Abstract
The approval of brentuximab vedotin (BV) and checkpoint inhibitors (CPI) has revolutionized the management of relapsed/refractory classical Hodgkin lymphoma (cHL) patients. In recent years these agents have rapidly moved to earlier lines of therapy, including post-autologous hematopoietic cell transplant (auto-HCT) consolidation, pre-HCT salvage, and the frontline treatment setting. This shift in practice means that double-refractory (refractory to both BV and CPI) cHL is becoming an increasingly common clinical problem. In patients who are not eligible for clinical trials, conventional cytotoxic and targeted therapies (off label) may be a potential option. In patients who are transplant eligible, early referral to allogeneic HCT should be considered given the significant improvement in transplant outcomes in the contemporary era. Cellular therapy options including CD30.chimeric antigen receptor T cells, Epstein-Barr virus-directed cytotoxic T cells, and CD16A/30 bispecific natural killer cell engagers appear promising and are currently in clinical trials.
Learning Objectives
Identify promising therapeutic agents for cHL refractory to both BV and checkpoint inhibitors
Highlight contemporary outcomes of allogeneic transplantation in patients with heavily pretreated cHL
Recognize emerging cellular therapies and summarize the results of clinical trials evaluating these therapies
CLINICAL CASE
A 32-year-old Caucasian man was diagnosed with stage IV-B classical Hodgkin lymphoma (cHL). He achieved a complete metabolic remission following six cycles of first-line treatment with adriamycin, vinblastine, bleomycin, and dacarbazine. The patient experienced a biopsy-proven relapse 9 months after finishing this chemotherapy. At the time of relapse, the patient had extranodal involvement in the axial skeleton and liver. His disease remained refractory to second-line treatment with ifosfamide, carboplatin, and etoposide. The patient achieved a second complete metabolic remission with a brentuximab vedotin (BV) and nivolumab combination and subsequently went on to receive autologous hematopoietic cell transplantation (auto-HCT) followedby BV consolidation. While on consolidation treatment, the patient relapsed again and was initiated on pembrolizumab, which resulted in a partial remission lasting approximately 18 months. At the current progression, the patient is fit, with excellent organ function, and his donor search has identified a haploidentical sibling.
Introduction
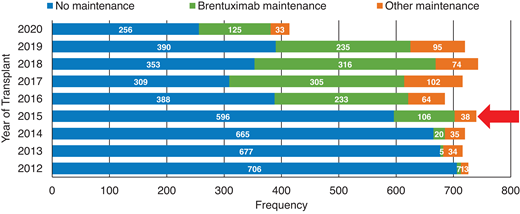
Although most patients with cHL are cured with initial chemotherapy, up to 20% to 25% of patients will relapse or have primary refractory (R/R) disease.1 While high-dose therapy and an auto-HCT consolidation can cure cHL patients responding to subsequent treatments, only about half of these patients will attain durable disease control.2,3 The advent of novel agents, especially BV and checkpoint inhibitors (CPI), in the past decade has not only revolutionized the management of R/R cHL patients; in recent years these agents have rapidly moved to earlier lines of therapy, including post-auto-HCT consolidation (Figure 1 depicts the increased utilization of BV consolidation post-auto-HCT in the United States based on reporting to the Center for International Blood and Marrow Transplant Research [CIBMTR] registry),3 pre-HCT salvage,4 and front-line treatment setting.5 This shift in practice means that the scenario described in the clinical case (above) of a cHL patient with double-refractory (refractory to both BV and CPI) disease is becoming a common clinical problem. Unlike patients with relapsed non-Hodgkin lymphoma (NHL; median age in the late 60s), the median age of relapsed cHL patients is typically in the 30s.6,7 The young age of these R/R patients means that in addition to selecting the next salvage option (typically expected to provide short-term disease control), the treating team must also consider therapeutic modalities that can provide durable disease control and hopefully cure. The latter may not be feasible for the subset of double-refractory patients with advanced age, compromised organ function, and/or poor performance status.
Increased use of BV as post-autologous transplant consolidation therapy in the US.Red arrow: year of AETHERA trial publication; 2020 data incomplete. Based on CIBMTR registry data.
