Abstract
An estimated 1 million people in the United States have functional or anatomic asplenia or hyposplenia. Infectious complications due to encapsulated organisms such as Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae can lead to fulminant sepsis and death, particularly in young children, in the period shortly after splenectomy, and in immunocompromised patients. Patients with asplenia are also at risk for less common infections due to Capnocytophaga, Babesia, and malaria. Antibiotic prophylaxis, vaccines, and patient and family education are the mainstays of prevention in these at-risk patients. Recommendations for antibiotic prophylaxis typically target high-risk periods, such as 1 to 3 years after splenectomy, children ≤5 years of age, or patients with concomitant immunocompromise. However, the risk for sepsis is lifelong, with infections occurring as late as 40 years after splenectomy. Currently available vaccines recommended for patients with asplenia include pneumococcal vaccines (13-valent pneumococcal conjugate vaccine followed by the 23-valent pneumococcal polysaccharide vaccine), meningococcal vaccines (meningococcal conjugate vaccines for serogroups A, C, Y and W-135 and serogroup B meningococcal vaccines), H. influenzae type b vaccines, and inactivated influenza vaccines. Ongoing booster doses are also recommended for pneumococcal and meningococcal vaccines to maintain protection. Despite the availability of prevention tools, adherence is often a challenge. Dedicated teams or clinics focused on patient education and monitoring have demonstrated substantial improvements in vaccine coverage rates for individuals with asplenia and reduced risk of infection. Future efforts to monitor the quality of care in patients with asplenia may be important to bridge the know–do gap in this high-risk population.
Learning Objectives
Describe infection risks associated with asplenia
Understand the latest recommendations for immunizing children and adults with asplenia
Introduction
Asplenia or hyposplenia occurs when there is a loss of function of the spleen that may be either anatomic or functional in nature. Anatomic asplenia is most commonly due to surgical removal secondary to trauma or for therapeutic reasons (eg, immune thrombocytopenic purpura [ITP], autoimmune hemolytic anemia, hereditary spherocytosis) or autoinfarction in sickle cell disease, and it is rarely due to congenital asplenia syndromes (eg, isolated congenital asplenia, heterotaxy syndromes). Functional asplenia or hyposplenism can be secondary to hematological diseases (eg, sickle cell disease, hemoglobinopathies), oncologic conditions (eg, chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplant), immunological reasons (eg, antiphospholipid syndrome, severe celiac disease, autoimmune diseases), or untreated HIV infection.1,2 The spleen functions as a critical organ of the reticuloendothelial system, serving as a filter for senescent blood cells and opsonized bacteria. Its role in preventing infections includes the ability to trigger innate and adaptive immune responses to pathogens, including encapsulated bacteria.
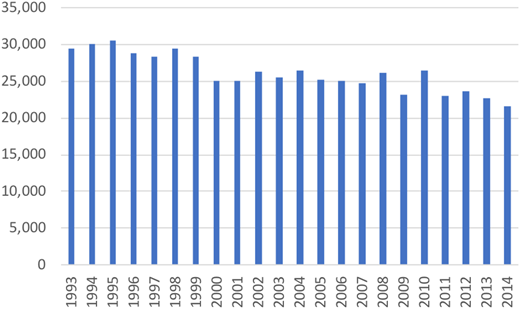
Currently, an estimated 1 million individuals in the United States are asplenic or hyposplenic, with ∼100 000 cases being due to sickle cell disease.3,4 With growing recognition of the harms associated with asplenia, indications for splenectomy have evolved over the past 2 decades.5 National data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project between 1993 and 2014 indicate a modest decline in the number of splenectomies performed each year from ∼30 000/y to 22 000/y (Figure 1).6 The downward trend may be due in part to advances in therapies such as the use of rituximab, immunosuppressive agents, or thrombopoietin receptor agonists for hemolytic anemia and ITP. Modest variability by country is noted regarding indications for splenectomy for medical conditions, with the United States and Italy performing splenectomies for ITP and lymphoma more commonly than other countries where spherocytosis and sickle cell disease are predominant indications for splenectomy.7 Aside from trauma, indications for splenectomy have been more commonly attributed to hematologic or oncologic indications.
