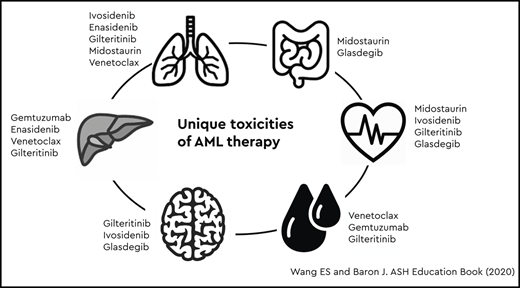
Abstract
The recent advent of myriad targeted therapies for acute myeloid leukemia (AML) has led to new hope for our patients but has also introduced new challenges in managing the disease. For clinicians, the ability to treat AML in the outpatient setting with novel agents of equal or greater efficacy than 7+3 has been transformative. Despite the enthusiasm, however, the reality is that many patients are still frail and remain at risk for treatment-related complications. Translating the results of clinical trials into improved outcomes for these individuals requires an understanding of how best to manage the adverse effects of these agents. Which patients benefit most and what to watch for? When to stop therapy? Using illustrative case presentations, this review details the unique toxicities associated with each of the approved mutation-specific and nonspecific targeted drugs for AML. The goal of this review is to help clinicians determine the risk:benefit ratio in decision making for individual patients with AML.
Learning Objectives
Learn how to manage the most common toxicities of targeted therapies for AML to continue therapy and improve clinical outcomes
Recognize unique and/or life-threatening adverse effects of targeted therapies that warrant rapid intervention and drug discontinuation
Introduction
Clinicians who treat patients with acute myeloid leukemia (AML) have for decades familiarized themselves with the risks of high-dose chemotherapy, specifically the need for prolonged hospitalization and the management of life-threatening cytopenia, sepsis, and organ failure. Over the years, innumerable prognostic models have been developed that purport to predict the “fitness” and appropriateness of individual patients to withstand 7+3 therapy.1,2 On the basis of these models, older patients with newly diagnosed AML were often offered palliation over definitive chemotherapy.3,4 Selecting appropriate patients capable of withstanding and benefiting from 7+3 therapy has remained one of the guiding tenets of AML therapy.5
The recent advent of myriad targeted therapies for AML therapy has led to new hope. For clinicians, the ability to treat AML in the outpatient setting with novel agents of equal or greater efficacy than 7+3 but with fewer toxicities in specific patient subsets has been transformative.6,7 Despite the enthusiasm, however, the reality is that many patients are still frail and remain at risk for treatment-related complications. Translating the results of clinical trials into improved outcomes for these individuals requires an understanding of how best to manage the distinctive adverse effects of these agents to prolong and preserve quality of life.
This review article discusses the unique toxicities associated with currently approved targeted therapies for AML, including both mutation-specific and nonspecific agents. Each section uses specific patient scenarios to illustrate the decision making for individual patients and includes specific recommendations for when to proceed with therapy and when to stop.
Therapies for AML
FLT3 tyrosine kinase inhibitors
Midostaurin
Case 1.
The patient is a 55-year-old woman with no significant medical history who presents with profound fatigue and easy bruising. Laboratory tests show a white blood cell count (WBC) of 170 000/μL, hemoglobin of 6 g/dL, and platelets of 18 000/μL with many peripheral blasts. Bone marrow biopsy confirms AML with normal karyotype and FLT3-internal tandem duplication (FLT3-ITD) mutation. She starts 7+3 treatment followed by midostaurin on days 8 to 21. Her course is complicated by pneumonia, which requires broad-spectrum antibiotics. On day 12, she develops worsening nausea, vomiting, abdominal bloating, and diarrhea. She is started on around-the-clock antiemetics but is unable to take oral medication, including midostaurin, for 3 days. On the evening of the fourth day since beginning treatment, she develops neutropenic fever, and a computed tomography (CT) scan demonstrates new right-sided colitis. A stool test is positive for Clostridium difficile toxin. She begins therapy with oral vancomycin and gradually improves. Midostaurin is resumed, and she completes 14 days of drug therapy.
Currently, 2 tyrosine kinase inhibitors (TKIs) of mutant FLT3 kinase have achieved regulatory approval in the United States for therapy of FLT3-mutant AML: midostaurin in combination with induction and consolidation chemotherapy in the newly diagnosed setting and gilteritinib for relapsed/refractory (R/R) disease. Given that FLT3 mutations are identified in 25% to 37% of newly diagnosed AML cases,8 both agents have been welcome additions to the therapeutic armamentarium.
Midostaurin is a broad-spectrum multikinase inhibitor re-purposed for its ability to block mutant FLT3 signaling pathways. When added to 7+3 induction and cytarabine consolidation, midostaurin significantly improved overall survival (OS) and event-free survival in individuals age 18 to 60 years with newly diagnosed AML characterized by FLT3-ITD and/or tyrosine kinase domain mutations.9 This agent was previously associated with significant gastrointestinal adverse effects leading to dose de-escalation from the originally planned dose of 100 mg twice per day continuously for 28 days to the currently recommended 50 mg twice per day for 14 days (days 8-21).10 Schlenk et al11 confirmed the event-free survival benefit of midostaurin added to first-line therapy in patients with FLT3-mutant AML up to age 70 years. Treatment in older individuals, however, was associated with cardiovascular (22%) and pulmonary events, primarily pneumonia. In addition, most did not complete the planned 12 months of midostaurin maintenance because of gastrointestinal toxicity and infections.
We adopt a 3-pronged approach to administer all 14 days of midostaurin therapy during induction and consolidation.9 Antiemetics are given before each dose, and the medication is taken with food. Because nausea and vomiting are potentiated by the smell of the capsules, this is mitigated by airing out midostaurin capsules outside their blister pack for 10 to 15 minutes before administration. Once concomitant gastrointestinal disorders (i.e., C difficile infections) have been ruled out, around-the-clock use of antidiarrheal agents may be enacted. Once-per-week electrocardiograms (ECGs) should be performed to check QTc intervals with replacement of any concomitant QTc-prolonging medications, such as azoles and fluoroquinolones. Electrolyte supplementation is recommended to reduce the risk of arrhythmias in older patients. Life-threatening cardiac events and drug-induced interstitial pneumonitis should prompt discontinuation. At our institute, we routinely perform chest CT scans on all patients with newly diagnosed AML before therapy as a baseline study and to assess for asymptomatic fungal pneumonitis.12 On the basis of data supporting the feasibility of continuous administration of midostaurin starting on day 8 until 48 hours before next chemotherapy,11 we typically make up missed doses to ensure 14 days of midostaurin with each cycle (Table 1).
