Abstract
Innovations in immuno-oncology for lymphomas have outpaced therapeutic developments in any other cancer histology. In the 1990s, rituximab, a CD20 monoclonal antibody, drastically changed treatment paradigms for B-cell non-Hodgkin lymphomas (B-NHLs). In parallel, the concept that T cells could be genetically reprogrammed and regulated to address tumor cell evasion was developed. Twenty years later, this concept has materialized—3 customized engineered CD19 chimeric antigen receptor T-cell (CART) constructs have been embraced as third-line therapies and beyond for aggressive B-NHL. Responses with CARTs are durable in 30% to 40% of patients, with consistent results in older patients, primary refractory disease, high-grade B-cell lymphoma, and patients with concurrent secondary central nervous system disease, all features historically associated with poorer outcomes. Challenges associated with the administration of CARTs include cumbersome and time-consuming manufacturing processes, toxicities, and cost, not to mention a substantial risk of relapse. Fortunately, as our understanding of how to manipulate the immune system to achieve full antitumor potential has grown, so has the rapid development of off-the-shelf immunotherapies, with CD20/CD3 bispecific antibodies standing out above all others. These agents have shown promising activity in aggressive B-NHL and have the potential to circumvent some of the challenges encountered with customized engineered products. However, toxicities remain substantial, dosing schedules intensive, and experience limited with these agents. Novel customized and off-the-shelf therapeutics as well as rational combinations of these agents are underway. Ultimately, growing experience with both customized engineered and off-the-shelf immunotherapies will provide guidance on optimal methods of delivery and sequencing.
Learning Objectives
Understand the strengths and limitations of CD19 CARTs as a customized engineered product vs off-the-shelf immunotherapies such as bispecific CD20/CD3 antibodies for the treatment of aggressive B-NHL
Review clinical efficacy and safety data for CD19 CARTs and bispecific CD20/CD3 antibodies
Optimize a therapeutic algorithm for relapsed/refractory aggressive B-cell lymphoma with the inclusion of CARTs and off-the-shelf immunotherapies
CLINICAL CASE: PART 1
A 65-year-old male presented with lower back and flank pain, fevers, and weight loss. Magnetic resonance imaging of the lumbar spine showed a paraspinal mass. Positron emission tomography–computed tomography scans showed diffuse lymphadenopathy with bone marrow involvement and highest uptake in bulky retroperitoneal lymph nodes and the paraspinal mass. A core biopsy of the paraspinal mass confirmed high-grade B-cell lymphoma with dual rearrangements of MYC and BCL2 (also known as double-hit lymphoma). The patient was treated with six cycles of DA-EPOCH-R and achieved a complete metabolic response at the completion of therapy. Twelve months later, the patient relapsed. He was treated with two cycles of R-ICE with complete response (CR) and consolidated with an autologous stem cell transplantation (ASCT). Unfortunately, scans 3 months post-ASCT demonstrated disease recurrence. He was referred to our institution to discuss treatment options for his second relapse.
Introduction
To date, salvage high-dose chemotherapy with ASCT remains the standard second-line treatment for relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) regardless of underlying high-risk biologic features.1 However, few patients are cured with this intensive approach, and applicability is limited by comorbidities, advanced age, and/or chemotherapy-insensitive disease.2,3 In the era predating the use of immunotherapies, patients with refractory disease or relapse within 12 months of ASCT had dismal outcomes. In the SCHOLAR-1 multicenter retrospective study, the objective response rate (ORR) to the next line of therapy was 26% (CR, 7%), with a median overall survival (OS) rate of 6.3 months in such patients.2
Fortunately, the treatment landscape has rapidly evolved for R/R DLBCL, with customized engineered immunotherapies—more specifically, CD19 chimeric antigen receptor T cells (CARTs)—and off-the-shelf immunotherapies taking center stage.
