Abstract
Positron emission tomography (PET)–adapted chemotherapy and radiotherapy approaches are currently used for the initial treatment of early-stage Hodgkin lymphoma (HL) with progression-free survival and overall survival exceeding 85% and 95%, respectively. However, despite general agreement on the prognostic value of interim PET in HL, frontline treatment approaches vary among institutions with respect to how pretreatment clinical risk factors determine treatment selection, the definition of PET negativity, which chemotherapy regimen to initiate and how many cycles to administer, and when to incorporate radiation. Furthermore, as recent trials have confirmed improved efficacy and manageable toxicity when brentuximab and checkpoint inhibitors are combined with frontline regimens such as doxorubicin, vinblastine, and dacarbazine in advanced-stage HL, these agents are now under evaluation as frontline therapy in early-stage HL. A number of issues will affect the use of these agents in early-stage HL, including the costs, early and late toxicities with these agents, patient population (favorable or unfavorable risk groups), how to incorporate them (concurrently or sequentially), and whether they can ultimately replace cytotoxic therapy with similar efficacy and fewer late effects. Future treatment paradigms for early-stage HL may change significantly once randomized studies are completed incorporating these agents into frontline therapy. Ideally, frontline use of brentuximab and checkpoint inhibitors in early-stage HL will result in improved outcomes compared with current PET-adapted approaches with decreased risks of late toxicities that continue to afflict long-term survivors of HL.
Learning Objectives
Describe current frontline treatment approaches in patients with early-stage HL
Describe recent clinical trials and controversies surrounding the incorporation of brentuximab and checkpoint inhibitors into frontline therapy in early-stage HL
CLINICAL CASE
A 20-year-old woman has bilateral cervical lymph node enlargement but no fevers, night sweats, or weight loss. She was initially treated with antibiotics and steroids by her primary care physician without improvement. Core needle biopsy specimen of a right cervical lymph node was suspicious but not diagnostic for classic Hodgkin lymphoma (HL). Excisional lymph node biopsy confirmed classic HL, and positron emission tomography (PET) scan demonstrated numerous cervical, supraclavicular, mediastinal, and axillary nodes, including a left posterior cervical node of 1.2 × 1 cm with standardized uptake value (SUV) 7.3, a right supraclavicular node measuring 2.7 × 3.5 cm with SUV 10.6, a mediastinal node measuring 2.3 × 1.3 cm with SUV 5.5, and right and left axillary lymphadenopathy. No intra-abdominal or splenic uptake was noted. Her laboratory testing demonstrated a white blood cell count of 12,400/microliter, hemoglobin of 11.9 g/dL, platelet count of 471 000, and an elevated erythrocyte sedimentation rate (ESR) of 77 mm/h. Therefore, she has nonbulky stage IIA classic HL, with adverse features including involvement of 5 nodal sites and an elevated ESR.
Introduction
A number of controversies surround treatment decisions in patients diagnosed with early-stage HL, and institutions vary in their approaches to these patients. Clinical trials that guide treatment decisions in early-stage HL differ in the designation of pretreatment risk factors, eligibility criteria, definition of response based on interim PET, and how novel agents are incorporated. In addition, long-term follow-up is lacking from many of these trials, and recent studies continue to show that patients with classic HL remain at risk for late complications from therapy despite improvements and reductions in therapy. Therefore, current treatment approaches need to balance the competing risks of achieving high clinical efficacy while minimizing late toxicity. This review summarizes recent data regarding late toxicities in patients with early-stage HL, PET-adapted therapeutic approaches for these patients, the treatment of bulky HL, and the incorporation of brentuximab and checkpoint inhibitors into the treatment of early-stage HL. In addition, the review highlights ongoing questions in the field, including which chemotherapy backbone to initiate, the number of treatment cycles to administer, definitions of PET negativity, when to incorporate radiotherapy, how to treat high-risk patients who are interim or end-of-therapy PET positive, treatment of bulky HL, and how to include brentuximab and checkpoint inhibitors in frontline therapy.