FLT3 inhibitors: when to push through and when to stop
| Drug name . | Dose and frequency . | Toxicity . | When to push through . | When to stop . |
|---|---|---|---|---|
| Midostaurin9,11 | 50 mg orally twice per day on days 8-21 of 7+3 treatment and high-dose cytarabine consolidation | Pneumonitis, nausea/vomiting, diarrhea, fever, mucositis, infections, cardiac issues (in patients age 60 to 70 years) | Complete 14-day regimen during induction and consolidation | Unable to take oral medication because of nausea/vomiting or development of life-threatening cardiac issues; discontinue for possibly drug-related interstitial pneumonitis without infectious etiology or if there is evidence of R/R disease. |
| Gilteritinib7 | 120 mg orally once per day | Liver dysfunction, fever, PRES, DS, myalgia/arthralgia, fatigue, edema | First 6 cycles of therapy, mild to moderate renal impairment, mild DS responding to therapy | Severe DS with life-threatening complications or no improvement after 48 hours of steroid therapy; elevated liver function tests (AST and ALT >5× ULN, bilirubin >3× ULN). Restart at 80 mg; if pancreatitis is present, restart at 80 mg. QTcF >500 ms (1%), adjust medications and restart at 80-120 mg. Discontinue for PRES (1%) or if there is no response after 6 cycles. |
| Sunitinib21 * | 50 mg once per day for 4 weeks with 1 to 2 weeks off the drug | Edema, fatigue, oral ulcerations, decreased appetite, headache, gastrointestinal symptoms | Not recommended for AML therapy | Not recommended for AML therapy at this time. |
| Sorafenib19,46 * | 200-400 mg orally twice per day | Skin rash, fatigue, diarrhea, liver, myalgias, marrow hypoplasia, cytopenia | Off-label use for mutant FLT3-ITD AML in combination with other HMAs | Hold for excessive bleeding and severe cytopenias (consider resuming dose at 200 mg); hold or discontinue drug for severe persistent hypertension or cardiac ischemia or infarction; discontinue for severe skin reactions or if there is no response in 3 months. |
| Ponatinib22 * | 45 mg orally once per day | Pancreatitis, petechiae | Off label use | Hold drug for pancreatitis and resume at lower dose (30 mg); discontinue for life-threatening cardiac and peripheral vascular events. |
| Quizartinib18 † | 30-60 mg orally once per day | QTcF prolongation (dose related), DS | Per clinical trial only | Hold for significant QTC prolongation or development of cardiac arrhythmias (dose related) per clinical trial or for severe DS with life-threatening complications or no improvement after 48 hours of steroid treatment. |
| Crenolanib17 † | 100 mg orally three times per day plus induction/consolidation or salvage | Nausea, vomiting, transaminitis, fluid retention, gastrointestinal bleeding | Per clinical trial only | Hold for gastrointestinal bleeding or toxicity and consider dose reduction (80 mg three times per day) per clinical trial. |
| Drug name . | Dose and frequency . | Toxicity . | When to push through . | When to stop . |
|---|---|---|---|---|
| Midostaurin9,11 | 50 mg orally twice per day on days 8-21 of 7+3 treatment and high-dose cytarabine consolidation | Pneumonitis, nausea/vomiting, diarrhea, fever, mucositis, infections, cardiac issues (in patients age 60 to 70 years) | Complete 14-day regimen during induction and consolidation | Unable to take oral medication because of nausea/vomiting or development of life-threatening cardiac issues; discontinue for possibly drug-related interstitial pneumonitis without infectious etiology or if there is evidence of R/R disease. |
| Gilteritinib7 | 120 mg orally once per day | Liver dysfunction, fever, PRES, DS, myalgia/arthralgia, fatigue, edema | First 6 cycles of therapy, mild to moderate renal impairment, mild DS responding to therapy | Severe DS with life-threatening complications or no improvement after 48 hours of steroid therapy; elevated liver function tests (AST and ALT >5× ULN, bilirubin >3× ULN). Restart at 80 mg; if pancreatitis is present, restart at 80 mg. QTcF >500 ms (1%), adjust medications and restart at 80-120 mg. Discontinue for PRES (1%) or if there is no response after 6 cycles. |
| Sunitinib21 * | 50 mg once per day for 4 weeks with 1 to 2 weeks off the drug | Edema, fatigue, oral ulcerations, decreased appetite, headache, gastrointestinal symptoms | Not recommended for AML therapy | Not recommended for AML therapy at this time. |
| Sorafenib19,46 * | 200-400 mg orally twice per day | Skin rash, fatigue, diarrhea, liver, myalgias, marrow hypoplasia, cytopenia | Off-label use for mutant FLT3-ITD AML in combination with other HMAs | Hold for excessive bleeding and severe cytopenias (consider resuming dose at 200 mg); hold or discontinue drug for severe persistent hypertension or cardiac ischemia or infarction; discontinue for severe skin reactions or if there is no response in 3 months. |
| Ponatinib22 * | 45 mg orally once per day | Pancreatitis, petechiae | Off label use | Hold drug for pancreatitis and resume at lower dose (30 mg); discontinue for life-threatening cardiac and peripheral vascular events. |
| Quizartinib18 † | 30-60 mg orally once per day | QTcF prolongation (dose related), DS | Per clinical trial only | Hold for significant QTC prolongation or development of cardiac arrhythmias (dose related) per clinical trial or for severe DS with life-threatening complications or no improvement after 48 hours of steroid treatment. |
| Crenolanib17 † | 100 mg orally three times per day plus induction/consolidation or salvage | Nausea, vomiting, transaminitis, fluid retention, gastrointestinal bleeding | Per clinical trial only | Hold for gastrointestinal bleeding or toxicity and consider dose reduction (80 mg three times per day) per clinical trial. |
PRES, posterior reversible encephalopathy syndrome; DS, differentiation syndrome; HMAs, hypomethylating agents (azacitidine, decitabine)
Off-label use.
Clinical trial use only.
Gilteritinib
Case 1 (follow-up).
The patient completes induction chemotherapy with achievement of complete remission (CR). Her brother is a full match, and plans are underway for her to proceed to allogeneic stem cell transplantation (allo-SCT). However, soon after completing consolidation cycle 1 consisting of high-dose cytarabine and midostaurin, she presents with new thrombocytopenia. Unfortunately, a repeat bone marrow biopsy reveals 75% myeloblasts with the same FLT3-ITD mutation. She begins treatment with gilteritinib 120 mg once per day. After 2 weeks, she has new elevations in liver enzymes, specifically aspartate aminotransferase (AST) 325 and alanine aminotransferase (ALT) 359 (greater than 7 times the upper limit of normal [ULN]). Gilteritinib is held for 1 week, and follow-up laboratory tests show improvement to AST 76 and ALT 140. She resumes drug treatment at a dose reduction of 80 mg per day and proceeds with plans for an allo-SCT.
