Abstract
An effective antitumor immune response in patients with lymphoma would eradicate the malignant B cells and cure the patient of the disease. This, however, does not occur, and a suboptimal antitumor response results in persistence and subsequent progression of the patient’s disease. The goals of immunotherapy are therefore to restore an effective antitumor immune response by promoting immune recognition, optimizing immune activation, and supporting persistence of the immune response resulting in subsequent immunological memory. Multiple mechanisms, however, are present within the tumor microenvironment that account for an inadequate immune response. These include loss of major histocompatibility complex expression on tumor cells and subsequent inadequate antigen presentation, increased expression of immunosuppressive ligands on malignant cells, populations of immune cells with suppressive function present in the tumor, and cytokines secreted by the malignant cell or other cells in the microenvironment that promote immune exhaustion or suppress the immune response. Successful immunotherapeutic strategies are specifically addressing these issues by promoting antigen presentation, improving recognition of the malignant cell, directly activating T cells and natural killer cells, and blocking immune checkpoint signaling that would suppress the immune response. Many of these approaches have proven highly successful in patients with various subtypes of lymphoma and are now being incorporated into standard clinical practice.
Learning Objectives
Define the components of an effective T-cell–mediated immune response
Identify deficiencies in the immune response in lymphoma
Describe strategies to overcome the immune deficiencies including the use of immune checkpoint blockade, bispecific molecules, CD47/signal-regulatory protein α blockade, and chimeric antigen receptor T cells
Case presentation
To illustrate the potential clinical efficacy of modulating the immune system, the case of an 83-year-old male patient, initially diagnosed in August 2015 with diffuse large B-cell lymphoma (DLBCL), activated B-cell type, is presented. At diagnosis, the patient presented with extensive bone marrow involvement and was treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy for 6 cycles. He only achieved a partial remission, and a repeat bone marrow biopsy done at the end of therapy showed residual DLBCL. The patient was then treated with a combination of a Bruton tyrosine kinase inhibitor, acalabrutinib, plus an anti–programmed death-1 (PD-1) antibody, pembrolizumab, on a clinical trial as neither agent is approved for this indication. The hypothesis for using this combination was that acalabrutinib, similar to ibrutinib, may inhibit both Bruton tyrosine kinase and interleukin 2 (IL-2)-inducible T-cell kinase, thereby promoting a TH1-dominant T-cell response, whereas pembrolizumab would prevent suppression of activated T cells by blocking PD-1 signaling. The patient responded well to the combination and achieved a complete remission based on a negative bone marrow biopsy and a negative positron emission tomography scan. Per protocol, treatment was then discontinued and the patient was observed. In January 2019, the patient had biopsy-proven evidence of disease progression with multiple bone lesions seen on positron emission tomography scan. The patient was retreated with pembrolizumab alone, again achieved a complete remission, and remains on treatment and in complete remission to date.
This clinical case illustrates a number of important immunological points. First, intratumoral T cells are able to recognize and suppress the malignant B-cell clone when inhibitory signals that downregulate their function are blocked. Second, despite the intratumoral T cells suppressing the malignant clone, they do not appear to eradicate all malignant cells and patients commonly progress when treatment is stopped. Third, as the disease recurs, intratumoral effector cells remain susceptible to suppression, and fourth, no immunological memory appears to be generated by exposure to the B-cell malignancy. All of these issues need to be addressed if effective and curative immunotherapy is to be developed.
What constitutes an effective antitumor T-cell response in lymphoma?
Immune homeostasis, and, particularly, T-cell activation due to engagement with presented antigens, is a very closely regulated process not only to ensure lysis of infected or malignant cells, but also to prevent autoimmunity and indiscriminate tissue destruction.1-3 Although it is incredibly important for the immune system to be activated by threats to the host including pathogens and malignancies, it is equally important for the immune response to be regulated so that the level of activation remains appropriate to the threat. As the threat is contained, it is important for activation and cytotoxicity to proportionally decrease. To achieve this, there is a complex network of antigen-presenting cells (APCs) and immune-regulatory cells, all of which express or secrete immune-activating and -suppressing ligands, which modulate the immune response.