CARTs therapy
CARTs are autologous T cells that have been genetically reengineered using viral transduction to express a CAR that targets a specific tumor antigen. For B-cell lymphomas, the CAR includes an extracellular moiety derived from an anti-CD19 single-chain variable fragment for antigen recognition and intracellular domains including a costimulatory domain, CD28 or 41BB, in tandem with a CD3ζ-activating domain. The US Food and Drug Administration (FDA) has approved three constructs for the treatment of R/R aggressive B-cell lymphomas, including DLBCL, high-grade B-cell lymphoma, transformed follicular lymphoma, and primary mediastinal B-cell lymphoma, after 2 prior lines of systemic therapy, and they show high response rates with durable remissions. The first construct, approved in 2017, for this population was axicabtagene ciloleucel (axi-cel), containing a CD28 costimulatory domain. The multicenter phase 1/2 ZUMA-1 trial evaluated axi-cel and had the longest follow-up of all CART trials of greater than 4 years (n = 101); responses were durable, with a median OS of 25.8 months and a 4-year OS rate of 44%.4,5 The phase 2 JULIET study of tisagenlecleucel demonstrated that the efficacy is comparable for this 41BB-containing CART, with a more favorable toxicity profile.6,7 The TRANSCEND study, the largest CART trial, evaluated the 41BB construct lisocabtagene maraleucel (liso-cel), manufactured uniquely through the separate transduction, expansion, and administration of equal target doses of CD4+ and CD8+ CARTs. This trial established the applicability of CARTs to a broader population, including patients with prior allogeneic stem cell transplantation and those with secondary central nervous system involvement.8
CLINICAL CASE: PART 2
The patient was enrolled in the TRANSCEND trial with liso-cel. The patient underwent leukapheresis for T cells followed by bridging therapy with rituximab and high-dose steroids for rapidly progressive disease. Four weeks after leukapheresis, manufacturing was complete. Lymphodepleting chemotherapy with fludarabine at 30 mg/m2/d intravenously (IV) and cyclophosphamide at 300 mg/m2/d IV for 3 days was administered, followed by infu- sion of liso-cel at a dose of 100 × 106 cells. He experienced grade 2 cytokine release syndrome (CRS) with fever, mild hypotension requiring intravenous fluids, and mild hypoxia 6 days after the administration of CART and was managed effectively with the IL-6 receptor antagonist tocilizumab. This resolved within 5 days. He had no signs of immune effector cell-associated neurologic syndrome (ICANS). Restaging scans 30 days and 90 days post-CART demonstrated a complete metabolic response.
Limitations of CARTs and the emergence of off-the-shelf immunotherapies
Although CARTs have changed the treatment paradigm for R/R aggressive B-cell lymphomas, the therapeutic has its limitations. First, CARTs have to be engineered for each individual patient, with a potential for logistical delays from the time of patient identification to CART infusion as well as a risk of manufacturing failure. Second, significant toxicities are associated with CART therapy that include CRS and ICANS. Such toxicities may preclude patients with certain comorbid conditions. Most importantly, although CARTs offer durable responses in some, 60% to 70% of patients will still relapse.4,7,8
Since the approval of CARTs for aggressive B-cell therapy, the FDA has approved a wave of immunotherapies (with or without chemotherapy) and targeted approaches for R/R DLBCL. These include the combinations of anti-CD19 monoclonal antibody (mAb) tafasitamab and lenalidomide,9 anti-CD79b antibody-drug conjugate (ADC) polatuzumab vedotin with bendamustine and rituximab,10 and monotherapy with anti-CD19 ADC loncastuximab tesirine,11 or selinexor, an orally available selective inhibitor of nuclear export.12 Each of these options should be considered for patients who are either poor candidates for CART or who relapse after CART, although data supporting these applications are limited.
In addition to the mAbs and ADCs listed above, a number of other off-the-shelf immunotherapies have been evaluated in aggressive lymphomas (Figure 1).13-20 Bispecific T-cell-engaging antibodies (BsAbs) have emerged as a novel class of off-the-shelf immunotherapies with clear efficacy in R/R aggressive B-cell lymphomas, including for those patients relapsing after CART therapy. BsAbs are designed to simultaneously bind to CD3 epsilon, a component of the T-cell receptor complex, and CD20 on the cell surface of malignant B cells, creating an “immune synapse” that redirects T-cell cytotoxic activity against malignant B cells. Four agents evaluated in R/R aggressive B-cell lymphomas include mosunetuzumab, epcoritamab, glofitamab, and odronextamab.15-19 Unlike their predecessor blinatumomab, a CD19/CD3 bispecific T-cell engager, CD20/CD3 BsAbs have a longer half-life, allowing for greater ease of administration, and appear to offer higher response rates in R/R aggressive B-cell lymphomas.20 Furthermore, CD20/CD3 BsAbs have the potential to circumvent the shortcomings of CARTs while providing high rates of response.20
Evolving landscape for customized engineered and off-the-shelf immunotherapies in aggressive B-NHL. ADCC: antibody-dependent cell cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; BiTE, bispecific T-cell engager; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
Evolving landscape for customized engineered and off-the-shelf immunotherapies in aggressive B-NHL. ADCC: antibody-dependent cell cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; BiTE, bispecific T-cell engager; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
Herein, the focus is on comparing and contrasting features of CARTs and BsAbs as prototypes of custom engineered vs off-the-shelf immunotherapies, respectively, with the strengths and limitations of each modality outlined (Table 1).
