Abstract
Direct oral anticoagulants (DOACs) are a group of direct coagulation factor inhibitors including both direct thrombin inhibitors and direct factor Xa inhibitors. These medications may cause hemostasis assay interference by falsely increasing or decreasing measured values, depending on the analyte. Considering the potential for DOAC interference in a variety of hemostasis assays is essential to avoid erroneous interpretation of results. Preanalytic strategies to avoid DOAC interference include selecting alternatives to clot-based hemostasis assays in patients taking DOACs when possible and sample collection timed when the patient is off anticoagulant therapy or at the expected drug trough. Clinical laboratories may also provide educational materials that clearly describe possible interferences from DOAC, develop testing algorithms to aid in detection of DOAC in submitted samples, use DOAC-neutralizing agents to remove DOACs before continuing with testing, and write interpretive comments that explain the effects of DOAC interference in hemostasis tests. Using a combination of the described strategies will aid physicians and laboratorians in correctly interpreting hemostasis and thrombosis laboratory tests in the presence of DOACs.
Learning Objectives
Describe the patterns of interference in hemostasis assays due to direct thrombin inhibitors and direct Xa inhibitors
List potential strategies physicians and clinical laboratories can use to decrease DOAC interference in hemostasis assays
Since the introduction of the direct oral anticoagulants (DOACs) in the 2010s, clinical laboratories have struggled to mitigate the effects of these drugs in clot-based hemostasis and thrombosis assays. DOACs are a group of direct coagulation factor inhibitors that include both direct thrombin inhibitors (dabigatran) and direct Xa inhibitors (rivaroxaban, apixaban, edoxaban).1 The DOACs may cause assay interference by falsely increasing or decreasing measured values, depending on the analyte. Data from both individual laboratory studies and external quality assessment programs describe expected patterns with a variety of reagents (Table 1).1-8
Patterns of DOAC interference in hemostasis/thrombosis assays
| Expected change . | Assays . | Notes . |
|---|---|---|
| Clotting time prolongation | • aPTT (dabigatran > direct Xa inhibitors) • PT (rivaroxaban > edoxaban > apixaban) • Thrombin time (dabigatran) | Effects on clotting times are reagent dependent. aPTT and PT mixing tests are expected to show incomplete correction in the presence of DOACs. |
| False increase | • Clot-based protein C activity • Clot-based protein S activity • Antithrombin activity (in factor IIa–based assays with dabigatran, in factor Xa–based assays with direct Xa inhibitors) • Activated protein C resistance ratio | False increase in protein C, protein S, and antithrombin activities may result in misdiagnosis of a patient with true deficiency as normal. Falsely elevated activated protein C resistance ratio may result in misdiagnosis of a patient with factor V Leiden mutation as normal. |
| False decrease | • aPTT-based factor assays (VIII, IX, XI, XII) • PT-based factor assays (II, V, VII, X) | Dilutions in factor assays may show nonspecific inhibitor effect. |
| False positive (or potentially false negative) | • LA assays | Includes aPTT- and DRVVT-based assays, among other clotting time-based LA assays; effects are drug and reagent dependent. |
| No change | • Clauss fibrinogen activity (for most reagents, rare methods show false decrease in presence of high concentrations of dabigatran) • D-dimer • Chromogenic protein C activity • Free and total protein S antigen • Anticardiolipin, anti-β2GP1 ELISAs • von Willebrand activity and antigen assays • DNA-based assays (eg, factor V Leiden mutation, prothrombin G20210A mutation) |
