2 × Kd | 5.6 | 2 × Kd | 5.7 | |||||||
| ≥70 | KRd | 23.8 | Frail | KRd | 24.1 | 65-74 | Kd | 15.6 | Frail | Kd | 18.7 | 65-74 | 1 × Kd | 9.2 | Frail | 1 × Kd | 10.3 | |
| Rd | 15.9 | Vd | 9.5 | Vd | 6.6 | 2 × Kd | 8.4 | 2 × Kd | 6.6 | |||||||||
| Rd | 16.0 | ≥75 | Kd | 18.7 | ≥75 | 1 × Kd | 12.2 | |||||||||||
| Vd | 8.9 | 2 × Kd | 9.5 | |||||||||||||||
| OS (median, months) | <70 | Not given | Fit | KRd | 55.6 | <65 | Not given | Fit | Kd | NE* | <65 | Not given | Fit | Not given | ||||
| Rd | 43.3 | Vd | 42.2 | |||||||||||||||
| ≥70 | Frail | KRd | 36.4 | 65-74 | Frail | Kd | 33.6 | 65-74 | Frail | |||||||||
| Rd | 26.2 | ≥75 | Vd | 21.8 | ≥75 | |||||||||||||
not estimable.
Rd, lenalidomide and dexamethasone; Vd, bortezomib and dexamethasone.
Carfilzomib-based studies: comparison of grade >3 treatment emergent adverse event (TEAE) and cardiac events by age and fitness49
| . | ASPIRE50 . | ENDEAVOR51 . | ARROW52 . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade ≥3 TEAE, n (%) | <70 | KRd | 236 (81.7) | Fit | KRd | 102 (89) | <65 | Kd | 152 (68.2) | Fit | Kd | 91 (83) | <65 | 1 × Kd | 61 (59.2) | Fit | 1 × Kd | 33 (55) |
| Rd | 215 (77.6) | Rd | 96 (84) | Vd | 131 (63) | Vd | 76 (64) | 2 × Kd | 58 (56.3) | 2 × Kd | 41 (62) | |||||||
| ≥70 | KRd | 92 (89.3) | Frail | KRd | 86 (93) | 65-74 | Kd | 124 (76.1) | Frail | Kd | 142 (85) | 65-74 | 1 × Kd | 62 (68.9) | Frail | 1 × Kd | 64 (81) | |
| Rd | 99 (88.4) | Rd | 94 (94) | Vd | 128 (69.9) | Vd | 125 (79) | 2 × Kd | 64 (63.4) | 2 × Kd | 42 (70) | |||||||
| ≥75 | Kd | 63 (81.8) | ≥75 | 1 × Kd | 38 (84.4) | |||||||||||||
| Vd | 46 (70.8) | 2 × Kd | 23 (74.2) | |||||||||||||||
| Grade ≥3 cardiac failure, n (%) | <70 | KRd | 6 (2.1) | Fit | KRd | 5 (4) | <65 | Kd | 6 (2.7) | Fit | Kd | 4 (4) | <65 | 1 × Kd | 1 (1) | Fit | 1 × Kd | 1 (2) |
| Rd | 5 (1.8) | Rd | 2 (2) | Vd | 3 (1.4) | Vd | 2 (2) | 2 × Kd | 6 (5.8) | 2 × Kd | 1 (2) | |||||||
| ≥70 | KRd | 9 (8.7) | Frail | KRd | 9 (10) | 65-74 | Kd | 8 (4.9) | Frail | Kd | 15 (9) | 65-74 | 1 × Kd | 5 (5.6) | Frail | 1 × Kd | 3 (4) | |
| Rd | 2 (1.8) | Rd | 1 (1) | Vd | 3 (1.6) | Vd | 7 (4) | 2 × Kd | 2 (2) | 2 × Kd | 5 (8) | |||||||
| ≥75 | Kd | 8 (10.4) | ≥75 | 1 × Kd | 1 (2.2) | |||||||||||||
| Vd | 2 (3.1) | 2 × Kd | 2 (6.5) | |||||||||||||||
| Grade ≥3 ischemic heart disease, n (%) | <70 | KRd | 8 (2.8) | Fit | KRd | 4 (3) | <65 | Not given | Fit | Kd | 2 (2) | <65 | 1 × Kd | 1 (1) | Fit | 1 × Kd | 1 (1) | |
| Rd | 7 (2.5) | Rd | 3 (3) | Vd | 2 (<1) | 2 × Kd | 0 | 2 × Kd | 0 | |||||||||
| ≥70 | KRd | 5 (4.9) | Frail | KRd | 7 (8) | 65-74 | Frail | Kd | 8 (5) | 65-74 | 1 × Kd | 0 | Frail | 1 × Kd | 0 | |||
| Rd | 1 (0.9) | Rd | 1 (1) | Vd | 6 (4) | 2 × Kd | 1 (1) | 2 × Kd | 1 (2) | |||||||||
| ≥75 | ≥75 | 1 × Kd | 1 (2.2) | |||||||||||||||
| 2 × Kd | 1 (3.2) | |||||||||||||||||
| . | ASPIRE50 . | ENDEAVOR51 . | ARROW52 . | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade ≥3 TEAE, n (%) | <70 | KRd | 236 (81.7) | Fit | KRd | 102 (89) | <65 | Kd | 152 (68.2) | Fit | Kd | 91 (83) | <65 | 1 × Kd | 61 (59.2) | Fit | 1 × Kd | 33 (55) |
| Rd | 215 (77.6) | Rd | 96 (84) | Vd | 131 (63) | Vd | 76 (64) | 2 × Kd | 58 (56.3) | 2 × Kd | 41 (62) | |||||||