Increased use of BV as post-autologous transplant consolidation therapy in the US.Red arrow: year of AETHERA trial publication; 2020 data incomplete. Based on CIBMTR registry data.
In this article we discuss the treatment options for double-refractory cHL, beginning with potential salvage options and followed by optimal consolidation approaches. At the outset we acknowledge that there are no universally accepted definitions of BV or CPI refractory disease. In this review, double-refractory disease is loosely defined as cHL that does not achieve at least a partial remission or progresses while on BV and CPI (-based treatments). It is important to remember that cHL patients previously achieving remission with either BV or CPI (or regimens containing these drugs) and subsequently relapsing after a treatment-free interval may be candidates for rechallenge with these agents.
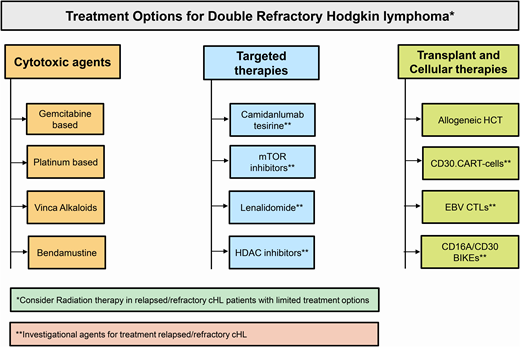
Salvage options in double-refractory cHL
Conventional cytotoxic therapy options
Clinical trial enrollment, if available, should be a priority in double- refractory cHL. Off trial, in a fit patient conventional multiagent chemotherapy as a bridge to allogeneic (allo) HCT is a reasonable option. In patients who are not candidates for allo-HCT, single-agent cytotoxic therapy with a palliative intent is reasonable. The choice of chemotherapy (regimen) is often empiric and depends on the patient's prior treatments, marrow reserve, and organ function. For instance, while both platinum- or gemcitabine-based therapies are active, platinum-containing regimens may be less desirable in patients with chronic kidney disease, and a gemcitabine-based option could be challenging in patients with a poor marrow reserve. A detailed discussion of salvage regimens is beyond the scope of this review, but Table 1 summarizes the commonly considered salvage options in double-refractory cHL patients.8-17
Conventional cytotoxic therapy options in R/R cHL
| Agent . | Study design . | Median age (range), years . | Total, N . | Prior AHCT (n) . | ORR% (CR%) . | Median PFS/EFS (mo) . | Reference . |
|---|---|---|---|---|---|---|---|
| Gemcitabine based | |||||||
| Gemcitabine | II | 35 (19-58) | 23 | 0 | 39 (9) | 6.7 | Santoro et al8 |
| GDP | II | 36 (19–57) | 23 | 0 | 69 (17) | NR | Baetz et al9 |
| GVD | I/II | 33 (19–83) | 91 | 40 | 70 (19) | 8.5* | Bartlett et al10 |
| IGEV | II | 30 (17-59) | 91 | NA | 81 (54) | 3-y FFP 53% | Santoro et al11 |
| Platinum based | |||||||
| ICE | II | 27 (12-59) | 65 | NA | 88 (26) | 58%† | Moskowitz et al12 |
| DHAP | II | 34 (21-64) | 102 | NA | 89 (21) | NR | Josting et al13 |
| ESHAP | II | 34 (18-66) | 22 | 2 | 73 (40) | 3-y DFS 27% | Aparicio et al14 |
| Vinca alkaloids | |||||||
| Vinblastine | II | 31 (23-48) | 17 | 17 | 59 (12) | 8.3 | Little et al15 |
| Vinorelbine | II | NR | 24 | 4 | 50 (14) | 6 | Devizzi et al16 |
| Alkylators | |||||||
| Bendamustine | II | 34 (21–75) | 35 | 27 | 53 (33) | 5.2 | Moskowitz et al17 |
| Agent . | Study design . | Median age (range), years . | Total, N . | Prior AHCT (n) . | ORR% (CR%) . | Median PFS/EFS (mo) . | Reference . |
|---|---|---|---|---|---|---|---|
| Gemcitabine based | |||||||
| Gemcitabine | II | 35 (19-58) | 23 | 0 | 39 (9) | 6.7 | Santoro et al8 |