| Expected change . | Assays . | Notes . |
|---|---|---|
| Clotting time prolongation | • aPTT (dabigatran > direct Xa inhibitors) • PT (rivaroxaban > edoxaban > apixaban) • Thrombin time (dabigatran) | Effects on clotting times are reagent dependent. aPTT and PT mixing tests are expected to show incomplete correction in the presence of DOACs. |
| False increase | • Clot-based protein C activity • Clot-based protein S activity • Antithrombin activity (in factor IIa–based assays with dabigatran, in factor Xa–based assays with direct Xa inhibitors) • Activated protein C resistance ratio | False increase in protein C, protein S, and antithrombin activities may result in misdiagnosis of a patient with true deficiency as normal. Falsely elevated activated protein C resistance ratio may result in misdiagnosis of a patient with factor V Leiden mutation as normal. |
| False decrease | • aPTT-based factor assays (VIII, IX, XI, XII) • PT-based factor assays (II, V, VII, X) | Dilutions in factor assays may show nonspecific inhibitor effect. |
| False positive (or potentially false negative) | • LA assays | Includes aPTT- and DRVVT-based assays, among other clotting time-based LA assays; effects are drug and reagent dependent. |
| No change | • Clauss fibrinogen activity (for most reagents, rare methods show false decrease in presence of high concentrations of dabigatran) • D-dimer • Chromogenic protein C activity • Free and total protein S antigen • Anticardiolipin, anti-β2GP1 ELISAs • von Willebrand activity and antigen assays • DNA-based assays (eg, factor V Leiden mutation, prothrombin G20210A mutation) |
CLINICAL CASES
Case 1: A 54-year-old woman was diagnosed with pneumonia and was bedridden due to the severity of her illness. A few weeks later, she developed a pulmonary embolism, prompting testing for lupus anticoagulant (LA) and treatment with rivaroxaban. Laboratory results for both her initial LA profile as well as follow-up testing 12 weeks later are included in Table 2.
LA panel results for clinical cases 1 and 2
| Test . | Case 1: initial LA panel . | Case 1: LA panel 12 weeks later . | Case 2: initial LA panel . | Reference interval . |
|---|---|---|---|---|
| PT(s) | 23.0 | 12.8 | 14.3 | 12.0-15.5 |
| DRVVT screen(s) | 57 | 35 | 91 | 33-44 |
| DRVVT 1:1 mix(s) | 52 | NA | 80 | 33-44 |
| DRVVT confirm(s) | Negative | NA | Positive | Negative |
| aPTT screen(s) | 61 | 38 | 119 | 32-48 |
| TT(s) | 15.7 | NA | 15.1 | 14.7-19.5 |
| aPTT 1:1 mix(s) | 49 | NA | 85 | 32-48 |
| PNP | Negative | NA | Positive | Negative |
| Hex phos neutralization | Positive | NA | NA | Negative |
| Test . | Case 1: initial LA panel . | Case 1: LA panel 12 weeks later . | Case 2: initial LA panel . | Reference interval . |
|---|---|---|---|---|
| PT(s) | 23.0 | 12.8 | 14.3 | 12.0-15.5 |
| DRVVT screen(s) | 57 | 35 | 91 | 33-44 |
| DRVVT 1:1 mix(s) | 52 | NA | 80 | 33-44 |
| DRVVT confirm(s) | Negative | NA | Positive | Negative |
| aPTT screen(s) | 61 | 38 | 119 | 32-48 |
| TT(s) | 15.7 | NA | 15.1 | 14.7-19.5 |
| aPTT 1:1 mix(s) | 49 | NA | 85 | 32-48 |
| PNP | Negative | NA | Positive | Negative |
| Hex phos neutralization | Positive | NA | NA | Negative |
DRVVT confirm, PNP, and hex phos neutralization are LA confirmatory reagents containing high phospholipid concentrations.
hex phos neutralization, hexagonal phase phospholipid neutralization test; NA, not applicable; PNP, platelet neutralization procedure;TT, thrombin time.
Case 2: A 37-year-old man sought treatment for an unprovoked pulmonary embolism and was evaluated for thrombophilia risk factors, including LA, while taking apixaban therapy. Laboratory results for his initial LA panel are included in Table 2. An anti-Xa activity assay calibrated for unfractionated heparin (UFH) showed measurable anti-Xa activity in the plasma sample submitted for the LA profile.
