Learning Objectives
Understand the current strategies of VTE treatment and prevention of recurrences in patients with MPN
Identify the pros and cons of traditional VTE treatment with VKAs vs newer approaches with DOACs in light of the available evidence
CLINICAL CASE
A 55-year-old man with a history of JAK2-mutated essential thrombocythemia (ET) presented to the emergency room for the sudden onset of severe pain and swelling in his right leg during the night. He denied any recent trauma, surgery, or infection. He was in good general condition except for a modest shortness of breath. An electrocardiogram showed a sinus tachycardia; the oxygen saturation was 95% in room air. At physical examination, a palpable cord at the right thigh was appreciable, associated with marked edema and erythema of the calf. A compression ultrasonography revealed an absence of color flow compatible with complete deep vein thrombosis of the femoral and popliteal veins of the right leg. A chest computed tomography scan also showed bilateral segmental pulmonary embolism. His past medical history was otherwise mute except for being a heterozygous carrier of the factor V Leiden variant and having hemorrhoids in the past. He was on low-dose aspirin as his sole medication. What is the best treatment of venous thromboembolism in this ET patient?
Introduction
Classical BCR/ABL-negative myeloproliferative neoplasms (MPN) include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). Patients with MPN are at high risk of thrombotic manifestations, which considerably affect morbidity and mortality, especially in younger patients.1,2 Up to 30% of patients present with a thrombotic event before or at MPN diagnosis.3 Although arterial thrombotic events (ATEs) are twice as common as venous events, MPN patients have a 4-fold increased risk of ATEs and a 10-fold increased risk of venous thromboembolism (VTE) shortly after diagnosis compared to the general population, and the thrombotic rate remains significantly elevated throughout the follow-up.2
MPN-related VTE manifests most often as deep vein thrombosis (DVT) of the legs and/or pulmonary embolism (PE; accounting for 40%-90% of all cases in different reports), although thromboses at unusual sites, ie, involving the splanchnic and cerebral districts, are remarkably frequent in these patients.4
In PV and ET, treatment is aimed at preventing thrombotic complications, and risk-stratification for treatment decisions is based on thrombotic risk factors, including an age over 60, a history of thrombosis, and, only in ET, the presence of the JAK2V617F mutation.5,6 In this regard, the substantial involvement of JAK2V617F and clonal hematopoiesis in thrombosis development in MPN has emerged.7
Current strategies of thromboprophylaxis include the use of low-dose aspirin (75-100 mg) once daily in both high- and low-risk PV patients (aged <60 years and no thrombosis history) and in low-/intermediate-risk ET patients (JAK2 mutated OR aged >60 years and no thrombosis history),5 based on 2 randomized controlled trials in PV and on 1 retrospective analysis in ET, respectively.8-10 Low-dose aspirin showed a considerable but non-statistically significant beneficial effect on mortality from thrombotic events and did not prevent major cardiovascular and venous thrombotic events, taken individually.8,11 Thromboprophylaxis also includes phlebotomy in all PV patients and myelosuppression with cytoreductive therapy in high-risk PV and ET patients.5,12