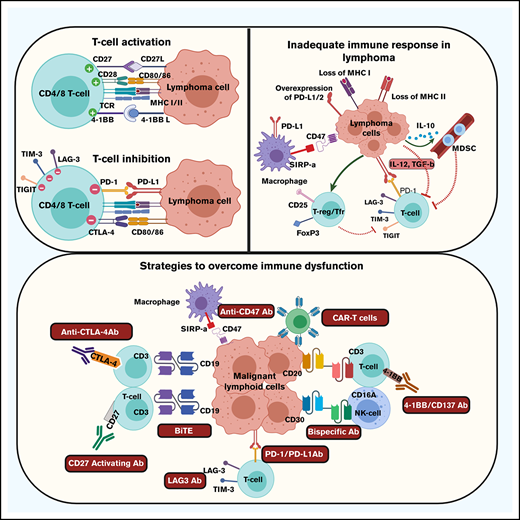
In patients with malignancies, such as lymphoma, optimal activation of, particularly, effector T cells is a complex process requiring 2 activating signals.3 The first signal is typically provided by recognition by the T-cell receptor of a tumor antigen presented in the context of the major histocompatibility complex (MHC) molecules expressed either on the surface of APCs or the lymphoma cell itself. Optimal activation of the T cell then requires a second costimulatory signal that is typically provided by engagement between B7 molecules, including B7-1 (CD80) and B7-2 (CD86), on APCs or lymphoma cells, and CD28 on the T cell.4 Receipt of these 2 signals results in activation, differentiation, and expansion of effector T cells (Table 1). Failure to deliver a specific second activating signal, despite the appropriate presentation of tumor antigens, typically results in T-cell anergy and subsequent apoptosis.
Necessary components of an effective antitumor immune response
| Components |
| • Recognition of tumor antigens as foreign |
| • Activation and expansion of immune effector cells |
| • Persistence of activated cells until all tumor cells are lysed |
| • Immunological memory to prevent recurrence |
| Components |
| • Recognition of tumor antigens as foreign |
| • Activation and expansion of immune effector cells |
| • Persistence of activated cells until all tumor cells are lysed |
| • Immunological memory to prevent recurrence |
The activation process can be modulated by additional costimulatory or coinhibitory signals that may be delivered to the T cell to further fine-tune immune stimulation and the subsequent activation of the cell.4-7 Activation of T cells typically results in upregulation of receptors that are primed to receive these immune-modulatory signals. Additional activating signals may be provided through CD137 (4-1BB), CD134 (OX40), CD27, and glucocorticoid-induced tumor necrosis factor receptor. In contrast, inhibitory signals are typically delivered through cytotoxic T-lymphocyte antigen 4 (CTLA4), PD-1, B- and T-lymphocyte attenuator (BTLA), lymphocyte-activation gene 3, T-cell immunoglobulin mucin-3, and T-cell immunoreceptor with immunoglobulin and ITIM domains.5 Additional activating and inhibitory receptors have been described and are also likely to be important. Activation of T cells results in upregulation of inhibitory signals in particular to regulate the intensity of the immune response. Signaling through these inhibitory signals suppresses immune activation, as inhibition of an activated T-cell response is quite appropriate after effective eradication of a biological threat.
Aside from appropriate activation and subsequent suppression of T cells, additional mechanisms are also important to optimize the antitumor immune response in lymphoma.3 Activated T cells need to proliferate to ensure adequate numbers are present to overcome the threat. Furthermore, tumor-specific T cells need to persist to ensure that malignant cells can be continually inhibited should they show any sign of re-expansion after initially being suppressed by the effector T cells. Ideally, immunological memory needs to develop so that effector T cells can rapidly re-expand should dormant malignant B cells reactivate and become a threat to the patient.
Mechanisms accounting for an inadequate immune response
The tumor microenvironment (TME) in patients with lymphoma appears to be an ideal niche for an adequate antitumor immune response.8-11 There is typically a substantial presence of immune cells in close proximity with the malignant clone and both APCs and malignant B cells are commonly located close to effector T cells. Despite this, malignant cells do not appear to be suppressed by the immune response and commonly continue to proliferate, resulting in disease progression.