Patterns of DOAC interference in hemostasis assays
For basic hemostasis assays such as prothrombin time (PT) and activated partial thromboplastin time (aPTT), response to DOACs varies considerably by drug, drug concentration, and reagent. In general, the aPTT may be prolonged with dabigatran but does not tend to be prolonged by direct Xa inhibitors.1,2,9 A normal aPTT is insufficient to exclude the presence of dabigatran.1 The PT may be prolonged with rivaroxaban and edoxaban but tends to be relatively insensitive to the presence of apixaban in all reagents evaluated to date.2,9 The difference between the observed PT prolongation in the clinical cases highlights the differential effects of rivaroxaban and apixaban on the PT. Dabigatran does not tend to prolong the PT.2,9 In mixing tests for both aPTT and PT, DOACs will appear as nonspecific inhibitors, with the inhibitor effect most pronounced at higher drug concentrations.1 Fibrinogen activity tends to be unaffected by DOACs, particularly the commonly used Clauss fibrinogen assay, which uses plasma dilution as well as a high concentration of thrombin to convert patient fibrinogen to fibrin.2-6 Factitiously decreased fibrinogen activity was reported by some laboratories at higher dabigatran concentrations (385 and 744 ng/mL) in an external quality assessment program.3 D-dimer assays are typically immunoassays, such as enzyme-linked immunosorbent assay (ELISAs) or latex immunoassays; these methods are not affected by the presence of DOACs.1
Among specialized hemostasis assays, those evaluating for thrombophilia risk factors are at particular risk for interference, given that many of these assays are clot based and may be measured in patients who have thrombosis and are treated with anticoagulant therapy.10-12 The clinical cases illustrate two typical examples of how LA assays are affected by DOACs. Dilute Russell viper venom time (DRVVT) and aPTT-based LA assays (eg, platelet neutralization procedure, hexagonal phospholipid neutralization) may show false-positive results in the presence of DOACs.10,12,13 False-positive DRVVT-based testing appears to be a particular risk with rivaroxaban, but there also appears to be risk of DRVVT false negatives for LA in samples containing apixaban.14 Just as with screening aPTT and PT, the mixing test steps in DRVVT and aPTT-based LA assays may show a nonspecific inhibitor pattern.13 Assays for clot-based protein C, protein S, and antithrombin may be falsely increased in the presence of DOACs, which may result in a false-negative result.2-6,11 That is, a patient with a true deficiency may have a normal result if plasma is tested for protein C, protein S, or antithrombin activity using clot-based assays in the presence of DOACs. DOACs do not affect chromogenic protein C activity assays or total protein C antigen assays.1,11 Protein S activity assays are clot based and experience interference by DOACs, whereas free and total protein S antigen assays are immunoassays and do not show DOAC interference.3,4,15 Antithrombin activity assays may be either factor IIa based or factor Xa based; the design determines which DOACs will interfere. Factor IIa–based assays show false elevation with direct thrombin inhibitors, whereas factor Xa–based assays show false elevation with direct Xa inhibitors.3-6 Activated protein C resistance assays also have the potential to give false-negative results in the presence of DOACs.11,16
Other clot-based hemostasis assays, such as factor assays, may show decreased activity in the presence of DOACs.1,3,4,11 Hemostasis assays based on ELISA or other immunoassay methods (eg, von Willebrand factor antigen, solid-phase antiphospholipid antibodies) will not show interference by DOACs; the results of these assays will not be affected if performed in a sample from a patient receiving DOAC therapy.1 Likewise, DNA-based assays (eg, factor V Leiden mutation, prothrombin G20210A mutation) will be unaffected by the presence of DOACs.
One particularly challenging area where direct Xa inhibitor interference can limit therapeutic monitoring of another drug is the special case of patients switching from a direct Xa inhibitor to therapy with UFH or low molecular weight heparin (LMWH).17 Early reports of efficacy of DOAC-Stop (D-S; Haematex Research) for removing the measurable anti-Xa activity effect of rivaroxaban and apixaban but not heparins raise the possibility that plasmas in patients transitioning between a direct Xa inhibitor and UFH or LMWH could be treated with D-S prior to measurement with a UFH- or LMWH-calibrated anti-Xa activity assay or aPTT for therapeutic monitoring.17-19 It is important to note that the degree to which activated carbon adsorbs LMWH is incompletely understood, and further study in this area is needed before routinely using DOAC-neutralizing compounds in samples also containing LMWH.19
What can physicians do to avoid DOAC interference in hemostasis assays?