Treatment of acute VTE and secondary prevention of VTE recurrence
Despite prophylaxis, the reported incidence of VTE is 0.6% to 1.0% in patient-years across the different MPN subtypes and is considerably higher than the annual incidence of 0.1% to 0.2% observed in the general population.4 Detected risk factors for a first VTE episode in MPN patients include an age >60, a previous history of VTE, a history of major bleeding, leukocytosis, inherited thrombophilia (in younger patients), and JAK2V617F (in ET and PMF).4
In light of a lack of prospective studies addressing the efficacy and safety of the newer direct oral anticoagulants (DOACs) in MPN patients, based on expert opinion the initial treatment for acute VTE in MPN patients should start with low-molecular-weight heparin (LMWH) or fondaparinux followed by vitamin K antagonists (VKAs), targeting an international normalized ratio (INR) of 2.5 for at least 3 to 6 months.4,13
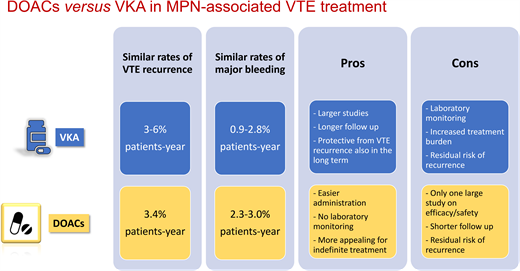
The efficacy and safety of VKAs in the MPN setting has been evaluated in 4 retrospective studies, including a very recent one (Table 1).14-17 The annual incidence rate of VTE recurrence ranged between 3% and 6% in patient-years.14-17 VKA treatment was associated with a significant reduction in VTE recurrences in all 4 studies and ATEs in 1 study.16
Principal studies including MPN patients on VKA treatment for usual site VTE
| Reference . | Study population . | N included in the study . | N on VKAs . | Overall thrombosis recurrence (A/V) . | VTE recurrence . | Major bleeding . | Median follow-up (years) . |
|---|---|---|---|---|---|---|---|
| De Stefano et al14 | PV/ET with at least 1 episode of thrombosis (ATE and VTE) | 494 | 90 | 33.6% (7.6% pt-y)* | 13.1% (3% pt-y)* | 5.4% (0.9% pt-y)* 7.7% (0.9% pt-y)† (2.8 pt-y)‡ | 5.3 |
| Hernandez-Boluda et al16 | PV/ET receiving VKA for a first VTE or ATE episode | 150 | 150 | 28% (6.0% pt-y)† | 24% (2.7% ON vs 9.0% OFF, p)† | 11.3% (overall 1.7% pt-y, 1.8% ON vs 1.5% OFF)† | 7.7 |
| De Stefano et al15 | PV/ET/PMF on systemic anticoagulation for a first VTE episode | 206 | 155 | 21.8% (6.5% pt-y)* 12.2% (4.7% pt-y)† | 17.4% (5.2% pt-y)* 9.6% (4.2% pt-y ON vs 9.6% pt-y OFF)† | 6.4% (2.4% ON vs 0.7 OFF)† | 3 |
| Wille et al17 | PV/ET/PMF with a first VTE episode | 78 | 40 | — | 20.5% (6.0% pt-y)* | 26.9% | 2 |
| Reference . | Study population . | N included in the study . | N on VKAs . | Overall thrombosis recurrence (A/V) . | VTE recurrence . | Major bleeding . | Median follow-up (years) . |
|---|---|---|---|---|---|---|---|
| De Stefano et al14 | PV/ET with at least 1 episode of thrombosis (ATE and VTE) | 494 | 90 | 33.6% (7.6% pt-y)* | 13.1% (3% pt-y)* | 5.4% (0.9% pt-y)* 7.7% (0.9% pt-y)† (2.8 pt-y)‡ | 5.3 |
| Hernandez-Boluda et al16 | PV/ET receiving VKA for a first VTE or ATE episode | 150 | 150 | 28% (6.0% pt-y)† | 24% (2.7% ON vs 9.0% OFF, p)† | 11.3% (overall 1.7% pt-y, 1.8% ON vs 1.5% OFF)† | 7.7 |
| De Stefano et al15 | PV/ET/PMF on systemic anticoagulation for a first VTE episode | 206 | 155 | 21.8% (6.5% pt-y)* 12.2% (4.7% pt-y)† | 17.4% (5.2% pt-y)* 9.6% (4.2% pt-y ON vs 9.6% pt-y OFF)† | 6.4% (2.4% ON vs 0.7 OFF)† | 3 |
| Wille et al17 | PV/ET/PMF with a first VTE episode | 78 | 40 | — | 20.5% (6.0% pt-y)* | 26.9% | 2 |
Entire cohort.
Patients on VKA only.
Patients on VKA and aspirin.
A/V, arterial/venous; N, number of patients; ON, on VKA; OFF, off VKA; Pt-y, patient-years.