Summary of clinical trials for CD19 CARTs and bispecific antibodies
| . | Axi-cel4,34 . | Tisa-cel6,7 . | Liso-cel8 . | Mosunetuzumab15 . | Epcoritamab16 . | Glofitamab17,18 . | Odronextamab19 . |
|---|---|---|---|---|---|---|---|
| Structure | CD19/CD3z/CD28 | CD19/CD3z/41BB | CD19/CD3z/41BB | Full-length humanized, IgG1 CD20/CD3 BsAb | CD20/CD3 BsAb | CD20/CD3 BsAb with 2:1 molecular configuration with bivalent binding to CD20 and monovalent binding to CD3 | Hinge-stabilized, fully human IgG4 CD20/CD3 BsAb |
| Route of administration | IV | IV | IV | IV or SC | SC | IV | IV |
| Trial/NCT | ZUMA-1 | JULIET | TRANSCEND | NCT02500407 | NCT03625037 | NCT03075696 | NCT02290951 |
| N = treated (aggressive histology) | 101 | 111 | 269 | 141 | 45 | 127 | 71 |
| Median lines prior therapy (range) | 3 (2-4) | Median not reported; range 1-6 | 3 (2-4) | 3 (1-14); 23 pts with prior CART | 3 (1-6); 6 pts with prior CART | 3 (1-13) | 3 (1-11); 29 pts with prior CART |
| Dosing | 2 × 106/kg × 1 dose | 0.6-6.0 × 108 × 1 dose | 0.5-1.0 × 108 × 1 dose | Step-up dosing on days 1, 8, and 15 of cycle 1, followed by fixed doses on day 1 of each 21-day cycle for up to 17 cycles | Flat dose in 28-day cycles (q1w: cycles 1–2; q2w: cycles 3–6; q4w thereafter) until disease progression or unacceptable toxicity | Step-up dosing on cycle (C) 1, day (D) 1 and 8 and then at the target dose from C2D1, q 3 weeks for up to 12 cycles | Step-up dose consisting of initial dose at week (W) 1, an intermediate dose at W2, and thereafter a fixed weekly dose until W12 followed by maintenance q2w |
| Median time to manufacture | Leukapheresis → infusion: 17 days | Enrollment → infusion: 21-day target | Leukapheresis → infusion: 24-day target | Immediate off-the-shelf immunotherapy | |||
| Median follow-up | 4 years | 40.3 months | 18.8 months | Not reported | 8.3 months | 13.5 months | 3.9 months |
| ORR (%) | 82 | 52 | 73 | 35 | 67 | 71 | • Without prior CART (n = 11): ORR 55% • Post-CART (n = 24): ORR 33% |
| CR (%) | 54 | 40 | 53 | 19 | 33 | 64 | • Without prior CART (n = 11): CR 55% • Post-CART (n = 24): CR 21% |
| Median DOR (mo) | 8.1 | Not reached | Not reached | Median time from first CR: 8.8 | Not yet mature | 5.5 | • Without prior CART: 10.3 • Post-CART: 2.8 |
| Median PFS (mo) | 5.8 | <6 months; not reached in CRs | 6.8 | Not yet mature | Not yet mature | 2.9 | Not yet mature |
| Median OS (mo) | 25.8 | 11.1 | 21.1 | Not yet mature | Not yet mature | Not yet mature | Not yet mature |
| CRS (%) | All grades: 93 ≥G3: 13 | All grades: 58 ≥G3: 22 | All grades: 42 ≥G3: 2 | All grades: 28 ≥G3: 1.4 | All grades: 58 ≥G3: 0 | All grades: 50 ≥G3: 3.5 | All grades: 62 ≥G3: 7 |
| ICANS (%) | All grades: 64 ≥G3: 28 | All grades: 21 ≥G3: 12 | All grades: 30 ≥G3:10 | All grades: 1.4 ≥G3: 0 | All grades: 6 ≥G3: 3 | All grades: 5.3 ≥G3: 0 | All grades: not reported ≥G3: 2.3 |
| Population applicability/considerations | DLBCL/PMBCL/TFL | DLBCL/TFL | DLBCL/PMBCL/transformation from indolent lymphoma; FL grade 3B; preferred for systemic + concurrent secondary CNS disease or post-alloSCT | Aggressive B-cell lymphoma prior to and post-CART | Aggressive B-cell lymphoma prior to and post-CART | Aggressive B-cell lymphoma | Aggressive B-cell lymphoma prior to and post-CART |
| . | Axi-cel4,34 . | Tisa-cel6,7 . | Liso-cel8 . | Mosunetuzumab15 . | Epcoritamab16 . | Glofitamab17,18 . | Odronextamab19 . |
|---|---|---|---|---|---|---|---|
| Structure | CD19/CD3z/CD28 | CD19/CD3z/41BB | CD19/CD3z/41BB | Full-length humanized, IgG1 CD20/CD3 BsAb | CD20/CD3 BsAb | CD20/CD3 BsAb with 2:1 molecular configuration with bivalent binding to CD20 and monovalent binding to CD3 | Hinge-stabilized, fully human IgG4 CD20/CD3 BsAb |