| GDP | II | 36 (19–57) | 23 | 0 | 69 (17) | NR | Baetz et al9 |
| GVD | I/II | 33 (19–83) | 91 | 40 | 70 (19) | 8.5* | Bartlett et al10 |
| IGEV | II | 30 (17-59) | 91 | NA | 81 (54) | 3-y FFP 53% | Santoro et al11 |
| Platinum based | |||||||
| ICE | II | 27 (12-59) | 65 | NA | 88 (26) | 58%† | Moskowitz et al12 |
| DHAP | II | 34 (21-64) | 102 | NA | 89 (21) | NR | Josting et al13 |
| ESHAP | II | 34 (18-66) | 22 | 2 | 73 (40) | 3-y DFS 27% | Aparicio et al14 |
| Vinca alkaloids | |||||||
| Vinblastine | II | 31 (23-48) | 17 | 17 | 59 (12) | 8.3 | Little et al15 |
| Vinorelbine | II | NR | 24 | 4 | 50 (14) | 6 | Devizzi et al16 |
| Alkylators | |||||||
| Bendamustine | II | 34 (21–75) | 35 | 27 | 53 (33) | 5.2 | Moskowitz et al17 |
Median EFS was not reached in the transplant-naive group, while it was 8.5 months in patients with prior transplant.
At a median follow-up of 43 months, as analyzed by intent to treat, the EFS was 58%.
ASCT, autologous hematopoietic cell transplantation; DFS, disease-free survival; DHAP, dexamethasone, high-dose cytarabine, cisplatin; EFS, event-free survival; ESHAP, etoposide, methylprednisolone, high-dose cytarabine, cisplatin; FFP, freedom from progression; GDP, gemcitabine, dexamethasone, cisplatin; GVD, gemcitabine, vinorelbine, liposomal doxorubicin; ICE, ifosfamide, carboplatin, etoposide; IGEV, ifosfamide, gemcitabine, etoposide, vinorelbine; NA, not applicable; NR, not reported; PFS, progression-free survival.
Radiation therapy
In relapsed patients with limited treatment options, salvage radiation should be considered. Limited retrospective data show effective short-term disease control with radiation therapy, particularly in patients with localized nodal disease.18 Although preclinical models and case reports demonstrate synergism between radiation and CPIs,19-21 it is unclear if this is a feasible approach in double-refractory cHL patients.
Investigational agents for double-refractory cHL
Camidanlumab tesirine
Camidanlumab tesirine (cami) is an antibody-drug conjugate (pyrrolobenzodiazepine) directed against CD25. After impressive antitumor activity was noted in a phase 1, dose-escalation, and dose-expansion study of cami in R/R cHL patients (Table 2),22 a phase 2 study was conducted using 45 µg/kg q3 week dosing for two cycles followed by 30 µg/kg Q3 weeks (in cHL with prior BV and CPI treatments). Cami was active in R/R cHL patients based on the preliminary results (n = 51), with an overall response rate (ORR) of 83% and a complete response (CR) rate of 38%.23 Grade 3 or higher adverse events were reported in 63% (n = 32) of cHL patients.23 Of note, there were 3 (6.4%) patients with Guillain-Barré syndrome (GBS)/polyradiculopathy (n = 1 with grade 4 GBS, n = 1 with grade 2 GBS, and n = 1 with grade 2 radiculopathy) that led to an enrollment pause. Following a review of safety and efficacy data (by independent review), the enrollment pause was lifted. The study recently completed accrual, and final results are not yet available, but the preliminary results with cami are encouraging and may provide an additional therapeutic option for patients with R/R cHL, particularly those with dual-refractory disease. Strategies to mitigate the autoimmune neurological toxicity of this agent will enhance its risk/benefit profile.