Physicians can choose from a few different practical strategies to minimize DOAC interference in hemostasis assays. The simplest and best way to avoid DOAC interference is to avoid ordering and performing hemostasis assays in patients taking DOACs. Another option would be to stop DOAC therapy for 2 to 3 days prior to collecting a sample for hemostasis testing; however, given the risk of thrombosis with interruption or change of treatment, this option may not be clinically feasible in most cases.11,12,20 Briefly transitioning to an anticoagulant with less assay interference, such as LMWH, could also be an option but also may not be practical or feasible in many cases. In some clinical situations, testing in patients taking DOACs may be desired (eg, in patients requiring indefinite anticoagulation or detecting an LA in the setting of unprovoked thrombosis to gain information about recurrence risk).12 If testing is undertaken, physicians can consider both choice of assay and timing of sample collection to decrease the possibility of DOAC interference. For analytes in which there is a choice between a functional assay and an assay such as a chromogenic, an antigenic, or a DNA-based assay where interference is not expected, physicians can choose the assay without expected interference. For example, using this principle would favor mutation analysis for factor V Leiden over functional testing with activated protein C resistance assays in a patient taking DOACs in whom thrombophilia testing is desired. Simply substituting another test does not work in all cases in which alternate assays are available. Some tests that lack interference (such as protein C or antithrombin antigen) do not detect rare deficiencies due to protein dysfunction and would not represent appropriate substitutes for functional assays. If a measurement must be made, testing at expected drug trough concentration is recommended: either 12 hours postdose for DOACs dosed twice daily or 24 hours postdose for DOACs administered once daily.9,14 LA test systems (aPTT and DRVVT based) have demonstrated interference from DOACs even at low drug concentrations; therefore, testing a sample collected at an expected drug trough concentration may not completely eliminate the possibility of interference.21
What can clinical laboratories do to avoid DOAC interference in hemostasis assays?
Clinical laboratories likewise have multiple options for mitigating the effects of DOAC interference in hemostasis and thrombosis assays.14 A laboratory can provide educational material to physicians and other health care providers using the laboratory's services that include clear descriptions of expected patterns of DOAC interference, similar to Table 1.22 Laboratories may also design testing algorithms that allow detection of interfering anticoagulants in plasma samples in cases where a patient's history of anticoagulant therapy is not available.11,13 In clinical case 1, the prolonged PT offered a clue to the presence of an anticoagulant medication, either warfarin or a direct Xa inhibitor. Detecting anti-Xa activity in the sample is another, more sensitive way to identify a direct Xa inhibitor in a plasma sample; thrombin time can be used to identify the presence of direct thrombin inhibitors and heparins.11,13 Laboratories should also provide interpretive result comments that explain the potential for DOAC interference in the assays performed, particularly for LA profiles as recommended in current International Society on Thrombosis and Haemostasis (ISTH) and Clinical and Laboratory Standards Institute (CLSI) guidelines.13,23 Some laboratories may elect not to complete testing for a LA in the event DOAC interference is detected and may simply issue a comment stating that DOAC interference renders results of LA testing uninterpretable.23
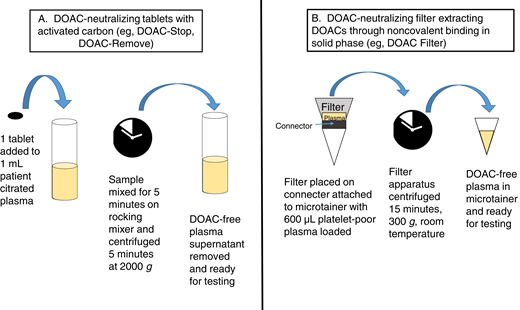
Recently, adsorbing agents that can neutralize DOAC effects in plasma samples in the hemostasis laboratory have been developed, although these agents are not yet FDA approved. Clinical laboratories have extensive experience with removing heparin effects from plasma samples using heparinase or polybrene.24 Using DOAC-neutralizing agents is an attractive possibility that could be incorporated into laboratory workflows that already use heparin neutralizers in a similar fashion. Currently available DOAC-neutralizing agents in tablet form include DOAC-Stop (D-S; Haematex Research) and DOAC-Remove (D-R; 5-Diagnostics AG).11 These agents are composed of activated carbon-containing adsorbent compounds. One D-S or D-R tablet must be added to 1 mL patient plasma, with time allowed for adsorption of DOACs