| ≥70 | KRd | 92 (89.3) | Frail | KRd | 86 (93) | 65-74 | Kd | 124 (76.1) | Frail | Kd | 142 (85) | 65-74 | 1 × Kd | 62 (68.9) | Frail | 1 × Kd | 64 (81) | |
| Rd | 99 (88.4) | Rd | 94 (94) | Vd | 128 (69.9) | Vd | 125 (79) | 2 × Kd | 64 (63.4) | 2 × Kd | 42 (70) | |||||||
| ≥75 | Kd | 63 (81.8) | ≥75 | 1 × Kd | 38 (84.4) | |||||||||||||
| Vd | 46 (70.8) | 2 × Kd | 23 (74.2) | |||||||||||||||
| Grade ≥3 cardiac failure, n (%) | <70 | KRd | 6 (2.1) | Fit | KRd | 5 (4) | <65 | Kd | 6 (2.7) | Fit | Kd | 4 (4) | <65 | 1 × Kd | 1 (1) | Fit | 1 × Kd | 1 (2) |
| Rd | 5 (1.8) | Rd | 2 (2) | Vd | 3 (1.4) | Vd | 2 (2) | 2 × Kd | 6 (5.8) | 2 × Kd | 1 (2) | |||||||
| ≥70 | KRd | 9 (8.7) | Frail | KRd | 9 (10) | 65-74 | Kd | 8 (4.9) | Frail | Kd | 15 (9) | 65-74 | 1 × Kd | 5 (5.6) | Frail | 1 × Kd | 3 (4) | |
| Rd | 2 (1.8) | Rd | 1 (1) | Vd | 3 (1.6) | Vd | 7 (4) | 2 × Kd | 2 (2) | 2 × Kd | 5 (8) | |||||||
| ≥75 | Kd | 8 (10.4) | ≥75 | 1 × Kd | 1 (2.2) | |||||||||||||
| Vd | 2 (3.1) | 2 × Kd | 2 (6.5) | |||||||||||||||
| Grade ≥3 ischemic heart disease, n (%) | <70 | KRd | 8 (2.8) | Fit | KRd | 4 (3) | <65 | Not given | Fit | Kd | 2 (2) | <65 | 1 × Kd | 1 (1) | Fit | 1 × Kd | 1 (1) | |
| Rd | 7 (2.5) | Rd | 3 (3) | Vd | 2 (<1) | 2 × Kd | 0 | 2 × Kd | 0 | |||||||||
| ≥70 | KRd | 5 (4.9) | Frail | KRd | 7 (8) | 65-74 | Frail | Kd | 8 (5) | 65-74 | 1 × Kd | 0 | Frail | 1 × Kd | 0 | |||
| Rd | 1 (0.9) | Rd | 1 (1) | Vd | 6 (4) | 2 × Kd | 1 (1) | 2 × Kd | 1 (2) | |||||||||
| ≥75 | ≥75 | 1 × Kd | 1 (2.2) | |||||||||||||||
| 2 × Kd | 1 (3.2) | |||||||||||||||||
CLINICAL CASE (conclusion)
The patient recovered from her hospital admission. Recalculation of the IMWG score remained intermediate-fit. The patient was started on a daratumumab, bortezomib, and dose-reduced dexamethasone for relapsed MM. Special attention to neuro- pathy was tracked closely. The patient benefited from physical therapy to assist with recovery from back pain and tolerated therapy without difficulty.
The multiplying therapies of myeloma have resulted in challenging clinical decisions. Moreover, the future of MM treatment decisions will soon include not only transplant decision making but also cellular therapy candidacy. How to best prescribe these therapies for aging adults has yet to be determined. To reconcile differences in health with aging, particularly for complex MM decisions, a comprehensive evaluation of health is required. The MM community has embraced several frailty scoring systems, each with limitations, yet importantly, these important first steps of embedding assessments in clinical trials are a start. In summary, myeloma treatments can be modified or informed by frailty scoring systems, and these tools demonstrate how geriatric principles can be incorporated into risk stratification to improve outcomes.
Conflict-of-interest disclosure
Shakira J. Grant: no conflicts to disclose.
Ciara L. Freeman received research funding from Lundbeck.
Ashley E. Rosko: no conflicts to disclose.
Off-label drug use
Shakira J. Grant: nothing to disclose.
Ciara L. Freeman: nothing to disclose.
Ashley E. Rosko: nothing to disclose.
References
Author notes
Joint first authorship.