Abstract
Allogeneic hematopoietic cell transplantation (HCT) is particularly susceptible to racial, socioeconomic, and geographic disparities in access and outcomes given its specialized nature and its availability in select centers in the United States. Nearly all patients who need HCT have a potential donor in the current era, but racial minority populations are less likely to have an optimal donor and often rely on alternative donor sources. Furthermore, prevalent health care disparity factors are further accentuated and can be barriers to access and referral to a transplant center. Research has primarily focused on defining and quantifying a variety of social determinants of health and their association with access to allogeneic HCT, with a focus on race/ethnicity and socioeconomic status. However, research on interventions is lacking and is an urgent unmet need. We discuss the role of racial, socioeconomic, and geographic disparities in access to allogeneic HCT, along with policy changes to address and mitigate them and opportunities for future research.
Learning Objectives
Understand the association of race, geography, and socioeconomic status with access to allogeneic transplantation in the United States
Highlight opportunities to evaluate, mitigate, and address social access-related barriers to allogeneic transplantation
CLINICAL CASE
A 40-year-old African American woman from rural Ohio has relapsed acute myeloid leukemia (AML). She is hospitalized at a regional hospital close to home to receive salvage chemotherapy. She is a single mother with a 10-year-old son and lives in an area that has one of the highest rates of poverty in Ohio. She does not have a car and uses public transportation. She used to earn an hourly wage as a waitress but has been unemployed with no health care benefits for the past year since being laid off at the onset of the COVID epidemic. She had been feeling very fatigued and had noticed spontaneous bruising for 4 weeks before the diagnosis of relapse; she was concerned about leukemia recurrence but did not want to see her oncologist given the lack of health insurance and concern about paying medical bills. She does not have any immediate family in the vicinity. Her oncologist has discussed an allogeneic hematopoietic cell transplantation (HCT) for her AML and the fact that she will need to be referred to a transplant center in Cleveland, which is 90 miles away from where she lives.
Introduction
Although allogeneic HCT is potentially curative for many patients with high-risk hematologic malignancies and other diseases, it is a highly specialized and complex procedure that requires comprehensive clinical infrastructure to facilitate referral, donor search, transplant hospitalization and supportive care, and posttransplant follow-up. The number of patients receiving allogeneic HCT continues to increase in the US every year with improvements in technology and supportive care, use of less intense conditioning regimens that allow transplantation in older and frail patients, and greater availability of suitable donors.1 However, it is also recognized that many patients who might otherwise benefit do not receive allogeneic HCT.2-7 Several patient-specific barriers to accessing HCT have been identified. Historically, a lack of suitably HLA-matched donors used to be a barrier to HCT, but alternative donors (eg, haploidentical, mismatched unrelated, and umbilical cord blood) are now used routinely, and nearly all patients have a suitable donor for transplantation. However, age-related, racial, economic, and other social disparities continue to limit access to allogeneic HCT in the US, and many patients who would otherwise benefit are not referred for and do not receive transplantation.
As highlighted by the case above, access to HCT is moderated by a complex interplay of several sociocultural, economic, disease, treating provider, hospital-related, and health-system-related factors. At a patient level, disparities in access can more factors that often tend to be closely related (eg, race/ethnicity, insurance status, education level, poverty, employment status; Table 1). Studies have established associations between age, sex, race/ethnicity, insurance coverage, and socioeconomic status (SES) and the utilization of HCT, and less evidence is available for other factors such as marital status, language barriers, distance from transplant center, and caregiver availability.7 Although the focus of this review is barriers to access, the same disparities also influence short-term and long-term outcomes following HCT, and the contemporary literature in this area is summarized in Table 2.