Investigational therapies in R/R cHL
| Agent . | Study design . | Median age (range), years . | Total (N) . | Prior AHCT (n) . | ORR% (CR%) . | Median PFS/EFS (mo) . | Reference . |
|---|---|---|---|---|---|---|---|
| Cami | I* | 38 (31-53) | 57 | NR | 75 (44) | NR | Hamadani et al22 |
| Cami | II | 36 (20-74) | 51 | 31 | 83 (38) | NR | Herrera et al23 |
| Everolimus | II | 32 (19-77) | 57 | 38 | 46 (9) | 8 | Johnston et al25 |
| Lenalidomide | II | 37 (18–74) | 15 | 10 | 13 | NR | Kuruvilla et al28 |
| Lenalidomide | II | 38 (20–83) | 42 | 31 | 30 | 8.2 | Fehniger et al29 |
| Vorinostat | II | 42 (20-71) | 25 | 11 | 4 | 7.2 | Kirschbaum et al32 |
| Panobinostat | II | 32 (18-75) | 129 | 129 | 27 (4) | 6.9 | Younes et al33 |
| Idelalisib | II | 42 (21-80) | 25 | 18 | 20 (4) | 2.3 | Gopal et al51 |
| Ibrutinib† | Retrosp | 35 (26-72) | 7 | 5 | 57 (43) | NA | Badar et al52 |
| Agent . | Study design . | Median age (range), years . | Total (N) . | Prior AHCT (n) . | ORR% (CR%) . | Median PFS/EFS (mo) . | Reference . |
|---|---|---|---|---|---|---|---|
| Cami | I* | 38 (31-53) | 57 | NR | 75 (44) | NR | Hamadani et al22 |
| Cami | II | 36 (20-74) | 51 | 31 | 83 (38) | NR | Herrera et al23 |
| Everolimus | II | 32 (19-77) | 57 | 38 | 46 (9) | 8 | Johnston et al25 |
| Lenalidomide | II | 37 (18–74) | 15 | 10 | 13 | NR | Kuruvilla et al28 |
| Lenalidomide | II | 38 (20–83) | 42 | 31 | 30 | 8.2 | Fehniger et al29 |
| Vorinostat | II | 42 (20-71) | 25 | 11 | 4 | 7.2 | Kirschbaum et al32 |
| Panobinostat | II | 32 (18-75) | 129 | 129 | 27 (4) | 6.9 | Younes et al33 |
| Idelalisib | II | 42 (21-80) | 25 | 18 | 20 (4) | 2.3 | Gopal et al51 |
| Ibrutinib† | Retrosp | 35 (26-72) | 7 | 5 | 57 (43) | NA | Badar et al52 |
Data shown for 30 µg/kg and 45 µg/kg cohorts.
A clinical trial is ongoing investigating the role of ibrutinib in R/R cHL (NCT02824029).
NA, not applicable; NR, not reported; Retrosp, retrospective.
Mammalian target of rapamycin inhibitors
Preclinical studies have shown that the PI3K-Akt-mammalian target of rapamycin (mTOR) pathway plays an important role in the growth and survival of Reed-Sternberg cells.24 Everolimus is an oral inhibitor of mTOR that is active in R/R cHL (Table 2).25 Recently, combination therapies with everolimus have been explored in R/R cHL with encouraging preliminary activity. In a phase 1/2 study of heavily pretreated cHL patients (n = 15), the combination of everolimus and itacitinib (an oral Jak inhibitor) produced an ORR and CR rate of 79% and 14%, respectively. Of note, 94% (n = 14) were double refractory in the study. The notable grade 3 or higher toxicities included thrombocytopenia (43%), neutropenia (21%), infection (7%), and hypertension (7%).26
Immunomodulatory agents
Lenalidomide modulates the immune microenvironment in lymphoid malignancies by interacting with ubiquitin E3 ligase cereblon and degrading Ikaros transcription factors.27 Single-agent lenalidomide has produced an ORR ranging from 13% to 30% across studies in R/R cHL (Table 2).28,29 The major toxicity is hematological adverse events, which are slightly higher with continuous dosing.29 Combination approaches have also been studied, mainly with histone deacetylase (HDAC) inhibitors associated with significant toxicity without any improvement in response rates.30
HDAC inhibitors
HDAC inhibitors are epigenetic modulators that induce cell death in cHL cell lines by inhibiting STAT6-mediated Th-2 cytokine and TARC (thymus and activation-regulated chemokine) production.31 While single-agent HDAC inhibitors have limited activity in R/R cHL (Table 2),32,33 the combinatorial approach with mTOR inhibitors seems to be promising. In a study that combined vorinostat with mTOR inhibitors (everolimus and sirolimus) in heavily pretreated cHL (n = 40), the ORR and CR rates were 55% and 33%, respectively.34 While the study included patients with prior BV failure, none of the patients in the study received CPI.