Gilteritinib is a new-generation oral inhibitor with potent and specific inhibitory properties against FLT3 and AXL-1 kinases.13 A randomized controlled phase 3 trial demonstrated higher overall response rates and significantly improved OS in patients with R/R FLT3-mutant AML who received gilteritinib vs conventional chemotherapy, including both high-intensity (mitoxantrone, etoposide, cytarabine) and low-intensity (azacitidine) regimens.7 In our experience, patients, particularly those with low counts at baseline, experience significant cytopenias and symptoms after starting gilteritinib therapy. Our practice is to monitor them once per week in the ambulatory setting. Other adverse effects include elevated liver function tests, fever, myalgia/arthralgia, fatigue, mucositis, edema, rash, and diarrhea. Orthostatic hypotension is common and can be safely managed with intravenous fluids, adjustment of blood pressure medications, and addition of midodrine and/or fludrocortisone. ECGs should be performed before starting gilteritinib, on days 8 and 15 of cycle 1, at the start of cycles 2 and 3, and in additional cycles. Interruption of doses for QTc interval prolongation >500 ms is recommended. Increases in liver function tests, specifically transaminase (AST/ALT) elevations greater than 5× ULN, total bilirubin levels greater than 3× ULN, or evidence of pancreatitis (4%) should prompt temporary drug holds and resumption of the drug at a lower dose (80 mg once per day).
Gilteritinib is linked to 2 severe but rare complications. Differentiation syndrome (DS; 3%) may occur as early as 2 days and as late as 75 days after drug initiation and has been reported with several FLT3 inhibitors.14 Symptoms include fever, shortness of breath, rapid weight gain, new pleural and/or pericardial effusions, heart failure, and hypotension. Laboratory tests typically show hyperleukocytosis with predominantly mature myeloid cells. Treatment consists of dexamethasone 10 mg intravenously or orally once every 12 hours for at least 3 days; if life-threatening complications and/or clinical deterioration occur despite 48 hours of steroid treatment, the drug should be held.15 Posterior reversible encephalopathy syndrome is a rare neurologic complication reported in 1% of patients receiving gilteritinib. Symptoms of posterior reversible encephalopathy syndrome include seizure and acute changes in mental status. Diagnosis should be confirmed by magnetic resonance imaging, with permanent drug discontinuation. Because many patients experience gradual improvement in bone marrow blasts and responses over time, continuing therapy for 6 months in the absence of overt disease progression or intolerable toxicity is suggested. Patients for whom gilteritinib therapy fails should be strongly encouraged to pursue clinical trials.
Other FLT3 TKIs
Several other TKIs (reviewed in Daver et al16 ) have been evaluated for treating FLT3-mutant AML, including newer-generation inhibitors (crenolanib17 and quizartinib18 ) and agents re-deployed in the off-label setting (i.e., sorafenib,19,20 sunitinib,21 and ponatinib).22 Table 1 lists common toxicities observed with these agents and provides suggestions for management.
BCL-2 inhibition
Case 2
The patient is a 77-year-old man with a history of coronary artery disease, atrial fibrillation, and chronic obstructive pulmonary disease. He presents to his primary physician with worsening hypoxia. Laboratory tests show severe anemia (7.1 g/dL) with a WBC of 3200 cells per μL and platelet count of 78 000/μL. He is referred for workup of pancytopenia. Bone marrow evaluation confirms AML (29% blasts) with numerous cytogenetic abnormalities including del5q. Molecular profiling reveals NRAS, IDH1, and DNMT3A mutations. He is admitted and starts venetoclax and azacitidine. On day 7, the absolute neutrophil count (ANC) drops to 280 cells per microliter. He is started on posaconazole with a dose reduction of venetoclax to 100 mg once per day. On day 10, his platelet count decreases to <5000/μL. He receives once-per-day platelet transfusions with no interruption of venetoclax. Day 21 bone marrow evaluation demonstrates <5% blasts. Venetoclax is held. Granulocyte colony-stimulating factor (G-CSF) is administered. Several days later, counts improve (ANC, 1000 cells per μL, platelets 40 000/μL), and he is discharged home.
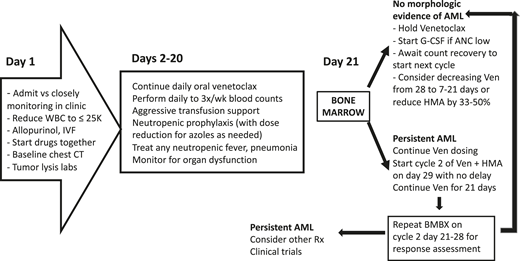
Addition of the oral B-cell lymphoma 2 (BCL-2) inhibitor, venetoclax, to hypomethylating agents (HMAs) or low-dose cytarabine has transformed the therapeutic landscape for older, unfit patients. These regimens deliver high response rates across diverse mutational subsets and median survival duration of more than 1 year.23 Despite this, managing toxicity in these frail patients causes significant trepidation among clinicians. In a phase 1b trial, the most frequent severe adverse events of venetoclax with HMAs were febrile neutropenia (43%), leukopenia (31%), anemia (25%), thrombocytopenia (24%), neutropenia (17%), and pneumonia (13%). Infections of all grades occurred in three-fourths of patients (45% grade 3 to 4), with pneumonia (18%) being the most common. Deaths occurred primarily because of infections. No dose-limiting toxicities were detected with venetoclax up to 1200 mg once per day. Toxicities were similar between azacitidine and decitabine23 (Table 2). Management of patients receiving venetoclax-based therapy varies. There are differences of opinion regarding the most appropriate setting for drug initiation (ie, outpatient vs inpatient), need for antifungal prophylaxis, timing of bone marrow evaluation, and duration and/or dosing of drugs in subsequent cycles.24,25 A schema of how we administer venetoclax plus HMA/low-dose cytarabine is depicted in Figure 1.