Total number of US hospitalizations associated with total splenectomy, 1993 to 2014.6
Total number of US hospitalizations associated with total splenectomy, 1993 to 2014.6
Infectious complications associated with asplenia or hyposplenia
Asplenia can lead to infectious and noninfectious complications, including infection, thrombosis, and pulmonary hypertension.8 We focus on the critical role of the spleen in infection, which is a major and potentially preventable complication of asplenia. The spleen contains 2 types of tissues with different functions. The white pulp is rich in T-cell lymphocytes, macrophages, and naïve B-cell lymphocytes. Antigen-presenting cells can enter the white pulp and activate T cells, which in turn activate naïve B cells and differentiate into plasma cells that generate immunoglobulin M antibodies followed by immunoglobulin G antibodies. B cells can also serve as antigen-presenting cells, as well as support phagocytic function to help opsonize encapsulated bacteria. The B-cell immune response is critical in the defense against encapsulated organisms. The red pulp is rich in macrophages and is responsible for filtering older, damaged red blood cells as well as phagocytosing opsonized bacteria. Because of its role in removing damaged erythrocytes, the spleen also plays an important role in the defense against intraerythrocytic parasitic infections such as malaria and Babesia.
The most feared complication of asplenia is overwhelming infection, due to either functional or anatomic asplenia, and is associated with mortality rates as high as 50% in the absence of prevention strategies such as vaccines and antibiotic prophylaxis. Pneumonia and meningitis may be more likely to occur; purpura fulminans episodes are also often associated with asplenic or hyposplenic states.9 The incidence of sepsis after splenectomy in children is ∼1.8 to 3 per 100 person-years, with children aged <3 years having the highest risk for infection.10 In contrast, the risk for infection is generally higher in adults, particularly in adults aged ≥60 years (11 to 14 per 100 person-years).11 The age-dependent risk may reflect a combination of indications for splenectomy and the risk of exposure to pathogens such as Streptococcus pneumoniae. Patients with thalassemia, sickle cell disease, or malignancy as indications for splenectomy may portend a higher future risk of sepsis or overwhelming post-splenectomy infection.12,13 In addition, the incidence of sepsis after splenectomy is substantially higher in the first 1 to 3 years after splenectomy, though infection can occur as late as 50 years after the procedure.10,13,14 Partial splenectomy has been used as an alternative to total splenectomy, particularly for hematologic disorders, in order to reduce the burden of significant hemolysis or thrombocytopenia while salvaging splenic immune function.15 The benefits of partial splenectomy are likely greatest in subpopulations with the highest risk of infection (eg, young age, lack of alternative management strategies, risk of exposure based on geographic location or occupation). However, patients with partial splenectomy may subsequently require a total splenectomy if the hematologic response is not sufficient. Currently, there are limited data available to determine whether partial or total splenectomy substantially changes the benefit–risk balance in patients with hematologic conditions, and it remains a shared decision based on context, values, and preferences.16
Infections in patients with asplenia or hyposplenia are most commonly due to encapsulated bacteria, including pneumococcal, meningococcal, and Haemophilus influenzae infections. Capnocytophaga canimorsus secondary to dog bites is also reported as a cause of sepsis.17 Due to the role of the spleen in clearing damaged or infected erythrocytes, severe infections due to intracellular pathogens such as Babesia, Bartonella, and Plasmodia species have also been described in the literature.18-21
Management of patients with asplenia with fever
The most common presentation of infection in patients with asplenia is fever. These patients are at risk for rapid and fulminant progression due to encapsulated organisms.14 Although the risk is lifelong, the risk may be particularly high for certain patients based on the indication for splenectomy, age at time of splenectomy, interval since splenectomy, risk of exposure to encapsulated organisms (ie, travel, age, low population vaccine coverage rates), underlying comorbidities, immunocompromise, and prior episode of sepsis.10,22-25 Early detection and management of suspected infections is critical in the management of patients with asplenia. Early empiric treatment with antibiotics with any seemingly minor signs of infection, with specific recommendations for an emergency oral antibiotic supply on hand, should be given at the first sign of infection (Table 1). Patients with fever should subsequently seek emergency department care immediately after taking oral antibiotics for further evaluation, diagnostic testing, and administration of intravenous antibiotics. In general, ceftriaxone serves as the backbone of treatment, and some experts also recommend the addition of intravenous vancomycin, depending on local antimicrobial susceptibility patterns and clinical presentation. For patients with cephalosporin allergy, fluoroquinolones or carbapenems may be used as an alternative, although resistance patterns should be monitored for common pathogens. Patients may also be admitted pending diagnostic evaluation.