Sociodemographic factors associated with access to allogeneic HCT
| Referencea . | Population . | Access variable(s) . | Key findings . |
|---|---|---|---|
| Jabo et al3 | Age ≥15 years; patients with ALL/AML in California Cancer Registry; 2003-2012 | Age, race/ethnicity, geography, SES | Higher rate of HCT in patients aged ≤40 and in married patients; women more likely to receive HCT for ALL; lower rates of HCT in Hispanic and non-Hispanic Black patients; no association between distance and HCT utilization; low neighborhood quintile SES index associated with less utilization of HCT |
| Dehn et al10 | All ages; donor searches through Be the Match registry; 2016 | Race/ethnicity | White patients more likely to receive HCT compared to Black patients |
| Barker et al12 | Age ≤70 years; single-center study of patients undergoing unrelated donor search; 2005-2017 | Race/ethnicity | Patients of European ancestry more likely to receive 8/8 HLA-MUD HCT transplant than non-European ancestry and less likely to have no MUD or cord-blood grafts |
| Bhatt et al2 | Age 61-75 years; National Cancer Database, patients with AML; 2003-2012 | Age, race/ethnicity, geography, SES, insurance coverage | Lower likelihood of receiving HCT in patients who were older, non-White, of lower educational status, uninsured, on Medicaid/Medicare, or received care at nonacademic facility; no difference in HCT rates among urban vs rural facility; higher likelihood of receiving HCT in patients who lived ≥37 miles from facility; no association with median household income |
| Paulson et al5 | Age <66 years; patients with AML/ALL/MDS reported to CIBMTR and SEER; 2000-2010 | SES, geography | Higher county levels of poverty associated with lower transplant rates; rural vs urban status was not associated with HCT utilization |
| Delamater and Uberti18 | All ages; several public databases | Geography | Overall, 66% of US population lives within 60 minutes' travel time and 94% within 3 hours' travel time of HCT facility; geographic access to HCT facility varies by state |
| Getta et al27 | Age ≤70 years; single-center study of MDS patients; 2008-2015 | Age | Patients ≥65 years were less likely to be referred for HCT evaluation; marital status and insurance type were not associated with transplant referral |
| Referencea . | Population . | Access variable(s) . | Key findings . |
|---|---|---|---|
| Jabo et al3 | Age ≥15 years; patients with ALL/AML in California Cancer Registry; 2003-2012 | Age, race/ethnicity, geography, SES | Higher rate of HCT in patients aged ≤40 and in married patients; women more likely to receive HCT for ALL; lower rates of HCT in Hispanic and non-Hispanic Black patients; no association between distance and HCT utilization; low neighborhood quintile SES index associated with less utilization of HCT |
| Dehn et al10 | All ages; donor searches through Be the Match registry; 2016 | Race/ethnicity | White patients more likely to receive HCT compared to Black patients |
| Barker et al12 | Age ≤70 years; single-center study of patients undergoing unrelated donor search; 2005-2017 | Race/ethnicity | Patients of European ancestry more likely to receive 8/8 HLA-MUD HCT transplant than non-European ancestry and less likely to have no MUD or cord-blood grafts |
| Bhatt et al2 | Age 61-75 years; National Cancer Database, patients with AML; 2003-2012 | Age, race/ethnicity, geography, SES, insurance coverage | Lower likelihood of receiving HCT in patients who were older, non-White, of lower educational status, uninsured, on Medicaid/Medicare, or received care at nonacademic facility; no difference in HCT rates among urban vs rural facility; higher likelihood of receiving HCT in patients who lived ≥37 miles from facility; no association with median household income |
| Paulson et al5 | Age <66 years; patients with AML/ALL/MDS reported to CIBMTR and SEER; 2000-2010 | SES, geography | Higher county levels of poverty associated with lower transplant rates; rural vs urban status was not associated with HCT utilization |
| Delamater and Uberti18 | All ages; several public databases | Geography | Overall, 66% of US population lives within 60 minutes' travel time and 94% within 3 hours' travel time of HCT facility; geographic access to HCT facility varies by state |
| Getta et al27 | Age ≤70 years; single-center study of MDS patients; 2008-2015 | Age | Patients ≥65 years were less likely to be referred for HCT evaluation; marital status and insurance type were not associated with transplant referral |
Table shows representative studies in US populations published since 2015.
MDS, myelodysplastic syndrome.