BCL-2, hedgehog, and CD33-directed therapies: when to push through and when to stop
| Drug name . | Dose and frequency . | Toxicity . | When to push through . | When to stop drug . |
|---|---|---|---|---|
| Venetoclax/HMAs (azacitidine or decitabine) or low-dose cytarabine23,47 | 400 mg orally once per day (azacitidine or decitabine) or 600 mg orally once per day (low-dose cytarabine) | Myelosuppression, tumor lysis, neutropenia, anemia, thrombocytopenia | First 2 cycles result in mild to moderate renal dysfunction, hepatotoxicity, cytopenias | Reduce dose by 50% for severe liver impairment (Child-Pugh score for cirrhosis). Hold if bone marrow evaluation on days 21 to 28 shows cytoreduction (<5% blasts). In responding patients, consider truncating duration of venetoclax therapy to 7 to 21 days in subsequent cycles; discontinue after 2 to 4 cycles if there is no response. |
| Glasdegib/low-dose cytarabine 20 mg subcutaneously twice per day × 10 days34 | 100 mg orally once per day | Fatigue, febrile neutropenia, dyspnea, anemia, dysgeusia, anorexia, QTc prolongation | Dysgeusia, fatigue, myalgias, cytopenias, QTc 480-500 ms during the first 6 cycles | Hold for QTc prolongation >500 ms and resume at 50 mg; discontinue for life-threatening cardiac arrythmias, or 30 days before donating blood, or for ANC <500 cells per μL and/or platelets <10 000/μL for >42 days in the absence of disease progression, or after 6 cycles of therapy if there is no response. |
| Gemtuzumab ozogamicin plus 7+3 therapy and consolidation (favorable- or intermediate-risk AML)37,48 | 3 mg/m2 (maximum, 4.5 mg/m2) intravenous infusion on days 1,4, and 7 of cycle 1 induction | Liver dysfunction, VOD/SOS, fever, myelosuppression, infusion reactions | Induction and consolidation for favorable- or intermediate-risk AML, mild or moderate hepatotoxicity, myelosuppression, or mild or moderate infusion reaction | Hold or discontinue gemtuzumab ozogamicin for severe hepatotoxicity or bleeding. Discontinue gemtuzumab ozogamicin for VOD/SOS or life-threatening anaphylaxis, or at least 2 months before planned allo-SCT. |
| Drug name . | Dose and frequency . | Toxicity . | When to push through . | When to stop drug . |
|---|---|---|---|---|
| Venetoclax/HMAs (azacitidine or decitabine) or low-dose cytarabine23,47 | 400 mg orally once per day (azacitidine or decitabine) or 600 mg orally once per day (low-dose cytarabine) | Myelosuppression, tumor lysis, neutropenia, anemia, thrombocytopenia | First 2 cycles result in mild to moderate renal dysfunction, hepatotoxicity, cytopenias | Reduce dose by 50% for severe liver impairment (Child-Pugh score for cirrhosis). Hold if bone marrow evaluation on days 21 to 28 shows cytoreduction (<5% blasts). In responding patients, consider truncating duration of venetoclax therapy to 7 to 21 days in subsequent cycles; discontinue after 2 to 4 cycles if there is no response. |
| Glasdegib/low-dose cytarabine 20 mg subcutaneously twice per day × 10 days34 | 100 mg orally once per day | Fatigue, febrile neutropenia, dyspnea, anemia, dysgeusia, anorexia, QTc prolongation | Dysgeusia, fatigue, myalgias, cytopenias, QTc 480-500 ms during the first 6 cycles | Hold for QTc prolongation >500 ms and resume at 50 mg; discontinue for life-threatening cardiac arrythmias, or 30 days before donating blood, or for ANC <500 cells per μL and/or platelets <10 000/μL for >42 days in the absence of disease progression, or after 6 cycles of therapy if there is no response. |
| Gemtuzumab ozogamicin plus 7+3 therapy and consolidation (favorable- or intermediate-risk AML)37,48 | 3 mg/m2 (maximum, 4.5 mg/m2) intravenous infusion on days 1,4, and 7 of cycle 1 induction | Liver dysfunction, VOD/SOS, fever, myelosuppression, infusion reactions | Induction and consolidation for favorable- or intermediate-risk AML, mild or moderate hepatotoxicity, myelosuppression, or mild or moderate infusion reaction | Hold or discontinue gemtuzumab ozogamicin for severe hepatotoxicity or bleeding. Discontinue gemtuzumab ozogamicin for VOD/SOS or life-threatening anaphylaxis, or at least 2 months before planned allo-SCT. |
VOD/SOS, veno-occlusive disease/ sinusoidal obstructive syndrome; HMAs, hypomethylating agents; alloSCT, allogeneic stem cell transplant
Management approach for venetoclax plus HMA/low-dose cytarabine. BMBX, bone marrow biopsy; IVF, intravenous fluid; Rx, prescription; Ven, venetoclax.
Management approach for venetoclax plus HMA/low-dose cytarabine. BMBX, bone marrow biopsy; IVF, intravenous fluid; Rx, prescription; Ven, venetoclax.
Despite its designation as a low-intensity regimen, we consider this intermediate-intensity therapy, falling somewhere between 7+3 treatment and HMA/low-dose cytarabine monotherapy. Although it is not required, our practice is to admit all patients for cycle 1. Regardless of the clinical setting, it is essential that all unfit, elderly individuals who start this regimen receive careful monitoring with frequent ideally daily count checks and easy accessibility to blood products. For optimal efficacy, it is important to start venetoclax plus HMA/low-dose cytarabine concurrently, not sequentially (ie, starting HMA/low-dose cytarabine first and adding venetoclax later). Tumor lysis remains a primary concern. Hydroxyurea is administered before therapy is started if the WBC exceeds 25 000 cells per μL. For the first 3 to 5 days, all patients receive frequent laboratory checks, intravenous fluids, and allopurinol. Although gradual daily dose escalation of venetoclax is recommended (ie, 100 mg to 200 mg to 400 mg to 600 mg, if indicated), we have noted no clinically significant tumor lysis with full-dose venetoclax given in the inpatient setting.
Prolonged, often severe, myelosuppression (typically ANC <500 cells per μL or platelets <10 000 to 20 000/μL) associated with venetoclax and HMA/low-dose cytarabine therapy can last several days to weeks with or without clinical sequelae. Management depends on bone marrow response to therapy. Most individuals remain hospitalized during cycle 1 because of the requirements of once-per-day transfusions and/or complications of cytopenias such as neutropenic fever, infection, and bleeding. Dose reductions of venetoclax for concurrent azole use are standard: 70 to 100 mg with posaconazole or voriconazole (ie, strong CYP3A4 inhibitors) and 200 mg with isavuconazole (ie, moderate CYP3A4 inhibitors). Given the life-threatening nature of pulmonary infections in these patients, we perform baseline chest CT scans on all patients before starting therapy with a low threshold to initiate antibiotics and antifungals for any suspect initial or subsequent CT findings.12
To assess response, we perform bone marrow evaluation on cycle 1 day 21. If the bone marrow shows disease control (defined as <5% marrow blasts and/or marrow cellularity <10%), we hold venetoclax for 2 to 4 weeks until hematologic recovery (ANC >500 cells per μL and platelets >50 000/μL) before starting cycle 2 of therapy with both drugs. We often administer growth factor (G-CSF; granulocyte-macrophage colony-stimulating factor [GM-CSF]) in the interim to hasten neutrophil recovery. In contrast, patients with refractory AML without cytoreduction (>5% to 10% blasts and cellularity >10%) on day 21 bone marrow evaluation are continued on once-per-day venetoclax regardless of blood cell counts. Cycle 2 is initiated with both drugs on day 29 with venetoclax administered once per day until repeat bone marrow assessment on days 21 to 28 of cycle 2. Given the median time to response of 1 to 2 cycles for venetoclax-based regimens, the presence of persistent AML after cycle 2 should prompt physicians to consider alternative therapy.