Late toxicities of treatment of classic HL in the modern era
Despite advances in frontline chemotherapy and radiotherapy for patients with early-stage HL, these patients still experience a lifelong risk of secondary malignancies and cardiovascular disease. In an analysis of Surveillance, Epidemiology, and End Results data on 20,007 survivors of HL aged 20 to 74 years diagnosed from 2000 to 2015, Dores et al1 ascertained that the risk of death due to noncancer causes and second neoplasms remains significantly elevated over the general US population, even in the modern treatment era. In this study, 60% of patients had stage I/II disease, and median follow-up was 8 years. Noncancer causes of mortality in early-stage patients included interstitial lung disease, infection, benign hematologic disease (cytopenias and clotting), heart disease, and diabetes.1 Other neoplasms accounted for 25% of all nonlymphoma deaths regardless of stage, with 145 cases of secondary neoplasms in early-stage and 144 cases in advanced-stage patients. Stage-specific mortality trends are declining in the modern treatment era with all-cause, noncancer, and other neoplasm standardized mortality ratios of 7.0, 2.9, and 2.9 for early-stage patients treated from 1983 to 1991 vs 2.9, 1.5, and 1.6 treated from 2001 to 2009, respectively. However, follow-up is short for those patients treated from 2001 to 2009, and these mortality rates still exceed rates in the general population.
Focusing on secondary neoplasms, the Children's Oncology Group published a 10-year follow-up on AHOD0031, a trial of 1711 patients aged up to 21 years treated with response-adapted therapy.2 All patients initially received doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide, and rapid early responders (determined by PET) were randomly allocated to 21 Gy involved field radiotherapy (IFRT) or observation. Slow responders were randomized to IFRT or dexamethasone, etoposide, cisplatin, and cytarabine plus IFRT. Ten-year event-free survival and overall survival (OS) were not significantly different in the rapid early responders treated with observation vs IFRT and also not different in slow responders treated with dexamethasone, etoposide, cisplatin, and cytarabine plus IFRT vs IFRT alone. The cumulative incidence of second neoplasms was 1.3% with 17 second malignancies (3 cases of acute myeloid leukemia, 11 solid tumors, and 3 cases of NHL). Sixteen of these malignancies occurred in patients treated with combined modality therapy (CMT) with doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide plus 21 Gy IFRT, and 9 of 11 solid tumors occurred within the radiation field. Most solid tumors were papillary thyroid carcinoma, but there was 1 breast cancer that did not develop until 13 years after study enrollment. Therefore, it is likely with longer follow-up that additional secondary neoplasms, including breast and lung cancers, will be observed despite a PET-adapted approach that limits both chemotherapy and radiation doses.
In 3905 Dutch patients aged 15 to 50 years who were treated between 1965 and 2000, with 60.5% receiving CMT, the cumulative incidence of subsequent solid neoplasms did not differ significantly (P = .71) among treatment eras: 1965 to 1976, 1977 to 1988, and 1989 to 2000.3 In addition, the risk for any second cancer remained high for up to 40 years after treatment for HL, with a 48.5% cumulative incidence. Bright et al4 also showed that the risk of solid tumors continues to increase annually, even up to 35 years after treatment for HL. In their study of 16 971 survivors of HL aged 15 to 39 years who were treated from 1971 to 2006, the cumulative incidence of subsequent primary neoplasms was 0.9% and 26.6% in females and 0.6% and 16.5% in males at 10 and 35 years from diagnosis. Taken together, these studies all demonstrate that the risk of second neoplasms continues to rise with long-term follow-up and that most of these second solid tumors are not observed until after at least 10 years from diagnosis. In addition, the risk of second malignant neoplasms continues to persist in the current treatment era despite improved chemotherapy and reduced radiation exposure.
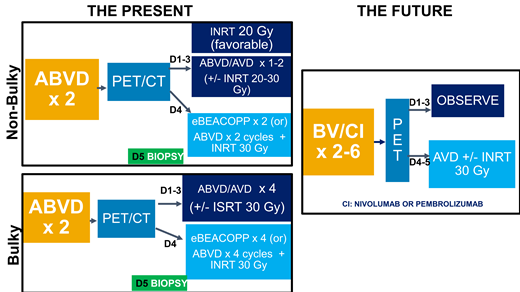
PET-adapted frontline treatment
As a result of the persisting evidence of late complications even with modern chemotherapy and radiation techniques and with the proven prognostic significance of interim PET in early-stage HL,5 many adolescent and young adult (AYA) and adult patients with early-stage HL currently receive PET-adapted therapy in an effort to further reduce chemotherapy and radiation exposure. The studies supporting PET-adapted therapy differ slightly in eligibility criteria, in the treatment regimen, and in defining PET negativity, making comparisons across studies challenging. Table 1 summarizes these studies that currently guide treatment in AYA and adult HL.