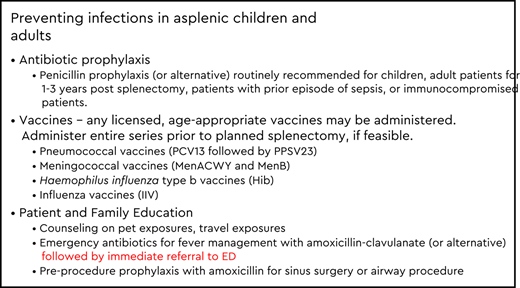
Antibiotic prophylaxis for patients with asplenia
| Routine prophylaxis* | |
| <3 y | PCN VK 125 mg twice daily (or amoxicillin 10 mg/kg by mouth twice daily) |
| ≥3 y | PCN VK 250 mg twice daily |
| Adults | PCN VK 250 mg by mouth twice daily (or amoxicillin 500 mg by mouth twice daily) |
| Adults with PCN allergy | Cephalexin 250 mg by mouth twice daily |
| Azithromycin 250 mg by mouth once daily | |
| Emergency antibiotics before ED arrival | |
| Child | Amoxicillin-clavulanate 45 mg/kg by mouth twice daily (maximum 875 mg per dose) |
| Child with PCN allergy | Cefdinir 7 mg/kg by mouth twice daily (max 300 mg per dose) |
| Levofloxacin 10 mg/kg by mouth twice daily (max 375 mg per dose) | |
| Adult | Amoxicillin-clavulanate 875 mg/125 mg by mouth twice daily |
| Adult with PCN allergy | Cefdinir 300 mg by mouth twice daily |
| Levofloxacin 750 mg by mouth once daily | |
| Moxifloxacin 400 mg by mouth once daily | |
| Preprocedural prophylaxis (for sinus surgery or airway procedure) | |
| Child | Amoxicillin 50 mg/kg 1 h before procedure (max 2 gram) |
| Adult | Amoxicillin 2 g 30-60 min before procedure |
| Routine prophylaxis* | |
| <3 y | PCN VK 125 mg twice daily (or amoxicillin 10 mg/kg by mouth twice daily) |
| ≥3 y | PCN VK 250 mg twice daily |
| Adults | PCN VK 250 mg by mouth twice daily (or amoxicillin 500 mg by mouth twice daily) |
| Adults with PCN allergy | Cephalexin 250 mg by mouth twice daily |
| Azithromycin 250 mg by mouth once daily | |
| Emergency antibiotics before ED arrival | |
| Child | Amoxicillin-clavulanate 45 mg/kg by mouth twice daily (maximum 875 mg per dose) |
| Child with PCN allergy | Cefdinir 7 mg/kg by mouth twice daily (max 300 mg per dose) |
| Levofloxacin 10 mg/kg by mouth twice daily (max 375 mg per dose) | |
| Adult | Amoxicillin-clavulanate 875 mg/125 mg by mouth twice daily |
| Adult with PCN allergy | Cefdinir 300 mg by mouth twice daily |
| Levofloxacin 750 mg by mouth once daily | |
| Moxifloxacin 400 mg by mouth once daily | |
| Preprocedural prophylaxis (for sinus surgery or airway procedure) | |
| Child | Amoxicillin 50 mg/kg 1 h before procedure (max 2 gram) |
| Adult | Amoxicillin 2 g 30-60 min before procedure |
ED, emergency department; PCN VK, penicillin V potassium.