In responding patients, both venetoclax and HMA/low-dose cytarabine should be administered indefinitely until intolerable toxicity or disease recurrence. In patients who maintain normal blood cell counts, no dose reduction or modification of treatment duration is recommended. Similar to others,24,25 we have 2 approaches to treating patients with prolonged cytopenias and no evidence of bone marrow AML: (1) shorten venetoclax duration initially from 28 to 21 days and then to 7 to 14 days on successive cycles to minimize cytopenias without dose reduction, and (2) reduce HMA dose by 33% to 50% and/or extend the time between HMA cycles from 4 to 5 weeks. Additional bone marrow evaluations are performed to rule out relapsed AML as the cause of prolonged cytopenias that are not responding to these measures and routinely every 4 to 6 months to confirm continuing response. In practice, regimen adjustments are highly individualized and shaped by patient preference, logistics, and clinical nuance.
IDH1 and IDH2 inhibitors
Case 2 (follow-up)
The patient achieves a CR with incomplete count recovery with venetoclax and azacitidine. Unfortunately, after 12 months, routine bloodwork shows that he has increased peripheral blasts. Bone marrow biopsy reveals relapsed AML with NRAS, IDH1, and DNMT3A mutations. He elects to take ivosidenib. Three weeks into therapy, he presents to the clinic with new onset fever, hypoxia, weight gain of 10 pounds, and evidence of diffuse bilateral infiltrates on chest CT scan. Laboratory tests demonstrate a WBC of 21 000 cells with predominantly neutrophils and no blasts. A respiratory viral panel is negative. He is started on empiric azithromycin for possible infection and dexamethasone 10 mg orally twice per day. Five days later, he feels much better and continues uninterrupted treatment with ivosidenib.
Ivosidenib and enasidenib are first-in-class oral small molecule inhibitors of mutant isocitrate dehydrogenase-1 (IDH1) and -2 (IDH2), respectively.26,27 Both agents work by decreasing abnormal production of the oncometabolite 2-hydroxyglutarate (2-HG), leading to differentiation of IDH-mutant myeloid blasts. Approximately 20% to 25% of patients on clinical trials develop hyperleukocytosis and clinical evidence of DS, a potentially lethal complication manifested by fluid retention, diffuse pulmonary infiltrates, hypoxia, and fever.28 Onset is delayed with a median time of 19 to 20 days (range, 1-86 days) after treatment initiation. Fatal outcomes occur in 5% to 6% of patients.29,30 Given the long half-life of these inhibitors, first-line treatment of DS is not to stop treatment with ivosidenib or enasidenib but to initiate dexamethasone 10 mg intravenously or orally twice per day for at least 3 days with hydroxyurea and/or leukapheresis for clinically relevant hyperleukocytosis. Inhibitors of IDH1 and IDH2 should be halted for life-threatening complications or lack of improvement after 48 hours, either temporarily or permanently depending on severity (Table 3).
IDH inhibitors: when to push through and when to stop
| Drug name . | Dose and frequency . | Toxicity . | When to push through . | When to stop . |
|---|---|---|---|---|
| Ivosidenib32 | 500 mg orally once per day | DS, QTc prolongation, cytopenias, high WBC | First 6 months, mild to moderate renal and hepatic dysfunction, and mild DS | Hold for severe DS with cardiopulmonary compromise that requires emergent hospitalization with or without renal dysfunction, for QTc prolongation >480 ms (resume when <480 ms). Discontinue for life-threatening cardiac arrythmias, for Guillain-Barré syndrome, or if there is no response (hematologic recovery/blast clearance) after 6 months. |
| Enasidenib33 | 100 mg orally once per day | DS, elevated bilirubin, cytopenias, high WBC | First 6 cycles, mild to moderate renal impairment, mild DS, bilirubin <3× ULN | Hold for severe DS with cardiopulmonary compromise requiring emergent hospitalization with or without renal dysfunction, for bilirubin 3× ULN or more and reduce dose to 50 mg once per day. Discontinue if there is no response (hematologic recovery or blast clearance) after 6 months. |
| Drug name . | Dose and frequency . | Toxicity . | When to push through . | When to stop . |
|---|---|---|---|---|
| Ivosidenib32 | 500 mg orally once per day | DS, QTc prolongation, cytopenias, high WBC | First 6 months, mild to moderate renal and hepatic dysfunction, and mild DS | Hold for severe DS with cardiopulmonary compromise that requires emergent hospitalization with or without renal dysfunction, for QTc prolongation >480 ms (resume when <480 ms). Discontinue for life-threatening cardiac arrythmias, for Guillain-Barré syndrome, or if there is no response (hematologic recovery/blast clearance) after 6 months. |
| Enasidenib33 | 100 mg orally once per day | DS, elevated bilirubin, cytopenias, high WBC | First 6 cycles, mild to moderate renal impairment, mild DS, bilirubin <3× ULN | Hold for severe DS with cardiopulmonary compromise requiring emergent hospitalization with or without renal dysfunction, for bilirubin 3× ULN or more and reduce dose to 50 mg once per day. Discontinue if there is no response (hematologic recovery or blast clearance) after 6 months. |
DS, differentiation syndrome; ULN, upper limit of normal; WBC, white blood cell count
Each inhibitor exhibits distinctive toxicities. More than one-third of patients receiving enasidenib develop asymptomatic increase of indirect bilirubin levels, which is generally self-limited and responds to dose reduction to 50 mg once per day. Almost 25% of patients treated with ivosidenib will have QTc prolongation >450 ms, with 10% developing a QTc interval >500 ms. ECG monitoring should be performed once per week for the first 3 weeks and then once per month for the duration of treatment. Ivosidenib can be resumed at a reduced dose of 250 mg once per day when QTc intervals return to within 30 ms of baseline or ≤480 ms. Another rare complication (incidence, 1%) of ivosidenib that requires permanent discontinuation is Guillain-Barré syndrome, which presents as progressive ascending muscle weakness, loss of reflexes, and sensory neuropathy leading to respiratory failure.31
It is important to recognize that both IDH inhibitors require long-term administration (at least 6 months) for optimal efficacy. In previous studies, the median time to CR with or without hematologic recovery was 2.8 months for ivosidenib and 1.9 months for enasidenib. Over time, many individuals experienced decreased transfusion requirements and fewer clinical events despite incomplete hematopoietic recovery and persistent blasts.32,33
Sonic hedgehog inhibitor (glasdegib)
Case 3
The patient is a 75-year-old man with a history of chronic obstructive pulmonary disease and multiple myeloma after chemotherapy who develops pancytopenia with circulating blasts. Bone marrow biopsy reveals a therapy-related myelodysplastic syndrome with 15% blasts and complex karyotype. Despite several cycles of azacitidine, his disease progresses to AML. Treatment with glasdegib 100 mg orally once per day is started with low-dose cytarabine, and he achieves a partial response after 2 cycles. He presents to the clinic today with progressive weight loss and lack of a sense of taste. ECG demonstrates QTc prolongation to 490 ms (previously 440 ms). Glasdegib is reduced to 50 mg orally once per day. He is prescribed zinc lozenges. Posaconazole treatment is converted to treatment with micafungin, and levofloxacin treatment is changed to treatment with amoxicillin clavulanate.