| Route of administration | IV | IV | IV | IV or SC | SC | IV | IV |
| Trial/NCT | ZUMA-1 | JULIET | TRANSCEND | NCT02500407 | NCT03625037 | NCT03075696 | NCT02290951 |
| N = treated (aggressive histology) | 101 | 111 | 269 | 141 | 45 | 127 | 71 |
| Median lines prior therapy (range) | 3 (2-4) | Median not reported; range 1-6 | 3 (2-4) | 3 (1-14); 23 pts with prior CART | 3 (1-6); 6 pts with prior CART | 3 (1-13) | 3 (1-11); 29 pts with prior CART |
| Dosing | 2 × 106/kg × 1 dose | 0.6-6.0 × 108 × 1 dose | 0.5-1.0 × 108 × 1 dose | Step-up dosing on days 1, 8, and 15 of cycle 1, followed by fixed doses on day 1 of each 21-day cycle for up to 17 cycles | Flat dose in 28-day cycles (q1w: cycles 1–2; q2w: cycles 3–6; q4w thereafter) until disease progression or unacceptable toxicity | Step-up dosing on cycle (C) 1, day (D) 1 and 8 and then at the target dose from C2D1, q 3 weeks for up to 12 cycles | Step-up dose consisting of initial dose at week (W) 1, an intermediate dose at W2, and thereafter a fixed weekly dose until W12 followed by maintenance q2w |
| Median time to manufacture | Leukapheresis → infusion: 17 days | Enrollment → infusion: 21-day target | Leukapheresis → infusion: 24-day target | Immediate off-the-shelf immunotherapy | |||
| Median follow-up | 4 years | 40.3 months | 18.8 months | Not reported | 8.3 months | 13.5 months | 3.9 months |
| ORR (%) | 82 | 52 | 73 | 35 | 67 | 71 | • Without prior CART (n = 11): ORR 55% • Post-CART (n = 24): ORR 33% |
| CR (%) | 54 | 40 | 53 | 19 | 33 | 64 | • Without prior CART (n = 11): CR 55% • Post-CART (n = 24): CR 21% |
| Median DOR (mo) | 8.1 | Not reached | Not reached | Median time from first CR: 8.8 | Not yet mature | 5.5 | • Without prior CART: 10.3 • Post-CART: 2.8 |
| Median PFS (mo) | 5.8 | <6 months; not reached in CRs | 6.8 | Not yet mature | Not yet mature | 2.9 | Not yet mature |
| Median OS (mo) | 25.8 | 11.1 | 21.1 | Not yet mature | Not yet mature | Not yet mature | Not yet mature |
| CRS (%) | All grades: 93 ≥G3: 13 | All grades: 58 ≥G3: 22 | All grades: 42 ≥G3: 2 | All grades: 28 ≥G3: 1.4 | All grades: 58 ≥G3: 0 | All grades: 50 ≥G3: 3.5 | All grades: 62 ≥G3: 7 |
| ICANS (%) | All grades: 64 ≥G3: 28 | All grades: 21 ≥G3: 12 | All grades: 30 ≥G3:10 | All grades: 1.4 ≥G3: 0 | All grades: 6 ≥G3: 3 | All grades: 5.3 ≥G3: 0 | All grades: not reported ≥G3: 2.3 |
| Population applicability/considerations | DLBCL/PMBCL/TFL | DLBCL/TFL | DLBCL/PMBCL/transformation from indolent lymphoma; FL grade 3B; preferred for systemic + concurrent secondary CNS disease or post-alloSCT | Aggressive B-cell lymphoma prior to and post-CART | Aggressive B-cell lymphoma prior to and post-CART | Aggressive B-cell lymphoma | Aggressive B-cell lymphoma prior to and post-CART |
alloSCT, allogeneic stem cell transplantation; DOR, duration of response; FL: follicular lymphoma; IgG: immunoglobulin G; PMBCL, primary mediastinal B-cell lymphoma; TFL, transformed follicular lymphoma; tisa-cel, tisagenlecleucel.
Off-the-shelf immunotherapies vs CARTs: ease of administration
CARTs are customized products engineered for each individual patient. The manufacturing process has been refined to ensure that the end products meet specifications for viability and composition.21 However, despite every precaution taken, successful manufacture is not guaranteed—the final product may not meet specifications or may entirely fail to generate. Furthermore, the process can be lengthy.