Duration of routine prophylaxis depends on age, time since splenectomy, degree of immunocompromise, or prior episode of sepsis.
Prevention: antibiotic prophylaxis
Daily antibiotic prophylaxis is typically recommended after splenectomy or for conditions such as sickle cell disease in which functional asplenia or autosplenectomy occurs on the basis of trials that demonstrated a 50% to 63% reduction in pneumococcal infection among patients with sickle cell disease receiving penicillin prophylaxis in children aged ≤5 years.26 Common regimens are listed in Table 1. However, there is little consensus on the duration of use. Lifelong prophylaxis is often recommended for patients with asplenia who previously experienced an episode of sepsis or who remain immunocompromised. In nonimmunocompromised populations, US and Australian guidelines recommend prophylaxis for 1 to 3 years after splenectomy or until a certain age threshold (ie, >5 years).22,27 In the United Kingdom, antibiotic prophylaxis is recommended for children aged <16 years, adults aged >50 years, and patients with an inadequate serologic response to pneumococcal vaccination.28 Of note, serologic testing is not routinely recommended for patients with asplenia. Rather, clinicians should consider whether patients are considered immunodeficient due to underlying disease or treatment and should ensure that on-time vaccination and continued antibiotic prophylaxis are administered. Additional investigation regarding the optimal duration of antibiotic therapy is warranted, given the epidemiologic shifts in colonization and infection due to newer vaccines.
Prevention: vaccines
Perhaps the most effective prevention method for patients with asplenia or hyposplenia is to vaccinate, ideally >2 weeks before a planned splenectomy. In addition to routine childhood and adult vaccines, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention and the Infectious Diseases Society of America recommends pneumococcal, meningococcal, H. influenzae type b (Hib), and influenza vaccinations for this at-risk population. The vaccine schedule recommended by ACIP depends on vaccination history, age at the time of splenectomy, and whether the procedure was elective or emergent (Tables 2 through 4). Conjugate vaccines are preferred for priming the immune response, particularly for patients with asplenia, given the higher opsonophagocytic activity geometric mean antibody titers elicited by conjugate vs polysaccharide vaccines. In addition, polysaccharide vaccines are poorly immunogenic and may lead to hyporesponsiveness to subsequent doses.29 If patients are unable to receive at least one dose of vaccine before splenectomy, administration of needed vaccines should be initiated ∼2 weeks after splenectomy. We describe specific recommendations below for the United States; however, it should be noted that recommendations may vary by country.30
ACIP recommendations for pneumococcal vaccines in children and adults with asplenia or hyposplenia49,50