Glasdegib is an oral sonic hedgehog inhibitor used in combination with low-dose cytarabine for older and/or unfit patients with AML. Glasdegib plus low-dose cytarabine significantly improved CR rates (17.0% vs 2.3%) and OS (8.8 vs 4.9 months; P = .0004) compared with low-dose cytarabine.34 We consider this regimen in individuals at high risk for complications of venetoclax-based therapy (eg, infections, bleeding), specifically patients with several comorbidities and baseline pancytopenia, as well as in individuals who request a 100% outpatient regimen. Inhibition of hedgehog signaling in normal tissues causes adverse effects such as muscle spasms (20%), ageusia/dysgeusia (loss or alteration of the sense of taste, 24%), thinning or loss of hair (10%), and asthenia (fatigue). Other toxicities include anemia, hemorrhage, febrile neutropenia, edema, thrombocytopenia, nausea, dyspnea, constipation, and rash. Five percent of patients develop QTc prolongation >500 ms.34 Management of glasdegib includes close monitoring of ECGs before and after initiation of therapy, antispasmodics, nutritional evaluation and supplementation, and anecdotal treatment with zinc lozenges to alleviate changes in sense of taste. Permanent discontinuation of glasdegib should be implemented for life-threatening cardiac arrythmias or severe neutropenia or thrombocytopenia lasting more than 42 days without overt bone marrow disease. Embryo-fetal toxicity remains a concern. It is recommended that patients do not donate blood products within 30 days of their last dose to avoid exposing pregnant women to the drug. Patients should receive 6 months of therapy for clinical response in the absence of progression (Table 2).
CD33 antibody-drug conjugate
Case 3 (follow-up)
The patient eventually develops worsening cytopenias, and a repeat bone marrow biopsy shows relapsed CD33+ AML (FLT3 and IDH wild-type). After discussion, he elects to start gemtuzumab ozogamicin monotherapy and receives the first dose in the clinic. He receives premedication with acetaminophen, diphenhydramine, and corticosteroid. After infusion, he is monitored every 10 to 15 minutes. Approximately 45 minutes after infusion, he develops fever, chills, and shaking rigors. His oxygen level drops to 85%. The nurse calls for additional orders. Additional doses of acetaminophen, diphenhydramine, and corticosteroid are given and he gradually improves. Vital signs return to normal, and he is discharged home 2 hours after infusion. One week after therapy, he has mild scleral icterus associated with acute increases in liver function tests: total bilirubin 4.5 mg/dL, AST 287, and AST 321. Hepatitis panel is negative. Right upper quadrant ultrasound and amylase/lipase levels are unremarkable. He is started on ursodiol (ursodeoxycholic acid) 3 times per day with gradual improvement and normalization of liver function tests.
Gemtuzumab ozogamicin is an antibody-drug conjugate composed of a monoclonal antibody directed against CD33 expressed on myeloid cells covalently linked to the DNA damaging agent calicheamicin. Gemtuzumab ozogamicin has been approved for treating patients with newly diagnosed CD33+ AML characterized by favorable-risk (specifically core binding factor) and intermediate-risk cytogenetics in combination with 7+3 induction and consolidation chemotherapy.35-38 In the United States, gemtuzumab ozogamicin is also indicated as monotherapy for unfit patients with newly diagnosed AML and patients with R/R AML. In combination with chemotherapy, gemtuzumab ozogamicin is dosed on a hyperfractionated regimen of 3 mg/m2 (maximum dose, 4.5 mg/m2) per dose on days 1, 4, and 7. Adverse effects included veno-occlusive disease (VOD, also known as sinusoidal obstructive syndrome [SOS]), prolonged platelet recovery, severe hemorrhage, and infusion reactions during and up to 24 hours after infusion. Premedications (ie, acetaminophen, diphenhydramine, and corticosteroid) should be given before each dose of gemtuzumab ozogamicin with frequent checks of vital signs and clinical monitoring before and immediately after infusion. Patients should be carefully monitored on at least a once-per-week basis to determine whether they need additional platelet transfusions to minimize bleeding risk as well as liver function tests. Gemtuzumab ozogamicin should be avoided in individuals with known hepatic dysfunction and for 2 months before allo-SCT. Any life-threatening anaphylaxis and VOD or SOS warrant permanent discontinuation of the drug (Table 3). Results of an expanded access trial in R/R AML confirmed the safety and feasibility of gemtuzumab ozogamicin administered alone or with chemotherapy in patients age 3 months or older. Hepatotoxicity was infrequent, with a VOD incidence of 0.5% to 1.1%39 (Table 2).
Financial toxicities
Prohibitively high cost is common to all agents, also known as financial toxicity. Currently, the average wholesale price for 1 tablet of gilteritinib is estimated to be $315.0040 ; ivosidenib, $548.4241 ; enasidenib, $1081.1942 ; glasdegib, $710.8543 ; midostaurin, $192.1844 ; and venetoclax 100 mg, $122.92.45 Therefore a 1-month supply would cost $7000 to $28 000. Addressing this issue requires dedicated clinical, pharmacy, social work, and case management staff. Given the aggressive clinical course of AML, timely insurance authorizations are needed. Philanthropic foundations, such as Patient Access Network Foundation, Cancercare, Good Days, and the Leukemia Lymphoma Society should also be pursued.
Conclusion
There are now myriad effective targeted agents for AML, an embarrassment of riches, after 4 decades of 7+3 treatment. Each of these agents has a distinctive mechanism of action and unique toxicity profile (summarized by organ toxicity in Table 4). Knowing when to push through and when to stop therapy is essential to striking the elusive balance between prolonging survival and preserving meaningful quality of life. It is our hope that these recommendations prove useful to both clinicians and patients who are navigating the difficult journey of AML therapy together.
