Abstract
Patients with sickle cell disease (SCD) have significant impairment in their quality of life across the life span as a consequence of serious disease burden with several SCD-related complications. A number of disease-modifying therapies are currently available, yet long-term clinical benefits in real-world settings remain unclear. Over the past few years, a number of important initiatives have been launched to optimize clinical trials in SCD in different ways, including: (1) established panels through a partnership between the American Society of Hematology (ASH) and the US Food and Drug Administration; (2) the ASH Research Collaborative SCD Clinical Trials Network; (3) the PhenX Toolkit (consensus measures for Phenotypes and eXposures) in SCD; and (4) the Cure Sickle Cell Initiative, led by the National Heart, Lung, and Blood Institute. Electronic patient-reported outcomes assessment is highly recommended, and patient-reported outcomes (PROs) should be evaluated in all SCD trials and reported using Standard Protocol Items Recommendations for Interventional Trials guidelines. Patient-centered outcomes research (PCOR) approaches and meaningful stakeholder engagement throughout the process have the potential to optimize the execution and success of clinical trials in SCD with considerable financial value. This article reviews several clinical trial considerations in SCD related to study design and outcomes assessment as informed by recent initiatives as well as patient-centered research approaches and stakeholder engagement. A proposed hematology stakeholder-engagement framework for clinical trials is also discussed.
Learning Objectives
Review key considerations for SCD clinical trials related to PROs, medication adherence, developmental issues in children, and the COVID-19 pandemic
Review efforts to optimize clinical trials and outcomes assessment in SCD, such as ASH-FDA panels, the ASH Research Collaborative Clinical Trials Network, the PhenX Toolkit, and the CureSCi
Review the evidence for patient-centered research (PCR) and stakeholder engagement and their potential role in successful trials and cost savings
CLINICAL CASE
A 19-year-old man with hemoglobin SS disease presents for his regular clinic visit. He has had no hospitalizations over the past 5 years since he started taking daily hydroxyurea with good adherence. He believes that hydroxyurea helped him a great deal with his quality of life, but he also understands that not every sickle cell patient feels the same. He has learned about other approved and emerging therapies, and he is fascinated by the science behind them. Given his interest in pursuing a career in medicine, he inquires about the possibility of getting involved in sickle cell research to help other sickle cell patients benefit from these therapies.
Introduction
Sickle cell disease (SCD) is an inherited hemoglobin disorder affecting about 100 000 individuals in the United States and more than 20 million people worldwide, mainly of African descent.1,2 SCD is a chronic, debilitating medical condition that affects patients across their life span and is associated with significant morbidity and early mortality.2,3 SCD patients suffer from a number of acute and chronic complications, including pain episodes, acute chest syndrome, cardiopulmonary disease, kidney damage, liver impairment, splenic sequestration, avascular necrosis, stroke, priapism, and other end organ damage.2,3 These complications lead to significant impairment in patient-reported outcomes (PROs) among children and adults with SCD, especially in the physical and psychosocial domains.4-6 Patients with SCD have increased health care utilization with frequent hospitalizations and emergency department visits.7-9 Treatment approaches include preventive strategies (eg, penicillin prophylaxis, transcranial Doppler screening), acute management (eg, opioids), disease-modifying therapies (ie, hydroxyurea, L-glutamine, voxelotor, and crizanlizumab), and curative options (eg, hematopoietic stem cell transplantation, gene therapy, or gene editing).3
Some earlier clinical trials in SCD faced a number of logistical challenges related to recruitment and retention.10-12 Barriers to successful trial completion included concerns from parents as to the necessity of the research, patient belief that research was only needed for those with more severe disease, or anxiety related to previous research experience.11-13 A number of facilitators were also identified from these earlier studies, including educating peers, explaining trial rationales more clearly, improving the readability of consent/assent forms, explaining study protocols using videos and other innovative illustrations, and leveraging patient-centered research (PCR) approaches that involve patients, parents/caregivers and other stakeholders (heretofore referred to as “stakeholders”) across all stages of the research process, from planning a study to the dissemination of findings.11-13 Despite some initial difficulties in research, several trials in SCD to date have been successfully completed, leading to significant progress in the field of SCD with new therapies now approved by the US Food and Drug Administration (FDA), providing clinical benefits to many SCD patients. Currently, there are several active, ongoing clinical trials with novel disease-modifying therapies and curative approaches, such as gene therapy, gene editing, and hematopoietic stem cell transplantation with various regimens and donors.14,15 Nevertheless, efforts to improve clinical trials, including involving stakeholders in trials, are still ongoing.
Over the past few years, a number of important initiatives have been launched to optimize clinical trials in SCD in different ways. First, consensus recommendations for evidence-based SCD end points have been developed as a result of a collaborative effort from 7 panels of patients, clinicians, and researchers established through a partnership between the American Society of Hematology (ASH) and the FDA.16,17 Second, the ASH Research Collaborative SCD Clinical Trials Network has focused on building partnerships with the SCD community and stakeholders, establishing collaboration across SCD centers, streamlining clinical trial operations with a single institutional review board approval, and facilitating data sharing with a centralized data repository through the ASH Research Collaborative Data Hub.18 Third, the PhenX (Phenotypes and eXposures) project has been funded by different sources, including the National Human Genome Research Institute, the National Institute on Drug Abuse, the Office of Behavioral and Social Sciences Research, the National Institute of Mental Health, the National Heart, Lung, and Blood Institute (NHLBI), the National Institute on Minority Health and Health Disparities, the National Cancer Institute, and the Tobacco Regulatory Science Program of the National Institutes of Health (NIH). In SCD, PhenX efforts have focused on selecting high-quality SCD-related outcome measures to be included in the Toolkit (consensus measures; www.phenxtoolkit.org), guided by the Sickle Cell Disease Research and Scientific Panel.19 Finally, the Cure Sickle Cell Initiative (CureSCi), led by the NHLBI, has centered on innovating genetic therapies, nurturing a collaborative, PCR environment, and establishing data standards for SCD clinical trials.20 In particular, interest in curative therapies in the SCD community has been growing as evidence has emerged and continues to materialize from ongoing clinical trials.