Sociodemographic and center factors associated with outcomes of allogeneic HCT
| Referencea . | Population . | Variable(s) . | Key findings . |
|---|---|---|---|
| Bona et al28 | Age ≤18 years; all diagnoses; first allogeneic HCT recipients reported to CIBMTR; 2006-2015 | SES | In children with malignant disease, high neighborhood poverty level associated with higher NRM and Medicaid insurance status associated with higher NRM and inferior OS (vs private insurance); no association between neighborhood poverty and HCT outcomes for nonmalignant disease |
| Hong et al8 | Age ≥18 years; all diagnoses; first allogeneic HCT recipients reported to CIBMTR; 2014-2016 | Several (county-level indicators of community health) | Patients residing in counties with worse community health status had inferior OS; among patients with hematologic malignancy, worse community health status was associated with inferior OS and higher risks of NRM |
| Madbouly et al29 | All ages; all diagnoses; allogeneic HCT using 10/10 allele matched MUD reported to CIBMTR; 1995– 2001 | Race/ethnicity (ancestry) | Higher recipient-donor African genetic admixture associated with lower OS and DFS and higher NRM |
| Majhail et al16 | Adult HCT centers; all diagnoses; allogeneic HCT reported to CIBMTR; 2008-2010 and 2012– 2014 | Center volume | Higher 100-day and 1-year OS in high-volume (>40 allogeneic HCT/year) vs low-volume centers; presence of survivorship program associated with higher 1-year OS |
| Khera et al30 | Adult; all diagnoses; first allogeneic HCT recipients at single center; 2000-2010 | Geography (distance from HCT center) | No association of distance and OS, NRM, or relapse; trend toward higher NRM with increased distance in nonmyeloablative HCT recipients |
| Bhatt et al31 | All ages; hematologic malignancy; single-center study of first auto and allogeneic HCT recipients; 2007-2011 | Time to insurance approval | Time to insurance approval for HCT varied between private and public payers but was not associated with OS |
| Referencea . | Population . | Variable(s) . | Key findings . |
|---|---|---|---|
| Bona et al28 | Age ≤18 years; all diagnoses; first allogeneic HCT recipients reported to CIBMTR; 2006-2015 | SES | In children with malignant disease, high neighborhood poverty level associated with higher NRM and Medicaid insurance status associated with higher NRM and inferior OS (vs private insurance); no association between neighborhood poverty and HCT outcomes for nonmalignant disease |
| Hong et al8 | Age ≥18 years; all diagnoses; first allogeneic HCT recipients reported to CIBMTR; 2014-2016 | Several (county-level indicators of community health) | Patients residing in counties with worse community health status had inferior OS; among patients with hematologic malignancy, worse community health status was associated with inferior OS and higher risks of NRM |
| Madbouly et al29 | All ages; all diagnoses; allogeneic HCT using 10/10 allele matched MUD reported to CIBMTR; 1995– 2001 | Race/ethnicity (ancestry) | Higher recipient-donor African genetic admixture associated with lower OS and DFS and higher NRM |
| Majhail et al16 | Adult HCT centers; all diagnoses; allogeneic HCT reported to CIBMTR; 2008-2010 and 2012– 2014 | Center volume | Higher 100-day and 1-year OS in high-volume (>40 allogeneic HCT/year) vs low-volume centers; presence of survivorship program associated with higher 1-year OS |
| Khera et al30 | Adult; all diagnoses; first allogeneic HCT recipients at single center; 2000-2010 | Geography (distance from HCT center) | No association of distance and OS, NRM, or relapse; trend toward higher NRM with increased distance in nonmyeloablative HCT recipients |
| Bhatt et al31 | All ages; hematologic malignancy; single-center study of first auto and allogeneic HCT recipients; 2007-2011 | Time to insurance approval | Time to insurance approval for HCT varied between private and public payers but was not associated with OS |
Table shows representative studies in US populations published since 2015.
DFS, disease-free survival; NRM, nonrelapse mortality; OS, overall survival.
In a discussion of disparities in access to transplantation, it is important to acknowledge the limitations of the existing literature. Data on patients who receive HCT are robust and captured well by institutional and national registries (eg, the Center for International Blood and Marrow Transplant Research [CIBMTR]). However, data on patients who are candidates for and may potentially benefit from HCT are not readily available. National registries and secondary databases (eg, the Surveillance, Epidemiology, and End Results Program [SEER] and single- or multipayer databases) often do not include the details required to determine whether HCT was indicated for a given patient (eg, disease risk, remission status, donor availability). Furthermore, sociodemographic barriers are a complex construct, are challenging to define, and are not captured reliably at an individual level; hence, most studies focus on population-level indicators to define disparities (eg, median household income based on zip code of residence). Studies in HCT recipients have evaluated composite measures that combine several health disparity factors, but these instruments need further validation.8,9 Qualitative studies are also needed to contextualize the quantitative literature, to deepen our understanding of access barriers, and to identify impactful and timely interventions to address them.