Toxicities of AML therapies by organ system
| Toxicity . | Responsible drug(s) . | Description . | Treatment of toxicity . | When to stop drug . |
|---|---|---|---|---|
| Hepatotoxicity | Gemtuzumab ozogamicin | VOD/SOS, elevated AST, ALT, bilirubin | Monitor liver function test results frequently, use ursodiol, avoid administration in patients with known hepatic dysfunction, use defibrotide for VOD/SOS | Hold or discontinue gemtuzumab ozogamicin for severe hepatotoxicity; discontinue for VOD/SOS and at least 2 months before planned allo-SCT. |
| Enasidenib | Asymptomatic elevation of indirect bilirubin | Dose reduce to 50 mg once per day | No need to stop drug. | |
| Venetoclax | Elevated liver enzymes, bilirubin | Supportive care with no dose reduction | Reduce dose by 50% for severe liver impairment (Child-Pugh score for cirrhosis). | |
| Gilteritinib | Elevated liver function tests (transaminases, bilirubin), pancreatitis | Hold for elevated liver function tests (AST and ALT >5× ULN, bilirubin >3× ULN) and restart at 80 mg once per day | Hold for elevated liver function tests (AST and ALT >5× ULN, bilirubin >3× ULN) and restart at 80 mg once per day; hold drug for pancreatitis. | |
| Nephrotoxicity | Venetoclax + chemotherapy | Tumor lysis syndrome | Allopurinol, intravenous hydration, tumor lysis laboratory tests, frequent monitoring | Severe renal dysfunction in setting of tumor lysis. |
| Embryo-fetal toxicity | Glasdegib | Embryo-fetal toxicity | Withhold therapy in all individuals suspected of being pregnant, contraceptives | Do not give to individuals who are pregnant or breastfeeding; do not give blood within 30 days of last dose. |
| Neurologic complications | Glasdegib | Fatigue (asthenia), ageusia/dysgeusia (loss or alteration of sense of taste), anorexia, myalgias | Symptomatic measures, nonsteroidal anti-inflammatory drugs, zinc lozenges, analgesics | Hold for significant debilitating weight loss. |
| Gilteritinib | Posterior reversible encephalopathy syndrome | MRI scans, antiseizure medications | Permanently discontinue drug. | |
| Ivosidenib | Guillain-Barré syndrome | Lumbar puncture, MRI, ventilatory support | Permanently discontinue drug. | |
| Cardiotoxicity | Glasdegib | QTc prolongation | Perform ECGs before, and 1 week after intitation of therapy, and monthly for 2 months, supplement electrolytes, adjust other medications | Hold for significant QTC prolongation or development of cardiac arrhythmias. Reduce dose to 50 mg once per day, and discontinue for life-threatening cardiac arrythmias. |
| Midostaurin | QTc prolongation | ECGs once per week, electrolytes, substitute other medications | Hold for significant QTC prolongation or development of cardiac arrhythmias (dose related) per clinical trial. | |
| Gilteritinib | Orthostatic hypotension | Intravenous fluids, adjust blood pressure medications, add midodrine and/or fludrocortisone | Hold for severe symptomatic orthostasis. | |
| Gilteritinib | QTc prolongation | Electrolyte supplementation, baseline ECGs before cycle 1, on days 8 and 15, and start of cycles 2 and 3 | Hold for QTc prolongation >500 ms. | |
| Ivosidenib | QTc prolongation | Electrolyte supplementation, adjust other medications, ECG at baseline and once per week for 3 weeks, then once per month during treatment | Hold for significant QTC prolongation >500 ms or development of cardiac arrhythmias; reduce dose to 250 mg once per day. | |
| Gastrointestinal toxicity | Midostaurin 50 mg twice per day × 14 days | Nausea, vomiting, diarrhea, mucositis | Antiemetics before each dose, take with food, air each tablet outside of blister pack, rule out gastrointestinal infection, make up missed doses | Discontinue for life-threatening cardiac and peripheral vascular events. |
| Glasdegib | Ageusia/dysgeusia (loss or alteration of sense of taste), anorexia | Symptomatic measures, nonsteroidal anti-inflammatory drugs, zinc lozenges, analgesics | Hold for significant debilitating weight loss. | |
| Pulmonary toxicity | Gilteritinib | DS (2-75 days) | Dexamethasone intravenously or orally, oxygen, respiratory support, hydroxyurea, leukopheresis | Severe DS with life-threatening complications or no improvement after 48 hours of steroid treatment. |
| Ivosidenib | DS (1-86 days) | Dexamethasone intravenously or orally, oxygen, respiratory support, hydroxyurea, leukopheresis | Severe DS with life-threatening complications or no improvement after 48 hours of steroid treatment. | |
| Enasidenib | DS (1-86 days) | Dexamethasone intravenously or orally, oxygen, respiratory support, hydroxyurea, leukopheresis | Severe DS with life-threatening complications or no improvement after 48 hours of steroid treatment. | |
| Midostaurin | Drug-related interstitial pneumonia | Consider baseline chest CT before therapy, and rule out infections | Discontinue drug. | |
| Venetoclax + chemotherapy | Respiratory infections, pneumonia, | Consider baseline chest CT, Infectious Disease consult, antibiotics, antifungals, fungal markers | Do not stop drug during cycle 1 until bone marrow evaluation is complete. | |
| Hematologic toxicity | Venetoclax + chemotherapy | Prolonged myelosuppression | Daily transfusion support, neutropenic prophylaxis | Hold if cycle 1 days 21 to 28 bone marrow evaluation shows cytoreduction (<5% blasts), consider truncating duration of venetoclax therapy to 7-21 d in subsequent cycles. |
| Glasdegib | Prolonged myelosuppression | Transfusion support, neutropenic prophylaxis | Discontinue for ANC <500 cells per μL and/or platelets <10 000/μL for >42 days in the absence of disease progression. | |
| Gemtuzumab ozogamicin | Prolonged thrombocytopenia with risk of severe hemorrhages | Transfusion support, monitor counts once per week with frequent platelet transfusions, and take precautions to avoid falls | Hold or discontinue gemtuzumab ozogamicin for severe bleeding. | |
| Immunologic toxicity | Gemtuzumab ozogamicin | Infusion reaction before and 24 hours after dosing | Premedication (acetaminophen, diphenhydramine, corticosteroid) before each dose, frequent vital signs, clinical monitoring | Discontinue gemtuzumab ozogamicin for life-threatening anaphylaxis. |
| Toxicity . | Responsible drug(s) . | Description . | Treatment of toxicity . | When to stop drug . |
|---|---|---|---|---|
| Hepatotoxicity | Gemtuzumab ozogamicin | VOD/SOS, elevated AST, ALT, bilirubin | Monitor liver function test results frequently, use ursodiol, avoid administration in patients with known hepatic dysfunction, use defibrotide for VOD/SOS | Hold or discontinue gemtuzumab ozogamicin for severe hepatotoxicity; discontinue for VOD/SOS and at least 2 months before planned allo-SCT. |
| Enasidenib | Asymptomatic elevation of indirect bilirubin | Dose reduce to 50 mg once per day | No need to stop drug. | |