This article aims to review several clinical trial considerations in SCD related to study design and outcome assessment as informed by recent initiatives, in particular PROs as well as PCR approaches and stakeholder engagement.
Clinical trial considerations
PROs and e-PROs
PROs have been defined as “outcomes reported directly by patients themselves and not interpreted by an observer.”21 Health-related quality of life (HRQOL) is a PRO that is defined as “a multidimensional concept that usually includes self-report of the way in which physical, emotional, social, or other domains of well-being are affected by a disease or its treatment.”21 A proxy- or parent/caregiver-reported outcome is also commonly used to evaluate PROs and/or HRQOL among pediatric populations. Major regulatory authorities have recognized the value of including PROs evaluation in clinical trials to inform clinical decision-making, pharmaceutical-labeling claims, and product reimbursement.22 The inclusion of PROs in clinical trial protocols should be well planned in advance and reported according to the Standard Protocol Items Recommendations for Interventional Trials guidelines.23 Evaluating PROs in clinical trials that involve SCD patients provides an opportunity to measure the impact of a given treatment on their individual functioning and well-being.5
A number of factors should be considered when assessing PROs in SCD clinical trials, such as eligibility criteria (eg, age), relevant domains of interest, psychometric properties (eg, responsiveness, validity, reliability, and floor/ceiling effects), minimal clinically important differences, generic vs disease-specific approaches, participants' burden (ie, survey length), and mode of administration.5 Generic measures provide insight into the burden of SCD compared to healthy individuals and those with other chronic medical conditions.5 On the other hand, disease-specific measures better examine the differences and effects of treatments or interventions across different patient groups and within individual SCD patients.5 Thus, a combined approach using generic and disease-specific instruments to evaluate SCD patients' PROs is highly recommended, with a preference for patient self-reporting over proxy reporting when possible.4,5 Further, the ASH-FDA panel for PROs has provided detailed guidance and recommendations on PROs selection in SCD clinical trials (Table 1).16
PROs recommendations in CureSCi CDEs (version 1.0)
| Subdomain . | Population . | Measure . | Classification . |
|---|---|---|---|
| Pain intensity | Adults and children ≥8 years old | NRS | Core |
| VAS | Supplemental | ||
| Pain impact/interference | Adults, SCD-specific | ASCQ-Me Pain Impact | Core |
| Children 5-18 years, SCD-specific | PedsQL Pain Impact SCD module | Core | |
| Children/adults, not SCD-specific | PROMIS Pain Interferencea,b | Core | |
| Pain: mixed | Children 5-18 years, SCD-specific | PedsQL Pain and Hurt, SCD modules | Core |
| Painful crises | Adults, SCD-specific | ASCQ-Me Pain Episodes | Core |
| Emotional impact of SCD | Adults, SCD-specific | ASCQ-Me Emotional Impact | Supplemental, highly recommended |
| Children, SCD-specific | PedsQL, SCD Module Emotions | Supplemental, highly recommended | |
| PedsQL, SCD Module Worrying | Supplemental | ||
| Negative affect: mixed | Children, not SCD-specific | PROMIS Physical Stress Experience1 | Supplemental, highly recommended |
| Low mood | Children/adults, not SCD-specific | PROMIS Emotional Distress: Depression1,2 | Supplemental, highly recommended |
| Anxiety | Children/adults, not SCD-specific | PROMIS Emotional Distress: Anxiety1,2 | Supplemental, highly recommended |
| Fatigue | Children/adults, not SCD-specific | Pediatric/Adult PROMIS Fatigue1,2 | Core |
| Children, SCD-specific | PedsQL Multidimensional Fatigue Scale | Core | |
| Sleep disturbance | Children/adults, not SCD-specific | PROMIS Sleep Disturbance1,2 | Supplemental, highly recommended |
| Adults, SCD-specific | ASCQ-Me Sleep Impact | Supplemental, highly recommended | |
| Adults, not SCD-specific | Pittsburgh Sleep Quality Index | Supplemental | |
| Epworth Sleepiness Scale | Supplemental | ||
| Children, not SCD-specific | Epworth Sleepiness Scale (CHAD) | Supplemental | |
| General function | Adults, not SCD-specific | Canadian Occupational Performance | Supplemental |
| Social function | Adults, SCD-specific | ASCQ-Me Social Functioning Impact | Supplemental |
| Physical function | Adults, SCD-specific | ASCQ-Me Stiffness Impact | Supplemental, highly recommended |
| Adults, not SCD-specific | PROMIS - Physical Function (PF) 12a2 | Supplemental | |
| Children, not SCD-specific | Pediatric PROMIS - PF Mobility1 | Supplemental | |
| Pediatric PROMIS - PF Upper Extremity1 | Supplemental | ||
| Global health/QOL | Adults, not SCD-specific | PROMIS 10 Global Health2 | Core |
| Children, not SCD-specific | PROMIS 7 + 2 Global Health* | Core | |
| Global cognition | Children, 0-3.5 years old | Bayley-III | Supplemental |
| Children, 2.5-7 years old | WPPSI-IV (4th edition) | Supplemental | |
| Children, 6-16 years old | WISC-V (5th edition) | Supplemental | |
| Adults | Wechsler Adult Intelligence Scale | Supplemental | |
| Children and adults† | NIH Toolbox | Supplemental, highly recommended | |
| Executive functioning | Children 3-7, 8-11, and ≥12 years | Flanker Inhibitory Control/Attention (NT) | Supplemental, highly recommended |
| Dimensional Change Card Sort Test (NT) | Supplemental, highly recommended | ||
| Children ≥9 years old | Trail Making Test, parts A and B | Supplemental | |
| Children and adults, 8-89 years | Delis-Kaplan Executive Function System | Supplemental | |