Prior research has shown that there are multiple mechanisms that account for the lack of an effective immune response to the malignant B-cell clone (Table 2). A substantial issue in both Hodgkin lymphoma and aggressive non-Hodgkin lymphomas is loss of MHC class I and class II molecules.12-17 In Hodgkin lymphoma, decreased or absent expression of MHC class I may be present in ∼75% of patients, whereas decrease or loss of MHC class II expression may be identified in one-third of patients. Decreased expression of MHC class I molecules particularly has been shown to be associated with decreased progression-free survival in patients treated with standard therapy.12 Similarly, loss of MHC class II molecules in DLBCL, primary mediastinal large B-cell lymphoma, and aggressive lymphomas in immune-privileged sites, is associated with an inadequate immune response and a poor outcome.14-17 Additional molecular and genetic changes in the tumor cell may further compromise antigen presentation as well as the ability of the immune system to respond in an adequate fashion. These results suggest that inadequate presentation of tumor antigens due to loss of MHC molecules is a substantial barrier to an effective antitumor immune response and is an issue in multiple subtypes of lymphoma.
Mechanisms responsible for the inadequate immune response in lymphoma
| Mechanisms |
| • Loss of MHC expression on malignant B cells |
| • Increased expression of immunosuppressive ligands on lymphoma cells |
| • Suppressive immune cell populations in the TME |
| • Secreted cytokines that promote immune exhaustion or suppression |
| Mechanisms |
| • Loss of MHC expression on malignant B cells |
| • Increased expression of immunosuppressive ligands on lymphoma cells |
| • Suppressive immune cell populations in the TME |
| • Secreted cytokines that promote immune exhaustion or suppression |
A second barrier to an effective antitumor immune response in lymphoma is the upregulation or overexpression of immunosuppressive ligands on the tumor cells or on other cells in the TME. Overexpression of programmed death ligand 1 (PD-L1; CD274) and PD-L2 (CD273) by malignant lymphoma cells is a mechanism by which malignant cells protect themselves from activated effector T cells and the expression is commonly driven by viral or genetic causes.18-21 Many intratumoral T cells in lymphoma express PD-1, the receptor for these ligands, making them susceptible to PD-L1/2 signaling.22 As PD-1 is increasingly expressed on T cells as they become activated, PD-L1 and PD-L2 signal through PD-1 to inhibit T-cell function, promote immune exhaustion, and result in subsequent T-cell apoptosis. Over time, many T cells in the microenvironment of lymphoma express multiple immune-inhibitory receptors, including PD-1, T-cell immunoglobulin mucin-3, and lymphocyte-activation gene 3, all of which are associated with immune exhaustion.23,24 These findings suggest that many of the T cells present at sites of lymphoma are in fact suppressed or exhausted.
Despite the presence of multiple immune cell populations at sites of lymphoma involvement, many of these cell types do not target the malignant B cell but rather have a suppressive and regulatory effect on the immune response. Many intratumoral T cells express FoxP3 and CD25, are regulatory T cells with the ability to suppress both CD8+ and CD4+ T-cell function, and are located in close proximity to the malignant cell.25 Malignant B cells may further induce FoxP3 expression in CD4+ T cells and promote differentiation of T cells to a suppressive phenotype.26 Monocytes and macrophages are typically abundant in the tumor and commonly promote malignant cell growth.27 They also commonly express PD-L1 and PD-L2, which inhibit the function of effector T cells; PD-L1+ macrophages may surround the malignant cell, effectively providing it with protection from effector T cells.28 Myeloid-derived–suppressive cells (MDSCs) also increase in patients with lymphoid malignancies and the presence of these cells profoundly inhibits T-cell activation and proliferation.29 The presence of all of these cells in the TME, therefore, counteracts the T-cell response and prevents eradication of the malignant cell.
Additionally, multiple cytokines are secreted by the malignant cell or by cells in the TME that directly suppress T-cell function or subsequently induce T-cell exhaustion. For example, IL-10 may be secreted by the malignant B cell and expands the population of MDSCs.30 Serum IL-10 levels have been shown to be increased in patients with lymphoma, and IL-10–induced MDSCs substantially suppress T-cell proliferation. Transforming growth factor β may be expressed on the surface of lymphoma cells and results in immune suppression.31 Transforming growth factor β may in fact activate T cells but these activated cells commonly express immune-exhaustion markers and are dysfunctional.31 Furthermore, T-cell–activating cytokines like IL-12 may initially promote T-cell function but with sustained exposure to IL-12, T cells become exhausted and poorly functional.23 Clearly, there are many immunological barriers to circumvent to ensure an optimal antitumor T-cell immune response.