PET-adapted therapeutic trials in early-stage HL
| Study (median follow-up) . | Patient population (N) . | Risk factors at enrollment . | PET negative . | Treatment arms . | PFS or FFTF . | OS . |
|---|---|---|---|---|---|---|
| UK RAPID6 (5.0 years) | Stage IA or IIA (n = 602) | Nonbulky | Deauville 1-2 | PET neg: ABVD × 3 PET neg: ABVD × 3 + 30 Gy IFRT PET pos: ABVD × 4 + 30 Gy IFRT | 3-year 90.8% 3-year 94.6% 3-year 83% | 3-year 99.0% 3-year 97.1% |
| CALGB 506048 (3.8 years) | Stage I or II (n = 164) | Nonbulky | Deauville 1-3 | PET neg: ABVD × 4 PET pos: ABVD × 2, eBEACOPP × 2, 30 Gy IFRT | 3-year PFS 91% 3-year PFS 66% | |
| EORTC9 (4.5-5.1 years) | Stage I or II (n = 1950) | Favorable (no risk factors) Unfavorable (any of the following) 1. Age ≥50 2. Bulk 3. >3 nodal sites 4. ESR ≥50 (or 30 if B-symptoms) | Deauville 1-2 | (F) PET neg: ABVD × 3 + 30 Gy INRT (F) PET neg: ABVD × 4 | 5-year PFS 99% 5-year PFS 87.1% | 5-year OS 100% 5-year OS 99.6% |
| (U) PET neg: ABVD × 4 + 30 Gy INRT (U) PET neg: ABVD × 6 | 5-year PFS 92.1% 5-year 89.6% | 5-year OS 96.7% 5-year OS 98.3% | ||||
| (F/U) PET pos: ABVD × 4 + 30 Gy INRT (F/U) PET pos: ABVD × 2, eBEACOPP × 2, 30 Gy INRT | 5-year PFS 77.4% 5-year PFS 90.6% | 5 year OS 89.3% 5 year OS 96.0% | ||||
| GHSG HD1610 (45 months) | Stage I or II (N = 1150) | Favorable (none of the following risk factors): 1. Bulk 2. Extranodal sites 3. >2 nodal areas 4. ESR ≥50 (or 30 if B-symptoms) | Deauville 1-2 | PET neg: ABVD × 2 + 20 Gy IFRT PET neg: ABVD × 2 PET pos: ABVD × 2 + 20 Gy IFRT | 5-year PFS 93.4% 5-year PFS 86.1% 5-year PFS 88.4% | 5-year OS 98.1% 5-year OS 98.4% 5-year OS 97.9% |
| GHSG HD1711 (46.2 months) | Stage I or II (N = 1100) | Unfavorable (with one of the following risk factors): 1. Bulk 2. Extranodal sites 3. >2 nodal areas 4. ESR ≥50 (or 30 if B-symptoms) | Deauville 1-2 | PET negative: eBEACOPP/ABVD × 4 + 30 Gy IFRT PET neg: eBEACOPP/ABVD × 4 PET pos: eBEACOPP/ABVD × 4 + 30 Gy IFRT | 5-year PFS 97.7% 5-year PFS 95.9% 5-year PFS 94.2% | 5-year OS 98.7% 5-year OS 98.8% 5-year OS 99.2% |
| Study (median follow-up) . | Patient population (N) . | Risk factors at enrollment . | PET negative . | Treatment arms . | PFS or FFTF . | OS . |
|---|---|---|---|---|---|---|
| UK RAPID6 (5.0 years) | Stage IA or IIA (n = 602) | Nonbulky | Deauville 1-2 | PET neg: ABVD × 3 PET neg: ABVD × 3 + 30 Gy IFRT PET pos: ABVD × 4 + 30 Gy IFRT | 3-year 90.8% 3-year 94.6% 3-year 83% | 3-year 99.0% 3-year 97.1% |
| CALGB 506048 (3.8 years) | Stage I or II (n = 164) | Nonbulky | Deauville 1-3 | PET neg: ABVD × 4 PET pos: ABVD × 2, eBEACOPP × 2, 30 Gy IFRT | 3-year PFS 91% 3-year PFS 66% | |
| EORTC9 (4.5-5.1 years) | Stage I or II (n = 1950) | Favorable (no risk factors) Unfavorable (any of the following) 1. Age ≥50 2. Bulk 3. >3 nodal sites 4. ESR ≥50 (or 30 if B-symptoms) | Deauville 1-2 | (F) PET neg: ABVD × 3 + 30 Gy INRT (F) PET neg: ABVD × 4 | 5-year PFS 99% 5-year PFS 87.1% | 5-year OS 100% 5-year OS 99.6% |
| (U) PET neg: ABVD × 4 + 30 Gy INRT (U) PET neg: ABVD × 6 | 5-year PFS 92.1% 5-year 89.6% | 5-year OS 96.7% 5-year OS 98.3% | ||||