| Children and adults, 7-89 years | Wisconsin Card Sort Test | Supplemental | |
| Processing speed | Children ≥7 years old | Pattern Comparison Processing Speed Test | Supplemental, highly recommended |
| Adults | Processing Speed Index | Supplemental | |
| Working memory | Children ≥7 years old | List Sorting Working Memory Test (NT) | Supplemental, highly recommended |
| Subdomain . | Population . | Measure . | Classification . |
|---|---|---|---|
| Pain intensity | Adults and children ≥8 years old | NRS | Core |
| VAS | Supplemental | ||
| Pain impact/interference | Adults, SCD-specific | ASCQ-Me Pain Impact | Core |
| Children 5-18 years, SCD-specific | PedsQL Pain Impact SCD module | Core | |
| Children/adults, not SCD-specific | PROMIS Pain Interferencea,b | Core | |
| Pain: mixed | Children 5-18 years, SCD-specific | PedsQL Pain and Hurt, SCD modules | Core |
| Painful crises | Adults, SCD-specific | ASCQ-Me Pain Episodes | Core |
| Emotional impact of SCD | Adults, SCD-specific | ASCQ-Me Emotional Impact | Supplemental, highly recommended |
| Children, SCD-specific | PedsQL, SCD Module Emotions | Supplemental, highly recommended | |
| PedsQL, SCD Module Worrying | Supplemental | ||
| Negative affect: mixed | Children, not SCD-specific | PROMIS Physical Stress Experience1 | Supplemental, highly recommended |
| Low mood | Children/adults, not SCD-specific | PROMIS Emotional Distress: Depression1,2 | Supplemental, highly recommended |
| Anxiety | Children/adults, not SCD-specific | PROMIS Emotional Distress: Anxiety1,2 | Supplemental, highly recommended |
| Fatigue | Children/adults, not SCD-specific | Pediatric/Adult PROMIS Fatigue1,2 | Core |
| Children, SCD-specific | PedsQL Multidimensional Fatigue Scale | Core | |
| Sleep disturbance | Children/adults, not SCD-specific | PROMIS Sleep Disturbance1,2 | Supplemental, highly recommended |
| Adults, SCD-specific | ASCQ-Me Sleep Impact | Supplemental, highly recommended | |
| Adults, not SCD-specific | Pittsburgh Sleep Quality Index | Supplemental | |
| Epworth Sleepiness Scale | Supplemental | ||
| Children, not SCD-specific | Epworth Sleepiness Scale (CHAD) | Supplemental | |
| General function | Adults, not SCD-specific | Canadian Occupational Performance | Supplemental |
| Social function | Adults, SCD-specific | ASCQ-Me Social Functioning Impact | Supplemental |
| Physical function | Adults, SCD-specific | ASCQ-Me Stiffness Impact | Supplemental, highly recommended |
| Adults, not SCD-specific | PROMIS - Physical Function (PF) 12a2 | Supplemental | |
| Children, not SCD-specific | Pediatric PROMIS - PF Mobility1 | Supplemental | |
| Pediatric PROMIS - PF Upper Extremity1 | Supplemental | ||
| Global health/QOL | Adults, not SCD-specific | PROMIS 10 Global Health2 | Core |
| Children, not SCD-specific | PROMIS 7 + 2 Global Health* | Core | |
| Global cognition | Children, 0-3.5 years old | Bayley-III | Supplemental |
| Children, 2.5-7 years old | WPPSI-IV (4th edition) | Supplemental | |
| Children, 6-16 years old | WISC-V (5th edition) | Supplemental | |
| Adults | Wechsler Adult Intelligence Scale | Supplemental | |
| Children and adults† | NIH Toolbox | Supplemental, highly recommended | |
| Executive functioning | Children 3-7, 8-11, and ≥12 years | Flanker Inhibitory Control/Attention (NT) | Supplemental, highly recommended |
| Dimensional Change Card Sort Test (NT) | Supplemental, highly recommended | ||
| Children ≥9 years old | Trail Making Test, parts A and B | Supplemental | |
| Children and adults, 8-89 years | Delis-Kaplan Executive Function System | Supplemental | |
| Children and adults, 7-89 years | Wisconsin Card Sort Test | Supplemental | |
| Processing speed | Children ≥7 years old | Pattern Comparison Processing Speed Test | Supplemental, highly recommended |
| Adults | Processing Speed Index | Supplemental | |
| Working memory | Children ≥7 years old | List Sorting Working Memory Test (NT) | Supplemental, highly recommended |
Pediatric PROMIS measures are available for children self-report ≥8 years old and proxy report.
Adult PROMIS measures are available.
ASCQ-Me, Adult Sickle Cell Quality of Life Measurement Information System; CHAD, children and adolescents; NRS, Numeric Rating Scale; NT, NIH Toolbox; PROMIS, Patient-Reported Outcomes Measurement Information System; VAS, Visual Analog Scale; WISC, Wechsler Intelligence Scale for Children; WPPSI-IV, Wechsler Preschool and Primary Scale of Intelligence.
Finally, the mode of PROs evaluation is another key consideration. The use of electronic approaches or e-PROs has been recommended by the e-PRO consortiums of the International Society for Quality of Life Research, the Professional Society for Health Economics and Outcomes Research, and the regulators (eg, the FDA).23-25 e-PROs have the following advantages: (1) more precise, complete, timely, and high-quality data, (2) better adherence to study protocol, (3) possible PRO reminders and real-time monitoring, (4) less recall bias, (5) fewer data entry errors, (6) leveraged computerized adaptive testing when needed, (7) integrated skip patterns for relevant questions, (8) lighter workloads for staff, (9) possible cost savings and environmental friendliness with less paper printing, and (10) high acceptability ratings from patients.5,24,26 Given the ubiquitous access to smartphones and tablets as well as the growing evidence and acceptability of mobile health interventions among SCD patients,27,28 e-PROs should be strongly considered in SCD clinical trials, whether providing patients with a dedicated device for e-PROs or allowing patients to download an app and use their own phone—an approach we call “Bring your own device,” or BYOD.