| Age at first dose . | Timing of first dose PCV13 . | Total doses of PCV13 . | Interval between PCV13 doses . | Timing of first dose of PPSV23 . | Total doses of PPSV23 . | Interval between PPSV23 doses . |
|---|---|---|---|---|---|---|
| No prior PCV13 or PPSV23 | ||||||
| 8 wk | — | 4 doses | 2-mo, 2-mo, 6-mo intervals | — | — | — |
| 6-18 y | — | 1 dose | — | >8 wk after PCV13 dose | 2 doses | 5 y |
| 19-64 y | — | 1 dose | — | >8 wk after PCV13 dose | 2 doses | 5 y |
| ≥65 y* | — | 1 dose | — | >8 wk after PCV13 dose | 1 dose | — |
| Any PCV13; no prior PPSV23 | ||||||
| 2-5 y + <3 prior PCV13 doses | ≥8 wk after prior PCV13 dose | 2 doses | 8-wk interval | >8 wk after prior PCV13 dose | 2 doses | 5 y |
| 2-5 y + 3 prior PCV13 doses | ≥8 wk after prior PCV13 dose | 1 dose | — | >8 wk after prior PCV13 dose | 2 doses | 5 y |
| 6-18 y | — | — | — | >8 wk after prior PCV13 dose | 2 doses | 5 y |
| 19-64 y | — | 1 dose | — | >8 wk after PCV13 dose | 2 doses | 5 y |
| ≥65 y* | — | 1 dose | — | >8 wk after PCV13 dose | 1 dose | — |
| Any PPSV23; no prior PCV13 | ||||||
| 6-18 y | ≥8 wk after prior PPSV23 dose | 1 dose | — | >8 wk after PCV13 dose | 1 dose | 5 y |
| 19-64 y | ≥1 y after prior PPSV23 dose | 1 dose | — | >8 wk after PCV13 dose | 1 dose | 5 y |
| ≥65 y* | ≥1 y after prior PPSV23 dose | 1 dose | — | >8 wk after PCV13 dose | 1 dose | 5 y |
| Age at first dose . | Timing of first dose PCV13 . | Total doses of PCV13 . | Interval between PCV13 doses . | Timing of first dose of PPSV23 . | Total doses of PPSV23 . | Interval between PPSV23 doses . |
|---|---|---|---|---|---|---|
| No prior PCV13 or PPSV23 | ||||||
| 8 wk | — | 4 doses | 2-mo, 2-mo, 6-mo intervals | — | — | — |
| 6-18 y | — | 1 dose | — | >8 wk after PCV13 dose | 2 doses | 5 y |
| 19-64 y | — | 1 dose | — | >8 wk after PCV13 dose | 2 doses | 5 y |
| ≥65 y* | — | 1 dose | — | >8 wk after PCV13 dose | 1 dose | — |
| Any PCV13; no prior PPSV23 | ||||||
| 2-5 y + <3 prior PCV13 doses | ≥8 wk after prior PCV13 dose | 2 doses | 8-wk interval | >8 wk after prior PCV13 dose | 2 doses | 5 y |
| 2-5 y + 3 prior PCV13 doses | ≥8 wk after prior PCV13 dose | 1 dose | — | >8 wk after prior PCV13 dose | 2 doses | 5 y |
| 6-18 y | — | — | — | >8 wk after prior PCV13 dose | 2 doses | 5 y |
| 19-64 y | — | 1 dose | — | >8 wk after PCV13 dose | 2 doses | 5 y |
| ≥65 y* | — | 1 dose | — | >8 wk after PCV13 dose | 1 dose | — |
| Any PPSV23; no prior PCV13 | ||||||
| 6-18 y | ≥8 wk after prior PPSV23 dose | 1 dose | — | >8 wk after PCV13 dose | 1 dose | 5 y |
| 19-64 y | ≥1 y after prior PPSV23 dose | 1 dose | — | >8 wk after PCV13 dose | 1 dose | 5 y |
| ≥65 y* | ≥1 y after prior PPSV23 dose | 1 dose | — | >8 wk after PCV13 dose | 1 dose | 5 y |
For adults aged ≥65 y, routine pneumococcal vaccination is recommended. For patients with asplenia or hyposplenia, consider PCV13 before PPSV23 administration. Only 1 dose of PPSV23 is recommended for adults aged 65 y with immunocompromising conditions.