| (F/U) PET pos: ABVD × 4 + 30 Gy INRT (F/U) PET pos: ABVD × 2, eBEACOPP × 2, 30 Gy INRT | 5-year PFS 77.4% 5-year PFS 90.6% | 5 year OS 89.3% 5 year OS 96.0% | ||||
| GHSG HD1610 (45 months) | Stage I or II (N = 1150) | Favorable (none of the following risk factors): 1. Bulk 2. Extranodal sites 3. >2 nodal areas 4. ESR ≥50 (or 30 if B-symptoms) | Deauville 1-2 | PET neg: ABVD × 2 + 20 Gy IFRT PET neg: ABVD × 2 PET pos: ABVD × 2 + 20 Gy IFRT | 5-year PFS 93.4% 5-year PFS 86.1% 5-year PFS 88.4% | 5-year OS 98.1% 5-year OS 98.4% 5-year OS 97.9% |
| GHSG HD1711 (46.2 months) | Stage I or II (N = 1100) | Unfavorable (with one of the following risk factors): 1. Bulk 2. Extranodal sites 3. >2 nodal areas 4. ESR ≥50 (or 30 if B-symptoms) | Deauville 1-2 | PET negative: eBEACOPP/ABVD × 4 + 30 Gy IFRT PET neg: eBEACOPP/ABVD × 4 PET pos: eBEACOPP/ABVD × 4 + 30 Gy IFRT | 5-year PFS 97.7% 5-year PFS 95.9% 5-year PFS 94.2% | 5-year OS 98.7% 5-year OS 98.8% 5-year OS 99.2% |
eBEACOPP, escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone; EN, XXX; F, favorable; FFTF, freedom from treatment failure; neg, negative; pos, positive; U, unfavorable.
In the UK RAPID study,7 602 nonbulky stage I to IIA patients (32.3% unfavorable by German Hodgkin Study Group criteria) received 3 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine followed by a PET scan. Patients with a Deauville score of 1 to 2 were considered PET negative and randomized to either 30 Gy IFRT or no further treatment, and patients with a Deauville score of 3 to 5 underwent a fourth cycle of ABVD and IFRT. Seventy-four percent of patients were PET negative, and 3-year progression-free survival (PFS) was 94.6% with IFRT and 90.8% with observation (P = .02). No OS benefit was observed, with a 3-year OS of 97.1% compared with 99% with and without radiation (P = .27). For the PET-positive patients, 3-year PFS was 83%. In an analysis of the RAPID study by Deauville score,7 inferior outcomes were seen only in patients with scores of 5, with 5-year PFS of 91.5%, 91.1%, 95.3%, 87.5%, and 61.9% in patients with scores of 1, 2, 3, 4, and 5, respectively.
Cancer and Leukemia Group B 50604 examined a PET-adapted approach in patients aged 18 to 60 years with nonbulky stage I and II HL with and without B-symptoms.8 All patients received 2 cycles of ABVD, interim PET, and, if PET negative (Deauville scores 1-3), 2 additional ABVD cycles without IFRT. PET-positive patients switched to escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) with IFRT. In total, 164 patients were enrolled, 26% with B-symptoms and 58% unfavorable by GHSG criteria. With the expansion of PET negativity to Deauville scores of 1 to 3, 91% of patients were PET negative compared with 76% if Deauville scores of 1 to 2 were used. Three-year PFS was 91% for PET-negative patients and 66% for PET-positive patients. For patients with Deauville scores of 1 to 2 (n = 113), 3 (n = 22), and 4 to 5 (n = 14), 3-year PFS was 94%, 77%, and 67%, respectively.