For both the ZUMA-1 and JULIET trials, a minimum absolute lymphocyte count was required, which may be prohibitive in heavily pretreated patients. Rates of manufacturing failure ranged from 1% to 7%. Conversely, in the TRANSCEND trial, despite laxity in eligibility with no requirement for minimum absolute lymphocyte count, manufacturing failure occurred in only 2 patients.4,7,8
Factoring in the time from patient identification, leukapheresis, and manufacturing to eventual administration, with expected logistical delays along the way, the turnaround time for CARTs is unpredictable and typically greater than 3 to 4 weeks. Accordingly, CARTs may not be feasible for patients with rapidly progressive disease. Bridging therapy is often needed during the manufacturing period, with limited guidance as to which therapies are most effective for this purpose. Allogeneic off-the- shelf CARTs would solve this particular issue, leading to timely accessibility, but their development remains in infancy.22
Bispecific antibodies as an off-the-shelf option can be used without ex vivo T-cell preparation, allowing immediate treatment. However, unlike CARTs, treatment duration with BsAbs is prolonged, creating issues for accessibility and ease of administration. These agents are administered every 1 to 4 weeks either IV or subcutaneously, with shorter intervals early in the treatment course, and may be pursued for 12 cycles and beyond, depending on the agent used and the durability of response.15-17,19
Off-the-shelf immunotherapies vs CARTs: toxicities and application in vulnerable populations
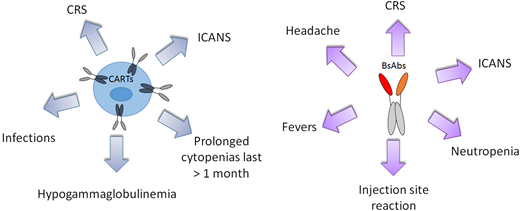
Side effects associated with CARTs include CRS and ICANS, prolonged cytopenia, and impairment of humoral immunity with increased risk of infection (Figure 2). CRS is the most common and has been described in 42% to 93% of patients, with grade ≥3 events occurring in 2% to 22% of patients.4,7,8 The pathophysiology of CRS has been attributed to an upsurge in cytokines and chemokines upon activation of CARTs after engagement with malignant cells with IL-6 as a primary driver.4,23 Rates of ICANS range from 21% to 64%, with grade ≥3 events described in 10% to 28%.4,7,8 The mechanism of ICANS is elusive but has been associated with high cytokine levels as well.4
CART or BsAb treatment-related adverse effects of interest with an incidence of ≥10% and ≥5%, respectively.
CART or BsAb treatment-related adverse effects of interest with an incidence of ≥10% and ≥5%, respectively.
Variability in the presentation, prevalence, and intensity of CRS and ICANS across CART constructs has been attributed to patient-related, disease-related, and product-specific factors. For product-specific factors, differences in T-cell expansion and proliferation kinetics conferred by the CD28 vs 41BB costimulation domains may explain the higher CRS and ICANS rates associated with axi-cel.4,7,8
Although the prevalence of CRS and ICANS is high with CARTs, these toxicities are effectively managed. Early mitigation strategies with anti-IL-6 therapy and/or steroids have improved the safety profile of CARTs without having an impact on CART function, efficacy, or persistence.24,25 Real-world data with CARTs and the movement toward outpatient CART administration are testaments to successful toxicity mitigation.26,27 For example, data captured from real-world experience with axi-cel showed that 43% of patients would not have met eligibility criteria for the registrational ZUMA-1 trial because of comorbidities. Despite the inclusion of older and sicker patients, toxicities and clinical outcomes were similar for these patients compared to outcomes in the pivotal trial.26 Additionally, the feasibility of CART administration has been demonstrated more formally in the older adult and unfit population. The phase 2 PILOT trial was the first to assess the safety and efficacy of liso-cel as a second-line therapy for transplant-ineligible patients with R/R aggressive lymphoma. This included patients ≥70 years of age or with impaired organ function including moderate cardiomyopathy (left ventricular ejection fraction ≥40%-50%) and/or pulmonary impairment (DLCO ≤60% but blood-oxygen saturation ≥ 92%).Rates of CRS, ICANS, and response were comparable to those of the TRANSCEND study with liso-cel in third line therapy and beyond.28
Taken collectively, such toxicities should not preclude the use of CARTs in older patients. Based on clinical trial and real-world experience, both CD28 and 41BB constructs appear to be reasonable options for older adult and/or frail patients or those who have comorbid conditions, recognizing the differences in toxicity profiles for these constructs. Practically, however, one might favor the use of 41BB constructs in patients with underlying neurologic comorbidities, given the lower rates of ICANS associated with these constructs. Additionally, the growing practice of outpatient administration of CARTs with preference for 41BB constructs in this context is likely to lead to wider access and utilization of this therapeutic.