Medication adherence
Adherence to any new medication is a critical component of the success of any clinical trial in SCD, yet often little attention is given to ways to monitor and optimize adherence during the course of a study. A number of objective and subjective adherence measures can be considered in the setting of a clinical trial, which might vary based on the study design (eg, randomized controlled trial vs real-world comparative effectiveness trial). Objective measures of medication adherence include biochemical measures (eg, drug levels, biomarkers), electronic monitoring (eg, electronic pill bottles or smartphone app logs), directly observed therapy (ie, in-person or mobile), digital pills (eg, Proteus), pill counts, and pharmacy records (eg, prescription refills).29 These measures provide a more accurate view of a patient's adherence behavior but require some additional resources. In contrast, subjective measures of medication adherence include a patient's self-reported adherence, using surveys (eg, paper and pencil or electronic) or interviews, and physician assessments.29 These measures are simple, short, and inexpensive and can provide insight into potential adherence barriers; nevertheless, social desirability and recall bias are important considerations. It is worth noting that recent collaborative, multidisciplinary efforts led to the development of the PROMIS Medication Adherence Scale (PMAS), and its psychometric evaluation is underway in several ongoing trials.30 PMAS is listed in CureSCi's common data elements (CDEs). Given that both objective and subjective measures of adherence have the potential to capture various aspects of medication-taking behavior, a multimodal strategy is highly recommended.29,31 Further, in randomized controlled trials evaluating the efficacy of a new medication in SCD, it is likely advantageous to use tools that can monitor and enhance adherence behavior to optimize the clinical benefits of a given therapy for study participants. In addition, this can provide a more precise assessment of the differences in study outcomes based on exposure or adherence to either experimental drug vs placebo or active comparator.
CDEs (CureSCi and PhenX Toolkit for SCD)
One of the goals of the NHLBI-led CureSCi is to standardize data collection forms for all clinical research studies in SCD, including those with promising genetic approaches.20 In 2021 the first set of CDEs were assembled and finalized. These CDEs serve as a critical resource for SCD clinical trials in the effort to improve the efficiency of clinical studies, enhance data quality, enable data sharing, and educate young investigators on various aspects of clinical research methodology.20 Table 2 includes an overview of the proposed CDEs in CureSCi. In addition, all core data elements that are essential for the initiation of any clinical research study in SCD are included in a Start-Up Resource Listing document.20 The PhenX Toolkit for SCD is another key NHLBI-funded initiative. The goal of the PhenX Measures for SCD Research project is to help researchers better understand the pathophysiology, natural history, and treatment approaches for SCD. The PhenX Toolkit in SCD is a framework for outcomes assessment and data sharing across various SCD research projects that allows for potential comparisons across studies.19
NIH-NHLBI CureSCi CDEs (version 1.0)
| Domain . | Subdomain . | Class . | Recommendations . | ||
|---|---|---|---|---|---|
| Participant characteristics | Demographics | C | Demographics | ||
| General health history | C | Baseline abnormal hematopoiesis | Behavioral history short form | ||
| Transfusion history | Medical history | Surgical history | |||
| S | Behavioral history | Medical history supplemental elements | |||
| Hospitalization form | Sleep assessment (ped form) | ||||
| Social history | C | Social status | |||
| S | Education school questionnaire | Social determinants screen | |||
| ACEs screen children (1-17 years) | ACEs screen adults ( ≥18 years) | ||||
| Acute anemia | Chronic anemia | ||||
| Disease and treatment-related events | Asthma | S | Asthma outcomes instrument recommendations (highly recommended) | ||
| Asthma outcomes | Over-read spirometry report form | ||||
| Fertility/bone | C | Endocrine, infertility, and bone health | |||
| Lung | C | 6-minute walk test | Pulmonary function test | ||
| Lung disease assessments guidelines | |||||
| S | PROMIS dyspnea functional limitations | ||||
| Pulmonary hypertension | PROMIS dyspnea severity | ||||
| Pain | C | Acute chest syndrome | SCD-related acute painful episodes | ||
| Priapism | C | Priapism core | |||
| S | Priapism Impact Profile (PIP) | Priapism questionnaire | |||
| Renal | C | Renal function assessments | |||
| Spleen | C | Acute spleen | Chronic spleen | ||
| S | Spleen assessment from the pediatric HU phase 3 clinical trial (BABY HUG) | ||||
| Other | S | Leg ulcers | Retinopathy | Avascular necrosis | |
| Chronic malnutrition | Guidelines malnutrition identification | ||||
| Assessments and examinations | Imaging diagnostics | C | Cardiac MRI | Echocardiogram | Brain MRI |
| S | Functional MRI | Brain MRA | Imaging TCD | ||
| Laboratory tests | C | Genetic diagnostic testing | Hemoglobin variant analysis | ||
| S | Immune function form | Lab assessments-genetics/assays | |||
| Nonimaging | S | Electrocardiogram | |||
| Physical exam | S | Physical exam | NIH Stroke Scale | ||
| Vital signs | C | Vital signs and blood gases | |||
| Treatment and interventions | Drugs | C | Prior and concomitant medications | ||
| S | PROMIS Medical Adherence Scale (PMAS) | Asthma medications list | |||
| Therapies | C | Drug product | Hematopoietic cellular transplant infusion | ||
| Genetics and assays summary of recommendations | |||||
| S | Adhesion and viscosity | Apheresis | Conditioning regimen | ||
| E | Adhesion molecules assay | ||||