| Venetoclax | Elevated liver enzymes, bilirubin | Supportive care with no dose reduction | Reduce dose by 50% for severe liver impairment (Child-Pugh score for cirrhosis). | |
| Gilteritinib | Elevated liver function tests (transaminases, bilirubin), pancreatitis | Hold for elevated liver function tests (AST and ALT >5× ULN, bilirubin >3× ULN) and restart at 80 mg once per day | Hold for elevated liver function tests (AST and ALT >5× ULN, bilirubin >3× ULN) and restart at 80 mg once per day; hold drug for pancreatitis. | |
| Nephrotoxicity | Venetoclax + chemotherapy | Tumor lysis syndrome | Allopurinol, intravenous hydration, tumor lysis laboratory tests, frequent monitoring | Severe renal dysfunction in setting of tumor lysis. |
| Embryo-fetal toxicity | Glasdegib | Embryo-fetal toxicity | Withhold therapy in all individuals suspected of being pregnant, contraceptives | Do not give to individuals who are pregnant or breastfeeding; do not give blood within 30 days of last dose. |
| Neurologic complications | Glasdegib | Fatigue (asthenia), ageusia/dysgeusia (loss or alteration of sense of taste), anorexia, myalgias | Symptomatic measures, nonsteroidal anti-inflammatory drugs, zinc lozenges, analgesics | Hold for significant debilitating weight loss. |
| Gilteritinib | Posterior reversible encephalopathy syndrome | MRI scans, antiseizure medications | Permanently discontinue drug. | |
| Ivosidenib | Guillain-Barré syndrome | Lumbar puncture, MRI, ventilatory support | Permanently discontinue drug. | |
| Cardiotoxicity | Glasdegib | QTc prolongation | Perform ECGs before, and 1 week after intitation of therapy, and monthly for 2 months, supplement electrolytes, adjust other medications | Hold for significant QTC prolongation or development of cardiac arrhythmias. Reduce dose to 50 mg once per day, and discontinue for life-threatening cardiac arrythmias. |
| Midostaurin | QTc prolongation | ECGs once per week, electrolytes, substitute other medications | Hold for significant QTC prolongation or development of cardiac arrhythmias (dose related) per clinical trial. | |
| Gilteritinib | Orthostatic hypotension | Intravenous fluids, adjust blood pressure medications, add midodrine and/or fludrocortisone | Hold for severe symptomatic orthostasis. | |
| Gilteritinib | QTc prolongation | Electrolyte supplementation, baseline ECGs before cycle 1, on days 8 and 15, and start of cycles 2 and 3 | Hold for QTc prolongation >500 ms. | |
| Ivosidenib | QTc prolongation | Electrolyte supplementation, adjust other medications, ECG at baseline and once per week for 3 weeks, then once per month during treatment | Hold for significant QTC prolongation >500 ms or development of cardiac arrhythmias; reduce dose to 250 mg once per day. | |
| Gastrointestinal toxicity | Midostaurin 50 mg twice per day × 14 days | Nausea, vomiting, diarrhea, mucositis | Antiemetics before each dose, take with food, air each tablet outside of blister pack, rule out gastrointestinal infection, make up missed doses | Discontinue for life-threatening cardiac and peripheral vascular events. |
| Glasdegib | Ageusia/dysgeusia (loss or alteration of sense of taste), anorexia | Symptomatic measures, nonsteroidal anti-inflammatory drugs, zinc lozenges, analgesics | Hold for significant debilitating weight loss. | |
| Pulmonary toxicity | Gilteritinib | DS (2-75 days) | Dexamethasone intravenously or orally, oxygen, respiratory support, hydroxyurea, leukopheresis | Severe DS with life-threatening complications or no improvement after 48 hours of steroid treatment. |
| Ivosidenib | DS (1-86 days) | Dexamethasone intravenously or orally, oxygen, respiratory support, hydroxyurea, leukopheresis | Severe DS with life-threatening complications or no improvement after 48 hours of steroid treatment. | |
| Enasidenib | DS (1-86 days) | Dexamethasone intravenously or orally, oxygen, respiratory support, hydroxyurea, leukopheresis | Severe DS with life-threatening complications or no improvement after 48 hours of steroid treatment. | |
| Midostaurin | Drug-related interstitial pneumonia | Consider baseline chest CT before therapy, and rule out infections | Discontinue drug. | |
| Venetoclax + chemotherapy | Respiratory infections, pneumonia, | Consider baseline chest CT, Infectious Disease consult, antibiotics, antifungals, fungal markers | Do not stop drug during cycle 1 until bone marrow evaluation is complete. | |
| Hematologic toxicity | Venetoclax + chemotherapy | Prolonged myelosuppression | Daily transfusion support, neutropenic prophylaxis | Hold if cycle 1 days 21 to 28 bone marrow evaluation shows cytoreduction (<5% blasts), consider truncating duration of venetoclax therapy to 7-21 d in subsequent cycles. |
| Glasdegib | Prolonged myelosuppression | Transfusion support, neutropenic prophylaxis | Discontinue for ANC <500 cells per μL and/or platelets <10 000/μL for >42 days in the absence of disease progression. | |
| Gemtuzumab ozogamicin | Prolonged thrombocytopenia with risk of severe hemorrhages | Transfusion support, monitor counts once per week with frequent platelet transfusions, and take precautions to avoid falls | Hold or discontinue gemtuzumab ozogamicin for severe bleeding. | |
| Immunologic toxicity | Gemtuzumab ozogamicin | Infusion reaction before and 24 hours after dosing | Premedication (acetaminophen, diphenhydramine, corticosteroid) before each dose, frequent vital signs, clinical monitoring | Discontinue gemtuzumab ozogamicin for life-threatening anaphylaxis. |
MRI, magnetic resonance imaging; DS, differentiation syndrome; VOD/SOS, veno-occlusive disease/sinusoidal obstructive syndrome.
Acknowledgments
The authors thank the clinicians, pharmacists, nurses, and patients at the Roswell Park Clinical Leukemia Service. Their daily invaluable input, discussions, and real-life experiences served as the basis for the above recommendations. They thank Ali McBride, BCOP, FASHP, FAzPA, University of Arizona Cancer Center for his assistance with the financial toxicities portion.
This work was supported by a National Institutes of Health, National Cancer Institute grant (P30CA016056) to Roswell Park Comprehensive Cancer Center. E.S.W. is supported by the Roswell Park Alliance Foundation (Jacquie Hirsch Leukemia Research Fund).
References
Competing Interests
Conflict-of-interest disclosure: E.S.W. served on advisory boards and/or provided consulting services for AbbVie, Astellas, Daiichi Sankyo, Dava Oncology/Arog, Genentech, Jazz, Kite Pharmaceuticals, Kura Oncology, Macrogenics, Pfizer, PTC Therapeutics, and Stemline; served on independent data review committees for clinical trials for AbbVie, Genentech, and Rafael Pharmaceuticals; and served as a speaker for Stemline and Pfizer. J.B. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.
Correspondence Eunice S. Wang, Department of Medicine, Roswell Park Comprehensive Cancer Center, Elm and Carlton Sts, Buffalo, NY 14263; e-mail: eunice.wang@roswellpark.org.