Bispecific antibodies can also produce CRS and neurologic toxicities, seemingly at lower frequencies and severity, although toxicity data are emerging and not yet mature. Additional toxicities described for BsAbs with an incidence of ≥10% include pyrexia, reaction at the injection site, headaches, and cytopenia (Figure 2). Rates of CRS for BsAbs range from 28% to 62%, with grade ≥3 events in 0% to 7% of patients, and tend to dissipate after 1 to 2 cycles of administration.15-17,19 CRS appears to be driven by IL-6 with BsAbs as well and is managed effectively with tocilizumab if needed.15-17,19 Rates of ICANS for BsAbs are not clearly reported; rates of grade ≥3 events range from 0% to 3%.15-17,19 Clinical and biologic predictors of CRS with BsAbs remain unclear. For mosunetuzumab, aggressive disease histology and a baseline elevated C-reactive protein appear to predict greater neurologic toxicity.29
Like CARTs, BsAbs have demonstrated feasibility in older patients and patients with comorbid conditions. As a single agent, mosunetuzumab was evaluated as frontline therapy in 19 patients aged ≥80 years or 60 to 79 years with functional impairments or comorbid conditions precluding the use of full-dose chemo-immunotherapy and demonstrated efficacy with remarkable tolerability.30
Furthermore, a number of strategies are being employed to optimize the dosing and tolerability of BsAbs. For example, step-up dosing for BsAbs is routine. The subcutaneous formulation of mosunetuzumab was shown to reduce the severity of CRS; CRS events were mild, transient, and delayed in onset and required minimal intervention with no grade ≥3 events reported.31 With glofitamab, the use of a cytoreductive anti-CD20 mAb and step-up dosing have been shown to mitigate CRS.17,18 Although it is unclear which strategy is most effective in decreasing toxicity, collectively, these strategies may allow for higher-dose drug administration and foreseeably improved response rates with BsAbs.
Off-the-shelf immunotherapies vs CARTs: efficacy and sequencing
For all 3 FDA-approved CD19 CARTs, the patterns and durability of response are similar (Table 1). Response rates range from 52% to 82%, with CR rates of 40% to 54%.4,7,8 Long-term follow-up data for CARTs suggest that these responses are durable, particularly for patients in CR. For the ZUMA-1 study, the median follow-up is now greater than 4 years, the median OS rate is 25.8 months, and the 4-year OS rate is 44% (n = 101).5 In the JULIET study, the median follow-up is 40.3 months (n = 115). Although the median OS was 11.1 months, the progression-free survival (PFS) rates at 24 and 36 months were 33% and 31%, suggesting that few patients who achieve a CR will relapse beyond 24 months.6 For the TRANSCEND study, with a shorter follow-up, median PFS and OS were 6.8 and 21.1 months, respectively.8
For BsAbs, response rates in aggressive lymphomas range from 33% to 71%, with CRs of 19% to 64%, and may depend on prior CART exposure. However, experience with BsAbs is still limited; follow-up is short and data on durability of response are lacking. Similarly, the impact of these agents on survival compared to CART is not clear. What is clear is that these agents do maintain their effects in patients with relapse after CART (Table 1).
For instance, results for mosunetuzumab in 30 patients who had received prior CART therapy were highlighted, and 18 patients were evaluated for response. In this subgroup, mosunetuzumab led to CART expansion and generated an ORR of 39% and a CR rate of 22% with long-lasting responses and tolerable safety.15 Similarly, odronextamab was evaluated in patients post-CARTs (n = 24) and demonstrated encouraging activity with an ORR of 33% and a CR rate of 21%.19
With clinical experience of sequencing strategies in R/R DLBCL essentially limited to CARTs as a third-line therapy followed by BsAbs, this sequence remains favored (Figure 3). One could consider CD20/CD3 BsAbs as a bridge to CARTs in patients with rapidly progressive disease or even as a bridge to allogeneic stem cell transplantation. Given the potential for T-cell exhaustion with programmed cell death ligand 1 upregulation in target cells seen with BsAbs, whether utilizing BsAbs prior to leukapheresis could have an impact on the quality of harvested T cells for CART manufacture is questionable.32 As both CARTs and BsAbs make their way to earlier lines of therapy, how best to sequence these agents will continue to evolve.30,33
Algorithm for preferred and alternative treatment options for R/R DLBCL that includes customized engineered and off-the-shelf immunotherapies.
Algorithm for preferred and alternative treatment options for R/R DLBCL that includes customized engineered and off-the-shelf immunotherapies.