The largest PET-adapted study, European Organization for Research and Treatment of Cancer H10, enrolled 1950 stage I to II patients, with 754 favorable and 1196 unfavorable HL.9 In the control arms, treatment consisted of 3 (favorable) or 4 (unfavorable) ABVD cycles and involved nodal radiotherapy (INRT), regardless of PET results. In the experimental arms, patients received 2 cycles of ABVD, interim PET, and, if PET negative (Deauville scores 1-2), 2 (favorable) or 4 (unfavorable) additional cycles of ABVD. PET-positive patients switched to escalated BEACOPP and INRT. Eighty-seven percent of favorable and 77.6% of unfavorable patients were PET negative. Five-year PFS rates in the favorable PET-negative patients were 99% with 3 cycles of ABVD and INRT vs 87.1% with 4 cycles of ABVD alone. In unfavorable PET-negative patients, 5-year PFS was 92.1% with 4 cycles of ABVD and INRT vs 89.6% with 6 cycles of ABVD. In both favorable and unfavorable cohorts, noninferiority for chemotherapy alone could not be demonstrated. Five-year OS was not significantly different at 99.6% and 98.3% with ABVD only and 100% and 96.7% with ABVD and INRT in favorable and unfavorable patients, respectively. For PET-positive patients, 5-year PFS with escalated BEACOPP was 90.6% compared with 77.4% with 3 to 4 cycles of ABVD and INRT (P = .002), with no OS benefit (P = .062).
The GHSG conducted the HD16 trial in 1150 patients with favorable stage I to II HL examining 2 cycles of ABVD and 20 Gy IFRT compared with PET-guided treatment with 2 cycles of ABVD, PET, and no radiotherapy if PET negative (Deauville scores 1-2) and 20 Gy IFRT if PET positive.10 In PET-negative patients, 5-year PFS was 93.4% with CMT and 86.1% with ABVD alone (P = .04). No OS benefit was noted with 5-year OS of 98.1% with CMT and 98.4% with ABVD (P = .12). Notably, PFS was significantly worse in patients with Deauville scores of 4 to 5, with 5-year PFS of 80.9%, 93.1%, and 93.2% for scores of 4 to 5, 1 to 3, and 1 to 2, respectively. The GHSG HD17 trial examined 2 cycles of escalated BEACOPP plus 2 cycles of ABVD followed by 30 Gy IFRT in unfavorable early-stage HL compared with an experimental arm omitting IFRT in PET-negative (Deauville scores 1-2) patients after 4 cycles of chemotherapy.11 In the PET-negative patients, 5-year PFS was 97.7% with CMT, not statistically different compared with 95.9% with chemotherapy alone. As in the HD16 trial, a Deauville score of 4 to 5 was a significant risk factor for poor PFS, whereas scores of 1 to 3 were not associated with PFS in the multivariable model.
Although the RAPID, EORTC H10, and GHSG HD16 trials all failed to show noninferiority in PFS with PET-adapted omission of radiotherapy compared with CMT, particularly in favorable patients, there was no OS benefit with inclusion of radiation. Therefore, the treating physician needs to weigh the risks of potential late toxicities with radiotherapy against the risk of relapse in their patients when determining if radiotherapy should be included. Based on data showing continued risk of late second malignancies with modern radiotherapy techniques and the lack of OS benefit with IFRT, my practice is to omit radiotherapy in PET-negative patients. On the basis of the secondary analyses of the association of PFS by Deauville score from the RAPID,11 HD16,9 and HD1710 studies, which demonstrated significantly worse PFS only in patients with Deauville scores of 4 to 5, I define PET negative as Deauville scores of 1 to 3 and often omit radiotherapy even in patients with a Deauville score of 3. With this approach, more than 90% of patients with early-stage HL can avoid radiotherapy. However, as all patients in the RAPID, HD16, and HD17 trials with a Deauville score of 3 received CMT and CALGB 50604 demonstrated an inferior PFS of 77% in 22 patients with a Deauville score of 3 treated with chemotherapy alone, the use of radiotherapy is certainly justified in patients with a Deauville score of 3 and should be discussed with the patient and a multidisciplinary treatment team and weighed against potential late effects.
In addition to the ongoing controversy over PET-directed radiotherapy, these trials also highlight other debates in early-stage HL, including number of chemotherapy cycles, which regimen to start (ABVD or escalated BEACOPP), and treatment of PET-positive patients. Although the low-risk patient could receive as few as 2 to 4 ABVD cycles, unfavorable patients in the EORTC and HD17 trials seem to have improved outcomes with 6 cycles of ABVD or escalated BEACOPP. In my own practice, I tend to administer 3 to 4 cycles of ABVD alone in nonbulky, unfavorable patients who are interim PET negative based on the UK Rapid and CALGB studies but recognize that 6 cycles can also be considered for these patients, particularly if omitting radiotherapy.