Strategies to overcome immune dysfunction
Although there are multiple mechanisms that inhibit an effective antitumor T-cell response in lymphoma, many of these specific mechanisms are being overcome with novel immunotherapeutic strategies (Table 3). To prevent immune suppression and thereby activate intratumoral T cells, blocking immune checkpoint signaling has been a successful strategy.8 Immune checkpoint blockade, particularly blocking PD-1 signaling, has been highly successful in patients with Hodgkin lymphoma. It is also been very effective in select non-Hodgkin lymphoma subtypes in which overexpression of PD-1 ligands is due to copy-number gain or amplification at the chromosome 9p24.1 locus or activation of the JAK/STAT pathway due to viral causes. Although blockade of other immune checkpoints such as CTLA4 and killer cell immunoglobulin-like receptor has been less effective,32 combination approaches that include activating signals through receptors such as CD27 and CD137 may be more promising.33,34
Clinical strategies in lymphoma to overcome immune suppression
| Strategies |
| • Target immune checkpoints to prevent immune suppression |
| • Promote tumor cell phagocytosis and antigen presentation by engaging macrophages |
| • Improve malignant cell engagement using CARs |
| • Activate T cells and NK cells using bispecific antibodies |
| Strategies |
| • Target immune checkpoints to prevent immune suppression |
| • Promote tumor cell phagocytosis and antigen presentation by engaging macrophages |
| • Improve malignant cell engagement using CARs |
| • Activate T cells and NK cells using bispecific antibodies |
A further way to activate T cells or natural killer (NK) cells and specifically direct them to lyse malignant B cells is to use molecules with bispecific binding ability. These antibodies are able to bind to both the malignant B cell and also to receptors such as CD3 or CD16 expressed on T cells and NK cells.35,36 These agents bring the effector cells into very close proximity to the malignant cell, thereby directly activating the effector cells while also directing them specifically to the malignancy. This therapy has shown substantial clinical efficacy in single-agent trials and has also shown substantial promise when used in combination with immune checkpoint blockade.
Because loss of MHC molecules effectively “hides” the malignant cell from activated T cells, immunotherapy that promotes presentation of tumor antigens to the immune system or allows for the T cells to directly engage with malignant cells is likely to be effective. Chimeric antigen receptor (CAR) T-cell treatment introduces an engineered receptor into T cells that provides all of the machinery necessary to activate the cell.37 The chimeric receptor construct has a binding domain that engages proteins on the lymphoma cell without the requirement for typical T-cell receptor signaling. The same receptor also includes a costimulatory domain that automatically provides the second required activation signal, thereby allowing for activation and expansion of the T cells. However, because CAR T cells are susceptible to similar suppressive signals as normal T cells, further strategies are now being tested to promote persistence of CAR T cells and to provide them with protection against suppression by blocking or removing inhibitory receptors such as PD-1.
An additional way to promote presentation of tumor antigens to the immune system, and thereby induce a more effective T-cell response, is to use a tumor-specific vaccine,38 often using dendritic cells to promote antigen presentation. This approach allows tumor-specific antigens to be presented in the appropriate immunological context and thereby induce an adaptive immune response. A similar strategy is to induce macrophages to phagocytose lymphoma cells and thereby promote tumor antigen presentation to T cells. Blockade of the CD47 “don’t-eat-me-signal” on lymphoma cells, which inhibits phagocytosis of the malignant cells by macrophages, has been found to substantially increase their ability to phagocytose malignant cells and triggers T-cell–mediated destruction of the tumor.39 Clinically, CD47 blockade in combination with rituximab in patients with lymphomas has shown very promising results.40
Conclusions
Optimizing immune function in lymphoma patients is clearly a new therapeutic frontier. Although all of the components for an antitumor immune response are present at sites of lymphoma involvement, there are many immunological barriers to the response being effective. These barriers include downregulation of MHC expression on malignant cells, increased expression of immunosuppressive ligands on lymphoma cells, suppressive immune cell populations in the TME, and secreted cytokines that promote immune exhaustion. Strategies to prevent suppression and promote immune activation, as well as approaches that increase malignant cell engagement by the immune system, are all resulting in promising activity. In the future, however, we will need to consider combination approaches to comprehensively overcome all of the immune dysfunction so that we will be able to achieve durable responses that will ultimately result in cures for lymphoma patients.
Correspondence
Stephen M. Ansell, Division of Hematology, Mayo Clinic, 200 First St, Rochester, MN 55905; e-mail: ansell.stephen@mayo.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.
This article was selected by the Blood Advances and Hematology 2020 American Society of Hematology Education Program editors for concurrent submission to Blood Advances and Hematology 2020. It is reprinted from Blood Advances 2020, Volume 4.