| Adverse events and toxicities | C | Cytopenia | Genotoxicity | Iron overload | |
| Infusion-related toxicity | Infection form | ||||
| S | Adverse events | New malignancy | Toxicity form | ||
| Cellular therapy essential data follow-up form | |||||
| Outcomes and end points | PROs | C | See details in Table 1 | ||
| Mortality | C | Death form | |||
| Domain . | Subdomain . | Class . | Recommendations . | ||
|---|---|---|---|---|---|
| Participant characteristics | Demographics | C | Demographics | ||
| General health history | C | Baseline abnormal hematopoiesis | Behavioral history short form | ||
| Transfusion history | Medical history | Surgical history | |||
| S | Behavioral history | Medical history supplemental elements | |||
| Hospitalization form | Sleep assessment (ped form) | ||||
| Social history | C | Social status | |||
| S | Education school questionnaire | Social determinants screen | |||
| ACEs screen children (1-17 years) | ACEs screen adults ( ≥18 years) | ||||
| Acute anemia | Chronic anemia | ||||
| Disease and treatment-related events | Asthma | S | Asthma outcomes instrument recommendations (highly recommended) | ||
| Asthma outcomes | Over-read spirometry report form | ||||
| Fertility/bone | C | Endocrine, infertility, and bone health | |||
| Lung | C | 6-minute walk test | Pulmonary function test | ||
| Lung disease assessments guidelines | |||||
| S | PROMIS dyspnea functional limitations | ||||
| Pulmonary hypertension | PROMIS dyspnea severity | ||||
| Pain | C | Acute chest syndrome | SCD-related acute painful episodes | ||
| Priapism | C | Priapism core | |||
| S | Priapism Impact Profile (PIP) | Priapism questionnaire | |||
| Renal | C | Renal function assessments | |||
| Spleen | C | Acute spleen | Chronic spleen | ||
| S | Spleen assessment from the pediatric HU phase 3 clinical trial (BABY HUG) | ||||
| Other | S | Leg ulcers | Retinopathy | Avascular necrosis | |
| Chronic malnutrition | Guidelines malnutrition identification | ||||
| Assessments and examinations | Imaging diagnostics | C | Cardiac MRI | Echocardiogram | Brain MRI |
| S | Functional MRI | Brain MRA | Imaging TCD | ||
| Laboratory tests | C | Genetic diagnostic testing | Hemoglobin variant analysis | ||
| S | Immune function form | Lab assessments-genetics/assays | |||
| Nonimaging | S | Electrocardiogram | |||
| Physical exam | S | Physical exam | NIH Stroke Scale | ||
| Vital signs | C | Vital signs and blood gases | |||
| Treatment and interventions | Drugs | C | Prior and concomitant medications | ||
| S | PROMIS Medical Adherence Scale (PMAS) | Asthma medications list | |||
| Therapies | C | Drug product | Hematopoietic cellular transplant infusion | ||
| Genetics and assays summary of recommendations | |||||
| S | Adhesion and viscosity | Apheresis | Conditioning regimen | ||
| E | Adhesion molecules assay | ||||
| Adverse events and toxicities | C | Cytopenia | Genotoxicity | Iron overload | |
| Infusion-related toxicity | Infection form | ||||
| S | Adverse events | New malignancy | Toxicity form | ||
| Cellular therapy essential data follow-up form | |||||
| Outcomes and end points | PROs | C | See details in Table 1 | ||
| Mortality | C | Death form | |||
ACEs: adverse childhood experiences; Class: classification; C: core; E: exploratory; HU: hydroxyurea; MRA: magnetic resonance angiography; MRI: magnetic resonance imaging; Ped: pediatric; S: supplemental; TCD: transcranial Doppler.
Publicly available at https://curesickle.org/system/files/Sickle_Cell_Disease_CDE_Highlight_Summary.pdf.
Developmental issues in pediatric trials
Some issues should be considered when planning outcomes assessment in an SCD clinical trial that involves children and adolescents, such as age-appropriate psychometric properties for PROs, patient or proxy reports or both, and developmental level.12 Children and adolescents experience many cognitive, psychological, and physical changes over time and show wide variability in their emotional, social, attentional, and intellectual levels of development.12 These differences have important implications for pediatric clinical trial design and implementation, especially in behavioral and interventional areas, where a one-size-fits-all approach is far from ideal.
Patient-centered research and stakeholder-engagement framework in hematology
Stakeholder involvement in different stages of sickle cell research has been limited, including in development, design, implementation, and dissemination. Most clinical trials in SCD have historically focused on surrogate end points, such as hospitalizations and emergency room visits, as markers of disease activity, with less emphasis on PROs or patient-centered outcomes research (PCOR). The problem with this approach is the possibility of missing what patients and other stakeholders care about the most, making clinical trial findings less relevant to many of them, at least in their view. The Patient-Centered Outcomes Research Institute (PCORI) was established by the US Congress in 2010 to address this issue. Since then, PCORI has funded several SCD projects at different stages and with a wide range of budgets and scopes (Table 3). Other government agencies also support PCOR projects with various levels of expected patient and stakeholder involvement (Table 4). The FDA Patient-Focused Drug Development initiative is another key effort to include patients' perspectives on their medical conditions, the symptoms that have the most impact on their daily lives, and the available therapies and to better understand the factors that drive treatment decisions and a willingness to participate in clinical trials.25 Moreover, PCOR highlighted important outcomes that were often overlooked by investigators, such as patient- or proxy-reported PROs, treatment satisfaction, caregiver/parent burden, work time off, transportation costs, and out-of-pocket costs.