For those patients with interim PET scores of 4 to 5, it remains unclear if these patients should switch therapy from ABVD to escalated BEACOPP. In EORTC H10, escalated BEACOPP and INRT in interim PET-positive patients improved PFS to 90.6% vs 77.4% with ABVD and INFRT. However, in the RAPID and HD16 trials, PET-positive patients received ABVD and IFRT without chemotherapy intensification, and PFS was 87.6% and 88.4%, respectively. In my own practice, I avoid the use of escalated BEACOPP even in interim PET-positive patients due to the potential risks of infertility and secondary malignancies with the regimen. I typically recommend IFRT for a Deauville score of 4 and biopsy followed by salvage chemotherapy and autologous transplant for a Deauville score of 5.
Treatment of bulky disease
In bulky HL, 4 studies suggest that radiation can be eliminated in PET-negative patients without compromising outcomes. The British Colombia Cancer Agency omitted radiotherapy in patients with stage I to II bulky, IIB, and III to IV HL who were PET negative (Deauville scores 1-3) after 6 cycles of ABVD.12 Eighty-four percent were PET negative and did not receive radiation. Five-year freedom from treatment failure was 89% for PET-negative compared with 56% for PET-positive patients. In PET-negative patients with bulk (n = 112), 5-year freedom from treatment failure was 89% compared with 88.5% for PET-negative nonbulky disease (n = 152).
In CALGB 50801, 101 patients with bulky stage I to II HL were treated with 2 cycles of ABVD followed by interim PET.13 PET-negative (Deauville scores 1-3) patients received 4 additional cycles of ABVD and no radiation, and PET-positive patients received 4 cycles of escalated BEACOPP and 30 Gy involved site radiotherapy (ISRT). Seventy-eight percent were PET negative, and 3-year PFS was 93.1% compared with 89.7% for PET-positive patients, confirming that a PET-adapted approach eliminating radiation leads to durable remissions in bulky HL.
In the Gruppo Italiano Terapie Innovative nei Linfomi/Fondazione Italiana Linfomi HD 0607 study,14 patients with stage IIB to IVB HL who were PET negative after 6 cycles of ABVD and who had a large nodal mass 5 cm or larger underwent randomization to 30 Gy IFRT or no further treatment. In the 296 PET-negative patients with a large nodal mass, there was no significant difference in 3-year PFS with a PFS of 93% without radiotherapy compared with 97% with IFRT (P = .29). Even when limiting the analysis to patients with bulk more than 10 cm, 3-year PFS was 94% with IFRT and 86% for observation (P = .34). Last, the UK RATHL study included 500 patients with bulky or high-risk stage II HL and demonstrated a 90.9% PFS in these patients if PET negative after 6 cycles of ABVD.15 Therefore, based on these studies, I omit IFRT in patients with bulky early-stage HL who achieve a negative PET.
Incorporation of novel agents
Unlike advanced-stage HL in which large randomized studies have examined brentuximab vedotin (BV) and checkpoint inhibitors combined with doxorubicin, vinblastine, and dacarbazine (AVD) or BEACOPP,16-18 only small studies have been completed incorporating these agents into frontline therapy for early-stage HL. Abramson et al19 evaluated combined AVD/BV without radiotherapy in 34 patients with nonbulky early-stage HL. Patients received 1 cycle of BV alone on days 1 and 15 followed by 4 cycles of combined AVD/BV. The complete response (CR) rate was 52% after the lead-in cycle of BV and 97% after 2 AVD/BV cycles. Three-year PFS was 94%. Grade 3 to 4 events included sensory neuropathy (23%), neutropenia (62%), and febrile neutropenia (35%), although neutropenic fever declined once growth factor support was mandated. Park and colleagues examined consolidation of patients with nonbulky (defined as ≤7.5 cm) early-stage HL with 6 cycles of BV after 2 to 6 cycles of ABVD.20 Of the 41 patients enrolled, 36 completed the planned 6 BV doses. With a consolidation approach, 95% achieved a CR, and 3-year PFS was 92%.