Off-the-shelf immunotherapies vs CARTs: efficacy in high-risk populations
Ahead of off-the-shelf immunotherapies, CARTs are being evaluated in several patient subsets with poor prognoses and high unmet needs. First, patients with double-expressor or high-grade B-cell lymphomas with rearrangements of MYC and BCL2 and/or BCL6, also known as double- and triple-hit lymphomas, were included in pivotal trials for all 3 FDA-approved CARTs. Response rates in these subsets were similar to those seen for all patients.7,8,34
Trials are also underway for CARTs in patients with intrinsic chemotherapy resistance. Both the ZUMA-7 (NCT03391466) and TRANSFORM (NCT03575351) trials have compared axi-cel or liso-cel, respectively, vs ASCT as second-line therapy for patients with primary refractory disease or relapse within 12 months of frontline therapy, with mature results eagerly awaited. The ZUMA-12 study is evaluating axi-cel in patients with large B-cell lymphoma who had either high-grade lymphoma or an international prognostic index score ≥3 and a positive interim positron emission tomography after 2 cycles of R-CHOP/R-CHOP-like therapy.35 Thus far, of 12 response-evaluable patients the ORR is 92%, with a CR rate of 75%. Longer-term follow-up of these trials will provide greater insight into the benefit of CARTs in primary refractory patients.
Experience with off-the-shelf immunotherapies is limited in these high-risk populations. In fact, for the L-MIND study, which evaluated the CD19 humanized antibody tafasitamab with lenalidomide, patients with primary refractory disease and/or high-grade B-cell lymphomas with rearrangements of MYC and BCL2 and/or BCL6 were excluded.9 Data for BsAbs remain immature, with inadequate analyses of subset populations with high-grade B-cell lymphoma and/or refractory disease. It is anticipated that this information will become more readily available with ongoing follow-up.
CLINICAL CASE: PART 3
Surveillance scans in our patient were conducted 180 days post-CART with concern for relapse in the retroperitoneum. A biopsy of a retroperitoneal node identified CD19− relapse of disease. The patient was subsequently offered a clinical trial with a novel CD20/CD3 bispecific antibody.
Off-the-shelf immunotherapies vs CARTs: mechanisms of resistance and future directions
Predictors of response and relapse with regard to both engineered products and off-the-shelf therapies remain elusive. For CARTs, it is clear that relapses can occur despite the persistence of reengineered T cells. CART exhaustion stemming from an immunosuppressive tumor microenvironment (TME) and host systemic inflammation along with intrinsic T-cell dysfunction may explain this phenomenon.6,36 These findings suggest opportunities for combinations with immuno-oncological agents such as checkpoint inhibitors, tyrosine kinase inhibitors, and immunomodulatory agents that may reinvigorate persistent CARTs, although this runs the risk of increased toxicity.37
Given that BsAbs also rely on the patient's own T cells, one expects T-cell exhaustion and dysfunction to be relevant mechanisms of resistance to said therapeutics as well.
Allogeneic CARTs afford the opportunity to minimize the contribution of T-cell dysfunction to relapse risk but may not be able to overcome the immunosuppressive effects of the TME (Table 2). Limitations associated with this modality also include a risk of increased immune toxicities, graft-versus-host disease, and possible rejection.21 Allogeneic natural killer (NK) CARs represent another immunocellular platform with several advantages over allogeneic CARTs—they can be selected from non-HLA related healthy donors, will not cause graft-versus-host disease, and are less prone to the inhibitory effects of the TME (Table 2).38 Similarly, bispecific dual-affinity retargeting (DART) proteins designed to target LAG3 and programmed cell death 1 may better overcome the negative effects of the TME and have demonstrated responses in CART-treated and naive patients.39 As an added benefit, all aforementioned products represent off-the-shelf options.