With regard to the overall duration of anticoagulation in these patients, while there is consensus on continuing lifelong treatment in patients with splanchnic vein thrombosis,18 the optimal treatment duration for VTE at the usual sites is uncertain. Two studies comparing VKA indefinite treatment vs discontinuation after 6 months showed a significantly greater incidence of recurrence in the group that discontinued VKAs (2.7%-4.2% in patient-years vs 9%-9.6%).15,16 Indeed, VKA suspension resulted in a 2- to 3-fold increased risk of recurrence.15,16 In addition, MPN patients showed a higher rate of 5-year recurrence after anticoagulant withdrawal compared to non-MPN patients (42% vs 29%, respectively).15 However, it is important to consider that the cumulative incidence of VTE recurrence in MPN patients receiving adequate VKA treatment still remains greater than that of the general population (7.8% vs 1.8%-3.5% at 1 year, respectively).15
In the studies shown in Table 1, the bleeding incidence during VKA treatment ranged between 0.9% and 2.8% patient-years and significantly increased only when administered in combination with aspirin (compared to patients off VKAs). Nevertheless, bleeding complications with VKAs look higher in MPN compared to non-MPN patients (up to 2.8% vs 1.2%-2.2% in patient-years, respectively),4 with disease-related factors contributing to the overall higher hemorrhagic risk in MPN patients compared to the general population.3 This is a very relevant aspect when choosing the type and duration of anticoagulant therapy. Improving the efficacy and safety of anticoagulation in MPN patients with VTE still represents an open issue.
In the last years, more therapeutic options for VTE have become available with the advent of the DOACs, including the factor IIa inhibitor dabigatran and the factor Xa (FXa) inhibitors rivaroxaban, apixaban, edoxaban, and others. In the non-MPN setting, DOACs have become the first treatment choice for DVT and PE.19 Moreover, FXa inhibitors have been tested specifically in the cancer population by means of RCTs, showing a good efficacy and safety profile compared to the standard therapy with LMWH.20-22 Thus, expert guidelines have recently included these drugs in the recommended treatment options for cancer-associated VTE.23,24 No prospective controlled studies on the use of DOACs have been conducted with MPN patients so far. In any case, some observational retrospective studies have been published in recent years evaluating the efficacy and safety of DOACs in MPN (Table 2).
Studies including at least 20 MPN patients on DOAC treatment for usual site VTE
| Reference . | Study population . | N on DOAC . | N on rivar . | N on apix . | N on edox . | N on dabig . | Overall thrombotic recurrence . | VTE recurrence . | Major bleeding . | Median follow-up (years) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Ianotto et al25 | PV/ET receiving DOAC for AF or VTE | 25* | 16 | 9 | — | — | 4% (1 stroke) | 0 | 12% | 2.1 |
| Curto-Garcia et al26 | PV/ET/PMF/MDS-MPN receiving DOAC for VTE | 32 | 17 | 14 | 1 | 0 | 3% (1 mesenteric ischemia) | 0 | 0% | 2.1 |
| Serrao et al27 | PV/ET/PMF receiving DOAC for AF or VTE | 71† | 26 | 21 | 14 | 10 | 0% | — | 0% | 1 |
| Barbui et al28 | PV/ET/PMF receiving DOAC for AF or VTE | 442‡ | 187 | 157 | 48 | 50 | 4.9% (2.1% pt-y) (AF) 9.2% (4.5% pt-y) (VTE) | 1.5% (0.6% pt-y) (AF) 7.1% (3.4% pt-y) (VTE) | 6.9% (3.0 pt-y) (AF) 5.0% (2.3% pt-y) (VTE) | 1.7 |
| Reference . | Study population . | N on DOAC . | N on rivar . | N on apix . | N on edox . | N on dabig . | Overall thrombotic recurrence . | VTE recurrence . | Major bleeding . | Median follow-up (years) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Ianotto et al25 | PV/ET receiving DOAC for AF or VTE | 25* | 16 | 9 | — | — | 4% (1 stroke) | 0 | 12% | 2.1 |
| Curto-Garcia et al26 | PV/ET/PMF/MDS-MPN receiving DOAC for VTE | 32 | 17 | 14 | 1 | 0 | 3% (1 mesenteric ischemia) | 0 | 0% | 2.1 |
| Serrao et al27 | PV/ET/PMF receiving DOAC for AF or VTE | 71† | 26 | 21 | 14 | 10 | 0% | — | 0% | 1 |
| Barbui et al28 | PV/ET/PMF receiving DOAC for AF or VTE | 442‡ | 187 | 157 | 48 | 50 | 4.9% (2.1% pt-y) (AF) 9.2% (4.5% pt-y) (VTE) | 1.5% (0.6% pt-y) (AF) 7.1% (3.4% pt-y) (VTE) | 6.9% (3.0 pt-y) (AF) 5.0% (2.3% pt-y) (VTE) | 1.7 |
13 patients receiving DOAC for AF, 4 for AF and stroke, and 8 for VTE.