Examples of SCD projects funded by the PCORI
| Project title . | Project type . | Budget . | Time line . |
|---|---|---|---|
| We'll Take the Village: Engaging the Community to Develop Better Health - Tier I | Pipeline to proposal | $15 000 | 2015 |
| We'll Take the Village: Engaging the Community to Better Health - Tier II | Pipeline to proposal | $25 000 | 2016-2017 |
| OMPASS: COMmunity Participation to Advance the Sickle Cell Story | Pipeline to proposal | $50 000 | 2017-2018 |
| National Sickle Cell Advocate Network (NSCAN) | Engagement award | $249 855 | 2016-2018 |
| Tennessee Sickle Cell Disease Network Project | Engagement award | $249 963 | 2014-2017 |
| Disseminating Results: Missed SCD Clinic Appointments and the Health Belief Model | Engagement award | $417 106 | 2019-2021 |
| Automating Quality and Safety Benchmarking for Children: Meeting the Needs of Health Systems and Patients | PCORnet demonstration | $1 264 641 | 2016-2021 |
| Engaging Parents of Children With SCA and Providers in Shared-Decision Making for HU | Research project | $1 962 454 | 2017-2023 |
| Comparative Effectiveness of a Decision Aid for Therapeutic Options in Sickle Cell Disease | Research project | $2 143 228 | 2013-2018 |
| PATient Navigator to rEduce Readmissions—The PArTNER Study | Research project | $2 054 803 | 2013-2018 |
| Patient-Centered Comprehensive Medication Adherence Management System to Improve Effectiveness of Disease Modifying Therapy With HU in Patients With SCD | Research project | $2 148 331 | 2013-2018 |
| Comparing Two Ways to Help Patients With SCD Manage Pain (CaRISMA) | Research project | $4 343 821 | 2019-2024 |
| Comparing Patient Centered Outcomes in the Management of Pain Between Emergency Departments and Dedicated Acute Care Facilities for Adults With SCD | Research project | $4 358 545 | 2014-2020 |
| National Pediatric Learning Health System (PEDSnet) - phase 1 | PCORnet: CDRN (phase I) | $6 459 893 | 2013-2015 |
| Mid-South Clinical Data Research Network - phase 1 | PCORnet: CDRN (phase 1) | $6 672 017 | 2013-2015 |
| Community Health Workers and Mobile Health for Emerging Adults Transitioning SCD Care (COMETS Trial) | Research project | $8 456 632 | 2017-2024 |
| Research Action for Health Network (REACHnet) | PCORnet: CDRN (phase 2) | $8 641 395 | 2015-2019 |
| Comparative Effectiveness of Peer Mentoring Versus Structured Education-Based Transition Programming for the Management of Care Transitions in Emerging Adults With SCD | Research project | $9 753 462 | 2017-2024 |
| Mid-South Clinical Data Research Network | PCORnet: CDRN (phase 2) | $10 064 128 | 2015-2019 |
| Project title . | Project type . | Budget . | Time line . |
|---|---|---|---|
| We'll Take the Village: Engaging the Community to Develop Better Health - Tier I | Pipeline to proposal | $15 000 | 2015 |
| We'll Take the Village: Engaging the Community to Better Health - Tier II | Pipeline to proposal | $25 000 | 2016-2017 |
| OMPASS: COMmunity Participation to Advance the Sickle Cell Story | Pipeline to proposal | $50 000 | 2017-2018 |
| National Sickle Cell Advocate Network (NSCAN) | Engagement award | $249 855 | 2016-2018 |
| Tennessee Sickle Cell Disease Network Project | Engagement award | $249 963 | 2014-2017 |
| Disseminating Results: Missed SCD Clinic Appointments and the Health Belief Model | Engagement award | $417 106 | 2019-2021 |
| Automating Quality and Safety Benchmarking for Children: Meeting the Needs of Health Systems and Patients | PCORnet demonstration | $1 264 641 | 2016-2021 |
| Engaging Parents of Children With SCA and Providers in Shared-Decision Making for HU | Research project | $1 962 454 | 2017-2023 |
| Comparative Effectiveness of a Decision Aid for Therapeutic Options in Sickle Cell Disease | Research project | $2 143 228 | 2013-2018 |
| PATient Navigator to rEduce Readmissions—The PArTNER Study | Research project | $2 054 803 | 2013-2018 |
| Patient-Centered Comprehensive Medication Adherence Management System to Improve Effectiveness of Disease Modifying Therapy With HU in Patients With SCD | Research project | $2 148 331 | 2013-2018 |
| Comparing Two Ways to Help Patients With SCD Manage Pain (CaRISMA) | Research project | $4 343 821 | 2019-2024 |
| Comparing Patient Centered Outcomes in the Management of Pain Between Emergency Departments and Dedicated Acute Care Facilities for Adults With SCD | Research project | $4 358 545 | 2014-2020 |
| National Pediatric Learning Health System (PEDSnet) - phase 1 | PCORnet: CDRN (phase I) | $6 459 893 | 2013-2015 |
| Mid-South Clinical Data Research Network - phase 1 | PCORnet: CDRN (phase 1) | $6 672 017 | 2013-2015 |
| Community Health Workers and Mobile Health for Emerging Adults Transitioning SCD Care (COMETS Trial) | Research project | $8 456 632 | 2017-2024 |
| Research Action for Health Network (REACHnet) | PCORnet: CDRN (phase 2) | $8 641 395 | 2015-2019 |
| Comparative Effectiveness of Peer Mentoring Versus Structured Education-Based Transition Programming for the Management of Care Transitions in Emerging Adults With SCD | Research project | $9 753 462 | 2017-2024 |
| Mid-South Clinical Data Research Network | PCORnet: CDRN (phase 2) | $10 064 128 | 2015-2019 |
Projects are organized by level of funding support from low to high.
CDRN, clinical data research network; HU, hydroxyurea; PCORnet, National Patient-Centered Clinical Research Network.
Various funding agencies, levels of patients, caregiver and stakeholder engagement, and potential benefits
| Funding agency . | Level of engagement . | Benefits . |
|---|---|---|
| Agency for Healthcare Research and Quality (AHRQ) | Desirable | Possibly beneficial |
| Center for Medicare and Medicaid Services (CMS) Innovation Center | Required | Beneficial |
| National Institutes of Health (NIH) | Potentially advantageous | Possibly beneficial |
| Patient-Centered Outcomes Research Institute (PCORI) | Expected | Beneficial |
| Pharmaceutical companies (industry) | Potentially advantageous | Beneficial |
| Professional societies and organizations | Desirable | Possibly beneficial |
| Funding agency . | Level of engagement . | Benefits . |
|---|---|---|
| Agency for Healthcare Research and Quality (AHRQ) | Desirable | Possibly beneficial |
| Center for Medicare and Medicaid Services (CMS) Innovation Center | Required | Beneficial |
| National Institutes of Health (NIH) | Potentially advantageous | Possibly beneficial |
| Patient-Centered Outcomes Research Institute (PCORI) | Expected | Beneficial |
| Pharmaceutical companies (industry) | Potentially advantageous | Beneficial |
| Professional societies and organizations | Desirable | Possibly beneficial |
Note: Level of engagement is defined as the depth and the extent to which patients, caregivers, and stakeholders are engaged in different stages of a given research project that is proposed for funding by any of the listed agencies.