Racial barriers to HCT
Race and ethnicity are particularly relevant when considering access to allogeneic HCT. First, racial disparities that are routinely prevalent in health care apply to HCT and in fact may be accentuated given the complexity and expense of the procedure along with its restricted availability in select centers in the US. Second, there is an element of donor availability associated with race and very specific to HCT. Unrelated donor registries are overrepresented by donors of European ancestry, and White patients have a higher chance of finding an HLA-matched unrelated donor (MUD).10-12 Using data from the National Marrow Donor Program registry, Gragert et al showed that the likelihood of finding a high-resolution HLA 8/8 allele MUD was 75% for White people of European descent and only 16% for Black people of South or Central American ancestry.11 Given that the majority of patients do not have an HLA-identical sibling donor, this disparity in MUD availability by race/ethnicity has significant implications on transplant utilization and, ultimately, survival and other outcomes after HCT. There is a possibility that this disparity may worsen in the future. In another analysis that modeled the likelihood of finding HLA-identical siblings and unrelated donors, Besse et al showed that the average number of siblings and sibling match probability vary by patient age and race, and young minority patients are at greatest risk for not finding an HLA-matched donor.13
In addition to race-related donor issues, minority populations often have social and economic barriers to referral and donor search. In general, Hispanic and Black populations in the US have lower median household income compared to Whites.14 This disparity often translates to exposure to adverse social determinants of health in the former, including a greater likelihood of residing in areas with high poverty levels, inadequate health care coverage, and lower levels of health literacy. Racial disparity in access to HCT has been well documented, including a recent study that showed adult Hispanic and Black patients with AML and acute lymphoblastic leukemia having a lower probability of proceeding with HCT.3 The mechanism by which these social determinants of health have an impact on the ultimate receipt of allogeneic HCT is complex, as demonstrated in a study by Clay et al.15 They showed that the reasons for not receiving a transplant differed by race. Patient decision/treatment reluctance and stable disease status not severe enough to warrant transplant were the most important reasons for not proceeding in European American patients, whereas comorbidities and physician decision were the main reasons for not proceeding in African American patients. Psychosocial or compliance concerns were identified more often in African American patients as a reason for not proceeding with HCT.
Socioeconomic barriers to HCT
In addition to race/ethnicity, the association of SES with access to HCT has been well documented, with most studies using US Census tract data to define SES. This is a limitation of the existing literature since SES is most accurate when it is patient self-reported. Regardless, using zip codes to estimate median household income is a well-validated method for defining SES in health care research. In the contemporary literature, Jabo et al have reported an inverse association of neighborhood SES with HCT utilization; compared to the highest-quintile SES, patients residing in the lowest quintile had a lower likelihood of undergoing HCT (adjusted relative risk, 0.63; 95% CI, 0.47-0.84 for acute lymphoblastic leukemia and 0.52; 0.43-0.64, for AML).3 Similarly, Paulson et al, using data from the CIBMTR, showed that residence in counties with high levels of poverty was associated with a lower probability of receiving HCT (in multivariable analysis, estimated rate ratio was 0.86 per 10% increase in county population below the poverty line; P < .01).5
Studies that have been able to investigate the role of social and economic determinants in greater detail suggest some mechanisms by which SES influences access to HCT. In a study using the National Cancer Database, Bhatt et al found that the primary payer for HCT coverage was significantly associated with HCT utilization.2 Compared to private insurance, patients were less likely to receive HCT if they had Medicaid (odds ratio [OR], 0.3; 95% CI, 0.3-0.5; P < .0001), Medicare (OR, 0.7; 0.6-0.8; P < .0001), uninsured (OR, 0.2; 0.1-0.5; P = .0003), and unknown insurance status (OR, 0.1; 0.1-0.3; P < .0001).
Geographic barriers to HCT
Given its specialized and highly regulated nature, need for experienced personnel, and infrastructural requirements, HCT is available through approximately 200 transplant programs in the US. There is a rationale for restricting HCT to select centers since a volume-outcome relationship has been demonstrated for this procedure.16,17 However, this does cause a barrier to some patients who need to travel long distances to access a transplant center. Overall, 48% and 79% of the US adult population and 43% and 72% of the pediatric population have access to an HCT facility within 30 and 90 minutes' travel time from their homes, respectively.18 There is significant variation by state; eg, >70% of adult residents in Arizona, Maryland, New Jersey, New York, Rhode Island, and Washington, DC, live within 30 minutes of an HCT, whereas 6% of the US population must travel >3 hours to access a transplant facility.18
Since the majority of the US population live in reasonably close proximity to an HCT center, studies have shown no definitive association between distance from the transplant program or rural/urban residence status and receipt of allogeneic HCT.2,3 Interestingly, a recent study has shown that patients able to travel longer distances (≥37 miles) for care were more likely to receive transplant, possibly indicating better patient status or better receipt of care in tertiary referral hospitals.2 As illustrated in the case at the beginning of this article, geographic disparities are likely accentuated in patients who are socioeconomically underserved to begin with.