Transplant and cellular therapy options
Allogeneic HCT
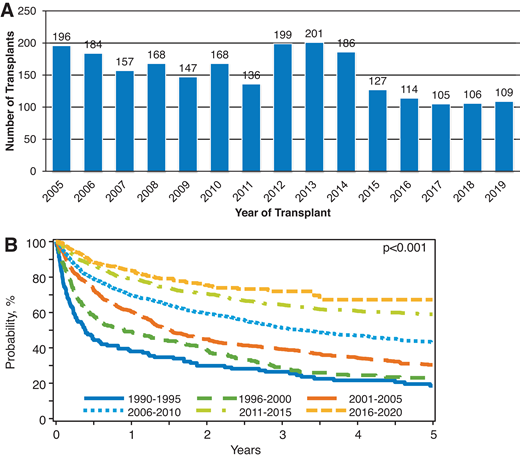
Adoptive immunotherapy in the form of an allo-HCT offers a potentially curative option for heavily pretreated R/R cHL patients, including those relapsing after a prior auto-HCT, BV, and CPI.6,35,36 Historically, allo-HCT in R/R cHL has been associated with high rates of nonrelapse mortality (NRM; 35%-45% at 1 year) and disease relapse (up to 50%).37 These rates (with an obvious negative impact on referral patterns) and the availability of novel agents in cHL have led to a decline in the use of allo-HCT in cHL in the US in the last decade (Figure 2A, CIBMTR registry data). However, in dual-refractory cHL, the role of allo-HCT warrants reappraisal, especially when focusing on modern outcome data from the CIBMTR showing remarkable improvement in survival outcomes (Figure 2B, CIBMTR registry data) for these cHL patients following allografting. Unlike the historical data in which NRM rates were almost 50% and 3-year overall survival (OS) 25% to 30%,37 in the contemporary series the NRM rates have declined to approximately 10%, and 3-year OS is approximately 60%.7 These improvements could be due to better conditioning approaches,7,38 advances in graft-versus-host disease (GVHD) prevention, and overall advances in supportive care and transplant practices. In addition, donor availability is no longer a barrier. Several studies looked at the outcomes of alternative donor allo-HCT for R/R cHL and reported excellent outcomes and low NRM (1 year, 10%-15%) among patients who received posttransplant cyclophosphamide (PTCy)-based haploidentical (haplo) allo-HCT.35,39
(A) Utilization of allo-HCT for cHL in the US. (B) Overall survival of patients undergoing allo-HCT in the US from 1990 to 2020. Based on CIBMTR registry data.
(A) Utilization of allo-HCT for cHL in the US. (B) Overall survival of patients undergoing allo-HCT in the US from 1990 to 2020. Based on CIBMTR registry data.
In current practice, most of the cHL patients undergoing allo-HCT have CPI exposure in the immediate pre-HCT period. Allo-HCT in this setting (post CPI) may be associated with an increased risk of early posttransplant complications, especially severe acute GVHD.40 It has been postulated that this phenomenon is likely due to the higher frequency of interferon-γ-producing effector T cells and a more pronounced T helper 1 differentiation in patients exposed to CPI before allo-HCT.41 PTCy has been shown to not only abrogate the CPI-induced immune activation but also promote the vigorous recovery of regulatory T cells, leading to immune tolerance and thereby suppressing GVHD to mild levels.41,42 In a recently published “real-world” analysis of cHL patients who underwent allo-HCT after CPI treatments, those who received haplo/PTCy had a lower cumulative incidence of relapse (2 year = 7%) and excellent OS (2 year = 85%). The time from CPI to allo-HCT was an important predictor of GVHD risk, wherein patients with a longer time from CPI exposure to allo-HCT (>80 days) had a decreased risk of severe acute GVHD.43 Given these improvements in recent years, early referral to transplant programs must be considered for fit cHL patients with dual-refractory disease that responds to available salvage treatments.