PCOR projects in SCD most often focus on investigator-initiated comparative effectiveness trials evaluating different established treatment approaches. Involving patients and stakeholders in clinical trial decisions, using measures such as PROs and other outcomes, is critical to ensure the relevance of these assessments to the larger SCD community. Moreover, in 2017 the Clinical Trials Transformation Initiative (CTTI) outlined, by phases of research, different approaches to incorporate patient and stakeholder input across the continuum of a clinical trial. The CTTI also reported some examples of potential benefits for PCOR, such as enhancing the relevance of research questions to patients and stakeholders, choosing the most appropriate primary and secondary study outcomes, improving strategies for engagement, recruitment, and retention, addressing barriers to participation, keeping study burden to a minimum, and optimizing overall clinical trial experience.32
Involving stakeholders with diverse backgrounds provides the needed insight into personal experiences managing SCD, cultural considerations, adherence barriers, and potential strategies to optimize the uptake of approved therapies as well as the research approach and acceptability of study assessments.33-35 An important part of stakeholders' engagement is clarifying the scientific rationale for the choice of study design and examining the feasibility of including specific outcomes for a given trial with clear expectations of time lines and levels of involvement.33-35 Stakeholders may participate in regular study calls (eg, steering committee) and engage in detailed research discussions in which they can offer potential solutions to unexpected challenges and hurdles hindering participants' recruitment, retention, and follow-up.33-35 Stakeholders may be given the opportunity to contribute to scholarly products from the research project and participate in educational initiatives for the dissemination of research findings.
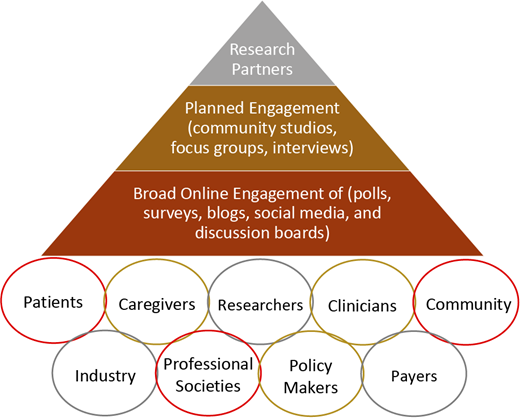
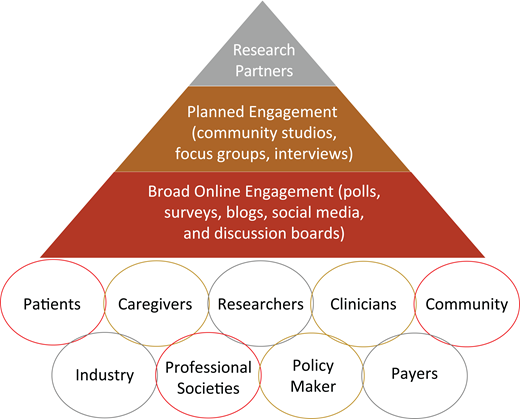
Figure 1 represents a proposed stakeholder engagement frame- work for clinical trials and research studies in hematology, including SCD. The first layer (base of the pyramid) includes the broader SCD community, with online engagement strategies such as polls, surveys, blogs, social media, and discussion boards. The second layer (middle) involves more planned stakeholder engagement for in-depth insight into different aspects of the research, with activities such as community studios, focus groups, and/or interviews. This engagement approach is essential to provide a safe environment for stakeholders to give unbiased and critical feedback based on their values, experiences, and backgrounds—especially those who have no internet access, are not active on social media, or have limited health literacy. Finally, the third layer (top) represents research partners who are driving the clinical trial or the research study, including investigators, industry partners, and selected, actively engaged patients and stakeholders. This hematology stakeholder-engagement framework (Figure 1) captures various potential stakeholders who either should or could be involved in clinical trials or research studies in hematology, with increasing levels of involvement as we move toward the top of the pyramid. Engagement of all these partners across different levels of the pyramid, including stakeholders and community organizations, is essential to ensure that clinical trials focus on meaningful outcomes for patients. This framework also facilitates collaboration and partnership between researchers and stakeholders while prioritizing outcomes of high value to patients.
Hematology stakeholder-engagement framework in clinical trials and research studies.
Hematology stakeholder-engagement framework in clinical trials and research studies.
Standardized training may be needed to ensure that different stakeholders are equipped with the skill set and adequate preparation needed to be actively involved in the trial or the project as research partners.33-35 A number of PCOR competencies and engagement principles have been reported in the literature (Table 5).36,37 Furthermore, establishing a detailed engagement plan might be helpful to outline the involvement of stakeholders across different stages of trials or research projects.