ACIP recommendations for meningococcal ACWY and meningococcal B vaccines in children and adults with asplenia or hyposplenia33,34,37,38,51
| Age at first dose . | Timing of first dose of meningococcal vaccine . | Total doses of meningococcal vaccine . | Interval between meningococcal vaccines . | Revaccination . |
|---|---|---|---|---|
| MenACWY-CRM (MENVEO) | ||||
| 8 wk | — | 4-dose series | 2-mo, 2-mo, 6-mo intervals | Revaccinate 3 y after primary series; additional boosters every 5 y if risk remains |
| 7-23 mo | — | 2-dose series | 12-wk interval and second dose at ≥12 mo of age | Revaccinate 3 y after primary series; additional boosters every 5 y if risk remains |
| ≥24 mo | — | 2-dose series | 8-wk interval | Revaccinate 3 y after primary series if most recent dose given before age 7 y; additional boosters every 5 y if risk remains |
| MenACWY-D (Menactra) | ||||
| 9-23 mo | Do not administer, because of immune interference with PCV13 | |||
| ≥24 mo | Administer >4 wk after completion of PCV13 series | 2-dose series | 8-wk interval | Revaccinate 3 y after primary series; additional boosters every 5 y if risk remains |
| MenB-4C (BEXSERO)* | ||||
| ≥10 y | — | 2-dose series | 1-mo interval | Revaccinate 1 y after primary series and revaccinate every 2-3 y if risk remains† |
| MenB-FHbp (Trumenba)* | ||||
| ≥10 y | — | 3-dose series | 1-mo, 4-mo intervals | Revaccinate 1 y after primary series and revaccinate every 2-3 y if risk remains† |
| Age at first dose . | Timing of first dose of meningococcal vaccine . | Total doses of meningococcal vaccine . | Interval between meningococcal vaccines . | Revaccination . |
|---|---|---|---|---|
| MenACWY-CRM (MENVEO) | ||||
| 8 wk | — | 4-dose series | 2-mo, 2-mo, 6-mo intervals | Revaccinate 3 y after primary series; additional boosters every 5 y if risk remains |
| 7-23 mo | — | 2-dose series | 12-wk interval and second dose at ≥12 mo of age | Revaccinate 3 y after primary series; additional boosters every 5 y if risk remains |
| ≥24 mo | — | 2-dose series | 8-wk interval | Revaccinate 3 y after primary series if most recent dose given before age 7 y; additional boosters every 5 y if risk remains |
| MenACWY-D (Menactra) | ||||
| 9-23 mo | Do not administer, because of immune interference with PCV13 | |||
| ≥24 mo | Administer >4 wk after completion of PCV13 series | 2-dose series | 8-wk interval | Revaccinate 3 y after primary series; additional boosters every 5 y if risk remains |
| MenB-4C (BEXSERO)* | ||||
| ≥10 y | — | 2-dose series | 1-mo interval | Revaccinate 1 y after primary series and revaccinate every 2-3 y if risk remains† |
| MenB-FHbp (Trumenba)* | ||||
| ≥10 y | — | 3-dose series | 1-mo, 4-mo intervals | Revaccinate 1 y after primary series and revaccinate every 2-3 y if risk remains† |
Use same product for all doses in a series
†MenB booster doses now recommended for person ≥ 10 y with asplenia as of June 2019.
ACIP recommendations for Hib vaccines in children and adults with asplenia or hyposplenia37,38,52
| Age at first dose . | Timing of first dose of Hib . | Total doses of Hib . | Interval between Hib doses . |
|---|---|---|---|
| ActHIB, HIBERIX, Pentacel | |||
| 2 mo | — | 4 doses | 2-mo, 2-mo, 6-mo intervals |
| PedvaxHIB | |||
| 2 mo | — | 3 doses | 2-mo, 8-mo intervals |
| Any licensed, age-appropriate Hib vaccine | |||
| 12-59 mo; ≤1 dose before age 12 mo | >8 wk after prior dose | 2 doses | 8-wk interval |
| 12-59 mo; ≥2 doses before age 12 mo | >8 wk after prior dose | 1 dose | — |
| ≥5 y; no prior Hib doses | — | 1 dose | — |
| Age at first dose . | Timing of first dose of Hib . | Total doses of Hib . | Interval between Hib doses . |
|---|---|---|---|
| ActHIB, HIBERIX, Pentacel | |||
| 2 mo | — | 4 doses | 2-mo, 2-mo, 6-mo intervals |
| PedvaxHIB | |||
| 2 mo | — | 3 doses | 2-mo, 8-mo intervals |
| Any licensed, age-appropriate Hib vaccine | |||
| 12-59 mo; ≤1 dose before age 12 mo | >8 wk after prior dose | 2 doses | 8-wk interval |
| 12-59 mo; ≥2 doses before age 12 mo | >8 wk after prior dose | 1 dose | — |
| ≥5 y; no prior Hib doses | — | 1 dose | — |
Pneumococcal vaccines: PCV13 and PPSV23