Novel dual-targeted autologous CARTs, allogeneic CARTs, and NK CARs currently in clinical trial
| Clinicaltrials.gov identifier . | Sponsor . | Phase . | Target . | Class . |
|---|---|---|---|---|
| Dual-targeted autologous CARTs | ||||
| NCT04186520 | Medical College of Wisconsin | 1/2 | CD19/CD20 | Autologous dual-target CART |
| NCT03287817 | Autolus Limited | 1/2 | CD19/CD22 | Autologous dual-target CART followed by limited duration of anti-PD-1 antibody pembrolizumab |
| Allogeneic CARTs | ||||
| NCT03939026 | Allogene Therapeutics | 1/2 | CD19 | Single-target allogeneic CART |
| NCT03398967 | Chinese PLA General Hospital | 1/2 | CD19/CD20 or CD19/CD22 | Dual-target allogeneic CART |
| NK CARs | ||||
| NCT03056339 | M. D. Anderson Cancer Center | 1/2 | CD19 | Cord blood CD19 NK CAR |
| NCT03774654 | Baylor College of Medicine | 1 | CD19 | NK CAR |
| Clinicaltrials.gov identifier . | Sponsor . | Phase . | Target . | Class . |
|---|---|---|---|---|
| Dual-targeted autologous CARTs | ||||
| NCT04186520 | Medical College of Wisconsin | 1/2 | CD19/CD20 | Autologous dual-target CART |
| NCT03287817 | Autolus Limited | 1/2 | CD19/CD22 | Autologous dual-target CART followed by limited duration of anti-PD-1 antibody pembrolizumab |
| Allogeneic CARTs | ||||
| NCT03939026 | Allogene Therapeutics | 1/2 | CD19 | Single-target allogeneic CART |
| NCT03398967 | Chinese PLA General Hospital | 1/2 | CD19/CD20 or CD19/CD22 | Dual-target allogeneic CART |
| NK CARs | ||||
| NCT03056339 | M. D. Anderson Cancer Center | 1/2 | CD19 | Cord blood CD19 NK CAR |
| NCT03774654 | Baylor College of Medicine | 1 | CD19 | NK CAR |
In rare circumstances, relapses after CD19 CARTs have been attributed to CD19 antigen escape. Mechanisms of antigen escape include altered CD19 membrane trafficking and/or internalization, expression of CD19 splice variants that lack the target epitope, or mutations in the CD19 gene that lead to disrupted membrane anchorage.40-43 Of note, concern for antigen escape/loss with use of CD19-mAb tafasitamab or CD19 ADC loncastuximab tesirine drives apprehension to utilize these agents prior to CARTs.
CD20 antigen loss has also been described in approximately 25% of patients treated with anti-CD 20 mAbs.44 Possible explanations include loss through clonal selection, epigenetic downregulation, internalization of CD20, or artifact due to rituximab-bound CD20.44,45 It has yet to be determined whether this is a relevant mechanism of resistance to CD20/CD3 BsAbs.
Several dual-targeting CARTs that concurrently target 2 antigens and would effectively address the challenge of antigen escape are now in development (Table 2). This includes a CD19/CD20 CART that has demonstrated a high response rate of 82% (CR, 64%) without added toxicity.46 Rational combinations of immunotherapies directed at multiple antigens are also a consideration.
Off-the-shelf immunotherapies vs CARTs: at what cost?
Both CARTs and BsAbs have been associated with a high financial burden. The cost of FDA-approved CARTs ranges from approximately $373,000 to $410,000 and is even higher when factoring in the price associated with the logistics of CART administration and the management of toxicities. In the TRANSCEND study, relevant trial-observed health care resource utilization and costs were significantly greater among patients with grade ≥3 CRS and/or ICANS (22.8%).47 These data favor the use of 41BB CARTs, which are associated with a low incidence of severe CRS/ICANS and support the development of safer CART options.
With BsAbs, some of these costs are circumvented, but many overlap, given the toxicity profile. The price tag for CD20/CD3 BsAbs has yet to be established. However, if one is to learn anything from the blinatumomab story, these may not be a cheaper alternative.
One also needs to consider the social burden associated with the administration of both CARTs and BsAbs. With CARTs, CRS and ICANs can have a delayed onset and require caregiver support for the first 1 to 2 months after administration.4,7,8 For BsAbs, these toxicities are milder and may not require such close attention. However, the frequency and duration of administration can be cumbersome. Ultimately, a clearer understanding of the cost-effectiveness of off-the-shelf and customized engineered immunotherapies is needed.
Conclusions
CARTs have changed the treatment landscape for R/R aggressive B-cell lymphomas, providing durable responses in patients with historically poor outcomes. CD20/CD3 BsAbs represent a promising new class of off-the-shelf immunotherapy that is highly active and offers the opportunity to circumvent some of the challenges faced with the administration of CARTs. Although experience favors the use of CARTs over other immunotherapies at present, further studies and longer-term follow-up are needed to elucidate optimal sequencing. Along with rational combinations, a number of other off-the-shelf immunotherapies, including novel CARs, are being explored to optimize ease of administration, safety, and efficacy, and they will undoubtedly lead to measurable impacts on patient outcomes.
Conflict-of-interest disclosure
Reem Karmali: speakers' bureau: AstraZeneca, BeiGene, Kite/Gilead, Morphosys; consultant: Kite/Gilead, BMS/Celgene/Juno, Karyopharm, Janssen/Pharmacyclics, Morphosys, Epizyme; research funding: Kite/Gilead, BMS/Celgene/Juno, Takeda.
Off-label drug use
Reem Karmali: mosunetuzumab, epcoritamab, glofitamab, and odronextamab are discussed.