PCR competencies and principles
| A. Competencies . | ||
|---|---|---|
| I. Knowledge . | II. Skills . | III. Attitudes . |
| Cultural context | Communication | Community values |
| Knowledge about disease | Conflict management | Emotional intelligence |
| Logistical considerations | Critical thinking | General attitudes toward PCR |
| Participatory approaches | Group participation | Openness and trust |
| Agenda setting | Leadership | Personal attributes |
| Research methodology | Project management | Personal growth |
| Understanding of data | Teamwork | Professional growth |
| Understanding PCR | Prioritization | Self-reflection |
| B. Principles | ||
| I. Shared learning experience | Involvement of patients, caregivers, and other stakeholders in all aspects of the research | |
| Researchers and team members learning about PCR methodology | ||
| Training for patients, caregivers, and other stakeholders on research principles | ||
| II. Collaborations | Cultural sensitivity and mutual respect | |
| Fair compensation for effort and time | ||
| Inclusion and diversity for all project-related activities and partnerships | ||
| Planning ahead for meetings, tasks, and milestones with realistic time lines | ||
| III. Bidirectional relationships | Patient, caregivers, and other stakeholders are involved as research partners | |
| Well-defined roles and strategies informed by collaborative discussions | ||
| IV. Trustworthiness | Clear and transparent communications | |
| Shared decision-making process | ||
| Sharing information and data openly | ||
| A. Competencies . | ||
|---|---|---|
| I. Knowledge . | II. Skills . | III. Attitudes . |
| Cultural context | Communication | Community values |
| Knowledge about disease | Conflict management | Emotional intelligence |
| Logistical considerations | Critical thinking | General attitudes toward PCR |
| Participatory approaches | Group participation | Openness and trust |
| Agenda setting | Leadership | Personal attributes |
| Research methodology | Project management | Personal growth |
| Understanding of data | Teamwork | Professional growth |
| Understanding PCR | Prioritization | Self-reflection |
| B. Principles | ||
| I. Shared learning experience | Involvement of patients, caregivers, and other stakeholders in all aspects of the research | |
| Researchers and team members learning about PCR methodology | ||
| Training for patients, caregivers, and other stakeholders on research principles | ||
| II. Collaborations | Cultural sensitivity and mutual respect | |
| Fair compensation for effort and time | ||
| Inclusion and diversity for all project-related activities and partnerships | ||
| Planning ahead for meetings, tasks, and milestones with realistic time lines | ||
| III. Bidirectional relationships | Patient, caregivers, and other stakeholders are involved as research partners | |
| Well-defined roles and strategies informed by collaborative discussions | ||
| IV. Trustworthiness | Clear and transparent communications | |
| Shared decision-making process | ||
| Sharing information and data openly | ||
Historically, industry-sponsored clinical trials did not often include significant sponsors of stakeholder engagement beyond small preliminary studies, and this hesitancy may have been due to a lack of familiarity with PCOR methodology and/or the unclear return on investment of study benefits. This has changed over the last decade, and currently, a number of ongoing clinical trials in SCD have established advisory boards with different stakeholders, including patients, caregivers, and advocacy groups. Furthermore, CTTI recently proposed an approach or a conceptual financial model to evaluate the value of stakeholder engagement in clinical trials.38 CTTI's model is based on an estimated expected net present value (ENPV) incorporating cost, time, revenue, and risk as crucial business drivers.38 In an example using an oncology development program, the authors reported a potential meaningful impact of stakeholder engagement by avoiding protocol amendments and enhancing enrollment, retention, and completion of study assessments. This positive impact in pre-phase 2 and pre-phase 3 trials was associated with an increase in NPV ($62 million and $65 million, respectively) and ENPV ($35 million and $75 million), adding substantial financial value to these trials. With a hypothetical initial investment of $100 000 dedicated to optimizing stakeholder engagement strategies in a clinical trial, there may be a return on investment, in both NPV and ENPV, that exceeds the investment 500-fold.38
COVID-19 pandemic and clinical trials
More recently, the COVID-19 pandemic has led to disruptions in our daily routines, personally and professionally, in different ways, including interrupting the execution of clinical trials.39,40 Most institutions stopped new enrollments and allowed the continuation of interventional trials when there were potential clinical benefits for the participants; however, many reported challenges related to delayed and rescheduled study visits, procedures, and assessments and overall difficulty reaching patients.40 Many of these vulnerable patients were at risk from exposure to COVID-19, and some were intentionally avoiding health care facilities or obeying stay-at-home-orders.39 This situation highlights the need for considerable adaptability using a hybrid strategy in designing future clinical trials to complete all planned study procedures.40 Some proposed strategies include (1) prioritizing primary outcomes over exploratory ones; (2) alternating strategies for outcomes assessment; (3) collecting remote data using phone interviews or online tools; (4) obtaining phone numbers and e-mail addresses for patients and 3 family members or friends to ensure maintained contact; (5) using different methods to contact participants, including text messaging, phone calls, e-mail, or social media; (6) employing telemedicine; (7) arranging home visits by health care workers wearing personal protective equipment; (8) allowing study medications to be taken at home; (9) making use of concierge services; (10) using local instead of central lab facilities; and (11) escalating incentives.12,40,41 Other approaches should be considered to achieve the highest level of retention and adherence to study interventions, and the statistical analysis plan for primary and secondary outcomes should be revised to reflect any expected meaningful effects or influence relate to the pandemic.41 For behavioral clinical trials, efforts should be directed to leverage widely available and user-friendly online surveys, databases, and web-based applications to optimize e-consenting and remote enrollment, with completion of all study assessments and delivery of study interventions conducted virtually.
CLINICAL CASE (continued)
The patient has been actively involved as an advisory board member in a few investigator-initiated trials at our institution. He had significant input in these trials, such as feedback on consent and assent forms and the selection of HRQOL domains that are more relevant to SCD patients. In addition, he was able to provide critical insight to inform our efforts to develop and refine a mobile app for adolescents and young adults as a behavioral intervention to monitor and improve medication adherence and HRQOL. His passion for medicine was reinforced, and he is determined to become a hematologist caring for adult SCD patients.
Conclusions
Several clinical trial considerations in SCD are key to success. PROs are significantly impaired among SCD patients and should be included in all clinical trials. A multimodal strategy is highly recommended to assess adherence outcomes. Developmental differences among children and adolescents with SCD should inform the study approach. Engaging patients and stakeholders in SCD clinical trials in meaningful ways is critical to ensure that their voices are heard and that study designs and outcomes are relevant to them, which is essential for future successful dissemination and implementation. This engagement is a dynamic, bidirectional commitment that is mutually beneficial to all involved partners, and it has the potential to improve health outcomes in the larger population of pediatric and adult SCD patients. Stakeholders can play a major role in closing the gap between data-heavy research findings from clinical trials and their implications in clinical practice. It is critical to keep stakeholders engaged and interested throughout the research process, and the sustainability of this partnership is key. Evaluating the financial value of stakeholder engagement is important to estimate the potential cost savings for SCD clinical trials, which might be of considerable financial value. Timely adaptations to address unusual circumstances, such as the COVID-19 pandemic, are often crucial.
Acknowledgments
This project was supported by a grant (K23HL150232, PI: Badawy) from the National Heart, Lung, and Blood Institute of the National Institutes of Health. The content is solely the responsibility of the author and does not necessarily represent the views of the National Institutes of Health.
I would like to thank Drs. Robert Liem, Jane Holl, David Cella, Tonya Palermo, and Alexis Thompson for their advice while writing this article.
Conflict-of-interest disclosure
Sherif M. Badawy: no competing financial interests to declare.
Off-label drug use
Sherif M. Badawy: nothing to disclose.