and subsequent centrifugation and removal of DOAC-free plasma for testing (Figure 1).10,18,25 DOAC-spiked plasmas that show false-positive LA results generally convert to negative LA results with DRVVT- and aPTT-based testing following D-S treatment, with results comparable to neutralization with idarucizumab or andexanet alfa.10,19,26,27 One tablet of D-S in 1 mL plasma can reportedly neutralize up to 708 ng/mL apixaban, 1060 ng/mL edoxaban, 1020 ng/mL rivaroxaban, and 360 ng/mL dabigatran.10 D-S or D-R has also been reported to decrease DOAC interference in chromogenic and clot-based factor VIII activity,18,26 1-stage factor IX activity,26 thrombin generation assays,28 activated protein C resistance,25,29 and antithrombin activity.29,30 D-S has been reported to cause decreased factor activities in treated plasmas, raising the possibility of adsorption of coagulation factors in addition to DOACs; however, D-S does not produce aPTT prolongation with all reagents tested.10,31 Caution in result interpretation is recommended when using DOAC-neutralizing agents in the clinical laboratory as complete neutralization was not observed in all cases.10,18 One even more recently described option, DOAC Filter (Diagnostica Stago), is a cartridge containing a solid phase designed to extract DOACs through noncovalent binding when 600 µL citrated plasma is added and centrifuged at 300 × g for 15 minutes.32 DOAC Filter treatment resulted in undetectable levels of DOACs as assessed by quantitative assays and no change in selected hemostasis assays (PT, aPTT, fibrinogen, antithrombin activity, protein C activity, activated protein C resistance, LA tests) in postfiltration samples.32 Further study is needed to better describe the efficacy of DOAC-neutralizing reagents and the risk for inadvertent adsorption of other coagulation factors from plasma in the clinical laboratory. It is also important to remember that performing additional assays to detect DOACs and use of neutralizing agents adds cost and time to testing and requires additional patient plasma. Laboratories will need to carefully consider how these methods would fit into existing test workflows.
DOAC-neutralizing processes with tablet-based adsorbing agents (A) and filter systems (B).
DOAC-neutralizing processes with tablet-based adsorbing agents (A) and filter systems (B).
CLINICAL CASE (continued)
Case 1: This patient had an initial positive LA profile with a follow-up profile 12 weeks later in which an LA was not detected; both profiles were performed while the patient was receiving rivaroxaban. Differential diagnostic considerations for this pattern include a transient LA, differences in timing of draw (eg, first profile drawn at drug peak and second profile drawn at drug trough), or nonadherence to the rivaroxaban regimen at the time of the second profile. The prolonged PT in the first LA profile suggests one of the latter 2 possibilities.
Case 2: This case demonstrates the relative insensitivity of the PT to apixaban. The measurable direct Xa activity suggests that the positive LA results obtained were caused by apixaban interference. A repeat sample collected when the patient is not taking apixaban, repeat sample collected at expected drug trough, or treating the current sample with a DOAC-neutralizing agent and repeating the LA profile are possible options to decrease apixaban interference.
Conclusion
Considering the potential for DOAC interference in a variety of hemostasis assays is essential to avoid erroneous interpretation of results. Preanalytic strategies to avoid DOAC interference include avoiding clot-based hemostasis assays in patients taking DOACs and sample collection timed when the patient is not taking anticoagulant therapy or at the expected drug trough (as opposed to peak or random samples). Strategies clinical laboratories may employ to avoid DOAC interference include providing educational materials that clearly describe possible interferences from DOACs, developing testing algorithms to aid in the detection of DOACs in submitted samples, using DOAC-neutralizing agents to remove DOACs before continuing with testing, and writing interpretive comments that explain the effects of DOAC interference in hemostasis tests. Using a combination of the described strategies will aid physicians and laboratorians in correctly interpreting hemostasis and thrombosis laboratory tests in the presence of DOACs.
Conflict-of-interest disclosures
Karen A. Moser: disclose no relevant conflicts of interest.
Kristi J. Smock: disclose no relevant conflicts of interest.
Off-label drug use
Karen A. Moser: nothing to disclose.
Kristi J. Smock: nothing to disclose.