Two pneumococcal vaccines are currently licensed for use in the United States: the 13-valent pneumococcal conjugate vaccine (PCV13) and the 23-valent pneumococcal polysaccharide vaccines (PPSV23). The routine childhood immunization schedule recommends routine use of PCV13 for all children to reduce the risk of invasive pneumococcal disease, pneumonia, and otitis media.31 For children with congenital asplenia, sickle cell disease, thalassemia, hereditary spherocytosis, or other inherited conditions resulting in functional asplenia, timely administration of pneumococcal conjugate vaccines is critical for disease prevention. In addition, due to the broader serotype coverage of PPSV23, booster doses of PPSV23 are also recommended after the PCV13 series. Among adults with functional or anatomic asplenia, PCV13 followed by PPSV23 is also recommended for use to reduce the risk of invasive pneumococcal disease. The sequence of administration is important for pneumococcal vaccines in order to optimize the immune response. Conjugate pneumococcal vaccines such as PCV13 should be administered before polysaccharide vaccines (PPSV23) whenever possible because patients who receive PPSV23 as the initial dose have lower antibody responses, shorter duration of immunity, and hyporesponsiveness to subsequent doses of either vaccine.32
Meningococcal vaccines: meningococcal conjugate vaccines for serogroups A, C, Y, and W-135 and serogroup B meningococcal vaccines
Vaccination with age- and formulation-appropriate meningococcal vaccines is recommended for individuals at increased risk for meningococcal disease. There are 4 licensed meningococcal vaccines currently available in the United States: 2 meningococcal conjugate vaccines for serogroups A, C, Y, and W-135 (MenACWY) and 2 serogroup B meningococcal vaccines (MenB). In healthy adolescents, ACIP routinely recommends MenACWY at 11 to 12 years of age and a booster at 16 years of age. MenB is also available for healthy teens and young adults 16 to 23 years of age under a shared decision-making recommendation. However, given the increased risk for severe morbidity and mortality attributable to meningococcal disease in children and adults with asplenia, both types of meningococcal vaccines (MenACWY and MenB) are specifically recommended for use in this at-risk population.
MenACWY-CRM (MENVEO) can be administered to children as young as 8 weeks of age and is recommended for use in children with functional or anatomic asplenia. Infants are recommended to receive a 4-dose series; children aged 7-23 months receive a 2-dose series with the second dose given at ≥12 months of age.33 MenACWY-D (Menactra) may be administered only to children aged ≥2 years due to interference with the immune response to PCV13 in children aged <2 years. The choice of MenACWY will depend on the age of the patient and vaccine availability, and either series is considered acceptable for protection against serogroups A, C, Y, and W-135. Because neither vaccine provides protection against serogroup B disease, however, ACIP also recommends MenB vaccination of children and adults with asplenia. MenB-4C vaccine (BEXSERO) protects against serogroup B meningococcal disease and is recommended for use as a 2-dose series for at-risk persons aged ≥10 years.34 Immunogenicity is similar for children with functional or anatomic asplenia when compared with healthy children.35 Given the absence of impact of MenB-4C on carriage, vaccination will remain a critical component of protection because reliance on herd immunity is less likely.36 MenB-FHbp vaccine (Trumenba) is recommended for use as a 3-dose series for persons at increased risk for serogroup B meningococcal disease aged ≥10 years. In contrast, healthy adolescents and young adults who are not at increased risk may receive a 2-dose series of MenB-FHbp, though for individuals with asplenia, a 3-dose series is strongly preferred to enhance protection in the short term. Either vaccine may be administered concomitantly with MenACWY vaccines, though the same MenB product is recommended for the entire series (ie, they are not interchangeable). Since June 2019, booster doses of MenB are also now recommended for those with asplenia 1 year after completion of a MenB primary series, followed by MenB booster doses every 2 to 3 years thereafter for as long as increased risk remains.37,38
Hib and other vaccines