35 patients receiving DOAC for AF; 36 for VTE.
203 patients receiving DOAC for AF; 239 for VTE.
Apix, apixaban; dabig, dabigatran; edox, edoxaban; rivar, rivaroxaban.
Three small studies described an overall thrombotic recurrence of 0% to 4% involving only arterial districts.25-27 Major bleeding was reported in 0% to 12% of patients, with 3 cases of clinically relevant nonmajor bleeding in association with aspirin.26 A recent large retrospective study including 442 patients treated with DOACs for atrial fibrillation (AF) or for VTE provided more extensive information on the rates of thrombohemorrhagic complications in this setting.28 Specifically, the incidence of a first VTE event in patients receiving a DOAC for AF was 0.6% in patient-years, while the incidence of recurrent VTE in patients receiving a DOAC for a prior VTE was 3.4% in patient-years, which was no different from the recurrence incidence observed in the VKA studies.14-17 Moreover, annual rates of major bleeding ranged from 2.3% to 3% in patient-years,28 also similar to VKAs. Therefore, on the basis of limited available evidence, DOACs and VKAs seem to have a comparable risk/benefit profile in the treatment and secondary prevention of VTE in MPN patients. Finally, 2 recent small studies tried to retrospectively compare the outcomes of MPN patients treated with VKAs or DOACs29,30 (Table 3). In the study by Huenerbein et al, despite a higher relapse rate seen in the VKA group compared to the DOAC group, thrombosis-free survival was no different between the 2 groups.29 In the study by Fedorov et al, the rates of thrombosis were also comparable between the 2 anticoagulant regimens. In both studies the rate of major bleeding was similar for the 2 anticoagulants.29,30 Interestingly, a significantly higher VTE recurrence rate was observed after the discontinuation of either drug.30
Retrospective studies comparing thrombosis recurrence and major bleeding in MPN patients receiving VKA or DOAC
| Reference . | Study population . | N on VKA . | N on DOAC . | Overall thrombotic recurrence . | VTE recurrence . | Major bleeding . | Median follow-up (years) . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| VKA . | DOAC . | VKA . | DOAC . | VKA . | DOAC . | |||||
| Huenerbein et al29 | PV/ET/PMF/ MPN-U on systemic anticoagulation for VTE or ATE | 45 | 26 | 48.8% | 15.3% | 24.4% | 11.5% | 8.88% | 7.6% | 3.2 |
| Fedorov et al30 | PV/ET/PMF/ MPN-U on systemic anticoagulation for VTE or ATE | 31 | 22 | 19.4% | 22.7% | — | — | 6.4% | 4.5% | 1.2 |
| Reference . | Study population . | N on VKA . | N on DOAC . | Overall thrombotic recurrence . | VTE recurrence . | Major bleeding . | Median follow-up (years) . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| VKA . | DOAC . | VKA . | DOAC . | VKA . | DOAC . | |||||
| Huenerbein et al29 | PV/ET/PMF/ MPN-U on systemic anticoagulation for VTE or ATE | 45 | 26 | 48.8% | 15.3% | 24.4% | 11.5% | 8.88% | 7.6% | 3.2 |
| Fedorov et al30 | PV/ET/PMF/ MPN-U on systemic anticoagulation for VTE or ATE | 31 | 22 | 19.4% | 22.7% | — | — | 6.4% | 4.5% | 1.2 |
MPN-U, myeloproliferative neoplasm-unclassifiable: PV, plycythemia vera.