Although not specifically a focus of this review, other social determinants of health can be related to racial, socioeconomic, and geographic disparities and ultimately affect access to allogeneic HCT. Some examples of such barriers include age, sex, patient preference, educational status, health literacy level, psychiatric disability, substance abuse, marital status, language barriers, and lack of compliance with medical care.7
Opportunities to address disparities in access to HCT
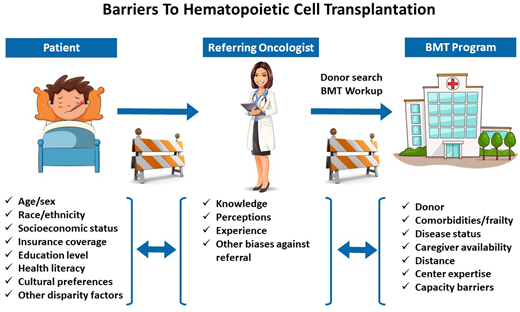
Research to date in the field of HCT has largely focused on understanding and defining health care disparities in access to and outcomes of allogeneic HCT, and acknowledging and quantifying them is an important first step. Less work has been done around investigating interventions to resolve or mitigate these disparities. Some reasons for this are related to the long-standing systemic inequities in health care that need to be addressed at the societal level, the lack of validated health care interventions to address these disparities, the difficulty in generalizing studies given that social determinants may vary at the local and individual levels, and the fact that such disparities are often outside the control of what a transplant center can realistically influence. Furthermore, the additional resources and effort required to bring in patients from disadvantaged populations for HCT may not be prioritized at the referring provider and transplant center level. Nevertheless, opportunities exist for interventions to ensure that patients with racial, socioeconomic, and geographic challenges receive appropriate transplant-related care (Figure 1).
Framework for investigating interventions to address racial, socioeconomic, and geographic disparities in access to allogeneic HCT.
Framework for investigating interventions to address racial, socioeconomic, and geographic disparities in access to allogeneic HCT.
A major time point for intervention to improve access is referral from a patient's oncologist to the transplant center so that indication and candidacy for transplantation can be determined, and a donor search can be initiated in a timely manner.19 Early referral in the disease course also allows for the identification of psychosocial and SES factors that may later hinder proceeding with transplantation so that they can be addressed early. Social workers and care coordinators are an integral part of the transplant team and play an important role in this early evaluation and in gathering appropriate resources for patients. Some examples of such interventions include referrals for grants to offset out-of-pocket costs, assessing caregiver support, and helping with local housing for patients who must temporarily relocate to be close to the transplant center.20,21 Particularly, programs often require a dedicated caregiver for HCT recipients, and interventions to identify and support caregivers are needed.21 Additionally, the development and implementation of tools to assess social challenges can facilitate individualized interventions for patients. Some instruments that have been evaluated in HCT recipients include the Psychosocial Assessment of Candidates for Transplantation scale, Transplant Evaluation Rating Scale, and Stanford Integrated Psychosocial Assessment for Transplant.22-25 Data are needed for special populations (eg, lesbian, gay, bisexual, transgender, queer patients; health-illiterate individuals; or nonnative English speakers) so that appropriate interventions can be designed to help them access HCT.
In this same context, optimizing health care delivery for allogeneic HCT recipients by improving care coordination among primary care physicians, referring hematologists/oncologists, and transplant centers can help mitigate the social barriers and challenges that patients and families face. Khera et al have described a patient-centered care framework to coordinate allogeneic HCT delivery.26 They lay out various phases in the HCT continuum and the roles of various health care providers. Their framework can be expanded to address social disparity-related factors that play a role throughout the transplant journey. It can also inform research on interventions since all stakeholders, including health care providers from outside the transplant center network, need to be engaged to address these disparities. A common thread for patients as they move through different phases and sites of care is their payer, and we need to explore opportunities for provider-payer collaborations to address barriers to transplant.
There is an urgent need for more research and funding to support the investigation of innovative interventions to address socioeconomic and geographic disparities in access to allogeneic HCT. There is also a societal responsibility to address health care system factors that operate at a systemic level and ultimately have an impact on access to HCT. Although cellular therapy was not the focus of this review, similar health care disparity factors also apply and may in fact be worse given the costs of newer chimeric antigen receptor T-cell therapies. Ultimately, the benefit of innovations in HCT and cellular therapy can be fully realized when all patients who may benefit actually receive these procedures.
CLINICAL CASE (continued)
The treating hematologist-oncologist contacted the transplant center early in the patient's treatment course. Local and transplant center social workers were able to enroll the patient in a state Medicaid program and refer her for grants and other services (eg, transportation assistance). An initial HCT consult was conducted through telemedicine, and a donor search was initiated. A close friend stepped in to serve as a dedicated caregiver during transplant. A MUD was identified, and the patient was able to successfully proceed with an allogeneic transplant.
Conflict-of-interest disclosure
Sanghee Hong: no competing financial interests to declare.
Navneet S. Majhail: no competing financial interests to declare.
Off-label drug use
Sanghee Hong: none discussed.
Navneet S. Majhail: none discussed.