Kumar et al21 conducted a pilot study of CMT using AVD/BV and 30 Gy ISRT in 30 patients with early unfavorable HL. Forty-seven percent of patients had bulky disease. Twenty-seven patients achieved CR, and 1-year PFS was 93.3%. This trial recently enrolled 3 additional cohorts testing the feasibility of reducing the ISRT dose to 20 Gy, reducing the radiation field with consolidation volume radiation, and eliminating ISRT.22 To date, 27% of the 117 patients enrolled across all 4 cohorts had bulky disease, and CR rates were 93% to 97% in the CMT cohorts and 97% with AVD/BV alone. With a median follow-up of 3.8 years, 2-year PFS was 94% in all patients, with a 2-year PFS of 96.6% in the chemotherapy alone arm, although follow-up in this no radiotherapy cohort is still short at 2.2 years.
Two studies have explored checkpoint inhibitors in early-stage HL. The GHSG conducted a trial in 109 patients with unfavorable or bulky HL examining concurrent nivolumab/AVD for 4 cycles or sequential therapy with 4 nivolumab cycles, 2 nivolumab/AVD cycles, and 2 AVD cycles.23 All patients received 30 Gy ISRT. Eighty-seven percent and 26% of patients achieved a CR after 2 nivolumab/AVD cycles or 4 nivolumab cycles, respectively. At the completion of treatment, the CR rates were 83% and 84%, and 2-year PFS was 100% and 98% in the combination and sequential arms, respectively. Grade 3 to 4 toxicities were similar with both approaches, and hypothyroidism was the most frequent late effect, persisting in 17% of patients. Allen and colleagues completed a phase 2 study of 3 doses of pembrolizumab followed by 4 to 6 cycles of AVD without radiotherapy in 12 early-stage and 18 advanced-stage patients, including 10 patients with bulky tumors more than 10 cm.24 After 3 cycles of pembrolizumab, the CR rate was 37% and improved to 100% after 2 cycles of AVD. Two-year PFS was 100%. Grade 3 to 4 events included neutropenia in 3 patients and transaminitis, lymphopenia, diarrhea, and Bell palsy in 1 patient each.
Therefore, BV and checkpoint inhibitors appear safe and effective in early-stage and bulky HL with PFS exceeding 90%; however; large randomized trials are necessary to establish the safety, cost-effectiveness, and long-term efficacy in comparison to current PET-adapted ABVD therapy. Although sequential administration may minimize toxicity, these prolonged treatment approaches may lead to significant education, childcare, financial, and employment constraints in an AYA population. Alternatively, as demonstrated by Abramson et al19 and Allen et al,24 financial costs and treatment risks may be minimized with 1 to 3 lead-in cycles of BV or checkpoint inhibitor prior to AVD, and these limited approaches should also be evaluated. In conclusion, although it is likely that the paradigm of treatment of early-stage HL will likely shift to incorporate BV and checkpoint inhibitors frontline, which agents to use, how to incorporate them (lead-in, sequential, or concurrent administration), which regimen (AVD or BEACOPP) to combine with, and whether conventional cytotoxic therapy can ultimately be eliminated are still under investigation.
CLINICAL CASE (continued)
The 20-year-old woman with nonbulky stage IIA classic HL with 2 adverse risk factors (5 nodal sites and elevated ESR) started ABVD chemotherapy, and after 2 cycles, PET was performed, demonstrating a right supraclavicular node of 2.1 × 1.5 cm with SUV 5.9 compared with 2.9 × 3 cm with SUV 7.3 previously, new right axillary lymph node of 1.5 × 1.1 cm with SUV 7.5, and worsening anterior mediastinal nodal mass with SUV 10.2 compared with 6.3 previously (Deauville score 5). Core needle biopsy specimen of the supraclavicular node confirmed persistent classic HL. She stopped ABVD therapy and began 4 cycles of combined BV/nivolumab salvage therapy. After 4 cycles, she achieved a CR with a Deauville score of 3. She proceeded to autologous transplant followed by 30 Gy proton therapy to bilateral neck, mediastinum, and axillary nodal sites. She initiated BV maintenance after radiation and remains on treatment for a planned maximum of 16 cycles.
Conflict-of-interest disclosure
Kristie A. Blum: no competing financial interests to declare.
Off-label drug use
Kristie A. Blum: Brentuximab, nivolumab, and pembrolizumab are not FDA approved as frontline therapy in early-stage HL.