Cellular therapies
Given the remarkable activity of chimeric antigen receptor modified T (CAR-T) cell therapy in R/R B-cell NHL, this approach is under investigation in cHL. In a phase 1/2 study of heavily pretreated cHL (median prior lines = 7) including BV, CPI, auto-HCT, and/or allo-HCT, CD30.CAR-T cell therapy was safe and well tolerated. The ORR was 72% in those receiving fludarabine-based lymphodepletion (n = 31) with a CR rate of 59%,44 but 1-year progression-free survival was only 36%, underscoring the need to improve the platform. Currently, a larger phase 2 study is ongoing to evaluate the efficacy of CD30.CAR-T cells in R/R cHL (NCT04268706).
Epstein-Barr virus (EBV)-directed cytotoxic T cells (CTLs) directed against LMP 1 and LMP 2 have activity in EBV+ cHL. EBNA1, LMP 1, and LMP 2 are attractive targets and have been clinically tested.45,46 In patients with EBV-associated cHL and NHL, CTLs with enhanced activity against LMP 1/2 were tested with promising results, wherein a response to therapy was noted in 13 of 21 patients with active disease, including 11 patients achieving CR.46 Another small study used LMP-specific CTLs expressing dominant-negative transforming growth factor β receptor to counteract transforming growth factor β-associated immune suppression in the tumor microenvironment with good clinical results.47 Currently, a phase 1 study using EBV CTLs expressing CD30 chimeric receptor for CD30+ lymphomas is ongoing (NCT01192464).
AFM13 is a first-in-class innate cell engager that activates the immune system and targets CD30+ hematologic cancers. AFM13 induces the specific and selective killing of CD30+ cells by engaging CD16 on natural killer (NK) cells and CD30 on the surface of the cHL cells (bispecific NK cell engagers, or BIKEs). Given the minimal single-agent activity of AFM13 in R/R cHL (11.5%),48 it has been combined with CPI (AFM13+ pembrolizumab in CPI-naive R/R cHL) and with cytokine- activated cord blood-derived NK cells.49 The preliminary safety and efficacy data on first-in-human cord blood-derived NK BIKEs (CD16A/CD30) in heavily pretreated cHL (n = 4) are provocative. The ORR/CR rate was 100% and 50%, respectively, among the 4 patients treated with this approach, with no evidence of cytokine release syndrome, neurotoxicity, or GVHD noted.50 Currently, the study is enrolling patients (NCT04074746).
CLINICAL CASE (continued)
The patient in the clinical case achieved a CR with cami on a clinical trial and went on to receive a reduced-intensity conditioning haplo-HCT from his sibling donor. At the last follow-up, the patient was in remission with mild active chronic GVHD.
Conclusions
Although the advent of BV and CPI has changed the therapeutic landscape of cHL management, these drugs are not curative (in an R/R setting). As these agents are moved earlier into the treatment lines, many more patients will become double refractory. Conventional cytotoxic and targeted therapies may be considered in these patients, who are otherwise not eligible for clinical trials or allo-HCT. In patients who are transplant eligible, allo-HCT should be strongly considered given the significant improvement in outcomes with allo-HCT in the contemporary era. Preliminary results with the cellular therapy options that include CD30.CAR-Ts, EBV CTLs, and CD30 BIKEs appear promising and are currently in clinical trials. As we continue on our path toward a greater understanding of the biology of cHL, we will be able to identify additional therapeutic targets with the goal of providing durable responses while minimizing toxicity in R/R cHL patients.
Conflict-of-interest disclosure
Narendranath Epperla: reports Speaker’s Bureau: Verastem and Beigene; Advisory Board: Karyopharm; Honorarium: Genzyme.
Mehdi Hamadani: research support/funding: Takeda Pharmaceutical Company; Consultancy: Incyte Corporation; ADC Therapeutics; Pharmacyclics, Omeros, Kite. Speaker’s Bureau: Sanofi Genzyme, AstraZeneca, BeiGene.
Off-label drug use
Narendranath Epperla: camidanlumab tesirine, AFM13, lenalidomide, everolimus, vorinostat, idelalisib, ibrutinib, panabinostat.
Mehdi Hamadani: camidanlumab tesirine, AFM13, lenalidomide, everolimus, vorinostat, idelalisib, ibrutinib, panabinostat.