In this setting it is important to recall that cytoreduction is recommended in PV/ET who have a history of thrombosis or are experiencing a first VTE episode in the follow-up.5 However, recent studies show that despite the demonstrated efficacy of hydroxyurea (HU) at cytoreduction to prevent primary and recurrent arterial events,14,31 its action in the prevention of first or recurrent venous thrombosis is more limited.32,33 Other cytoreductive or disease-modifying agents (ie, ruxolitinib, anagrelide, interferon alpha, and ropeginterferon) have proved valuable alternatives to HU for disease control, although for most of these drugs there is no controlled evidence showing their superiority over HU at preventing VTE. Since the incidence of ATEs and VTE remains high in MPN patients despite cytoreduction, further therapeutic proposals are needed, possibly addressing additional mechanisms besides myelosuppression and targeting other thrombogenic pathways.7
When it comes to choosing an anticoagulant agent, the pros and cons of treatment with VKAs or DOACs for MPN-associated VTE can be summarized as follows:
VKA pros:
Larger studies evaluating efficacy and safety
Longer follow-up
Reduced VTE recurrence
Reduced ATE recurrence
Long-term protection from VTE recurrence
VKA cons:
Laboratory monitoring of INR
Increased burden in case of lifelong treatment
Higher bleeding risk in MPN than in non-MPN patients
Residual risk of recurrence even with an INR in the therapeutic range
DOACs pros:
Easier to administer
Routine laboratory monitoring is unnecessary
Good efficacy and safety profile in studies with cancer patients
More appealing than VKAs for indefinite treatment
DOACs cons:
Limited studies evaluating efficacy and safety
Shorter follow-up
Higher bleeding risk in MPN than in non-MPN patients
Residual risk of recurrence even during treatment
In conclusion, the current evidence, although limited, shows similar patterns in terms of the efficacy and safety of VKAs and DOACs for the treatment and prevention of recurrent VTE in MPN. While we wait for a randomized comparison between the 2 regimens, treatment decisions should be guided according to individual factors (ie, renal function, bleeding risk profile, concomitant medications) as well as patient preferences. The optimal duration of anticoagulation is uncertain, but substantial evidence indicates that recurrence risk is particularly high in these patients even years after the index event. To help decide on the duration, it is wise to perform a careful assessment of the patient's risk factors for recurrence (such as unprovoked VTE, proximal DVT, pulmonary embolization, thrombophilia, etc) and to plan a periodical reassessment of risk factors for thrombosis and bleeding during the follow-up.
CLINICAL CASE (continued)
We discussed pros and cons with our patient, who was relatively young and in good form. We agreed to start HU and an FXa inhibitor for VTE treatment. This decision was based on the patient's preference for a less onerous regimen and on our experience with DOACs in cancer patients. We aimed to pursue a long-term treatment, considering his unprovoked proximal DVT and PE and the presence of additional risk factors such as JAK2 mutation and inherited thrombophilia. We suspended aspirin to reduce his bleeding risk. Nevertheless, we periodically assess his thrombosis and bleeding risk, willing to adjust our treatment strategies in case of changes in the patient's disease pattern or the availability of new evidence on anticoagulation modalities in MPN.
Conflict-of-interest disclosure
Francesca Schieppati: no competing financial interests to declare.
Anna Falanga: no competing financial interests to declare.
Off-label drug use
Francesca Schieppati: nothing to disclose.
Anna Falanga: nothing to disclose.