The incidence of Hib disease has declined precipitously since the introduction of Hib conjugate vaccine in the 1990s, and vaccination remains a mainstay of disease prevention in healthy and immunocompromised children. Given the risk of invasive disease due to H. influenzae and evidence for immunogenicity after vaccination in children with asplenia, Hib vaccine continues to be strongly recommended in this population (see Table 4). In addition, annual influenza vaccination of the patient and all household members is considered an important strategy for prevention in patients with asplenia or hyposplenia due to the risk posed by subsequent bacterial coinfection, such as pneumococcal pneumonia. Any age-appropriate, licensed, inactivated influenza vaccines can be administered yearly to children and adults aged ≥6 months.39 Live-attenuated influenza vaccines are not recommended for patients with asplenia, given the availability of inactivated influenza vaccines. For immunocompetent adults aged ≥50 years, recombinant zoster vaccine (RZV) is routinely recommended as a 2-dose schedule and is preferred over the live attenuated zoster vaccine.38 Although asplenia does not increase the risk for herpes zoster virus or postherpetic neuralgia, other associated immunocompromising conditions may increase an individual’s risk for disease. All patients with asplenia aged ≥50 years should receive RZV as a routinely recommended vaccine. ACIP is currently considering use of RZV in immunocompromised adult patients.
Prevention: patient and family education
Patients and families should receive education about the risks associated with asplenia and hyposplenia. Early recognition of signs and symptoms (fever, chills, rigors, vomiting, diarrhea) of infection and immediate referral for care are critical. Symptoms may be quite mild initially but may rapidly progress to fulminant sepsis, shock, and death. Antibiotics should be prescribed to have on hand for empiric treatment before seeking care in an emergency department (see Table 1). Patients are also recommended to wear a medical alert bracelet or carry a medical alert card that indicates the presence of asplenia and the need for prompt antimicrobial therapy, allergies to medications, and emergency contact information. If additional space is available, information about vaccination history (pneumococcal, MenACWY, MenB, Hib) and current medications (eg, antibiotic prophylaxis) may also be included. When prophylaxis is stopped, benefit–risk assessment and discussion with the patient should include the risks of continued antibiotic prophylaxis, availability of established medical care, local epidemiology of antibiotic-resistant bacteria, and reinforcement of early recognition and detection of infection in patients with asplenia.
Additional considerations for patients and families is ensuring they are aware of the need for preprocedural prophylaxis for sinus or respiratory tract procedures (eg, functional endoscopic sinus surgery, bronchoscopy). Dog bites or wounds licked by dogs are also indications for presumptive antimicrobial treatment, even in the absence of symptoms. Travelers should be counseled on the risks associated with tick-borne illnesses such as babesiosis and mosquito-borne illnesses such as malaria. The risk for infectious complications is lifelong in patients with asplenia, which reinforces the importance of taking preventive measures and ensuring that emergency antibiotics are available when traveling. Furthermore, all patients should receive education about the importance of vaccines in reducing the risk of sepsis and other preventable infectious diseases. Important sources of information about vaccines are provided by the Centers for Disease Control and Prevention (https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/sources.html), and specific patient education for adults with asplenia is provided by the Immunization Action Coalition (https://www.immunize.org/catg.d/p4047.pdf).
Quality of care for patients with asplenia
Multiple studies have demonstrated that best practice recommendations are challenging to implement.40-42 Vaccination rates are suboptimal (as low as 6%); adherence to antibiotic use may be low; and patient and family education and support may be sporadic in nature.43 One of the largest barriers to care reported by physicians is the lack of clarity about which physician is responsible for the management of patients with asplenia (eg, primary care, surgeons, hematologists, oncologists).44 Engagement of subspecialists in vaccine delivery is critical to protect patients with asplenia from infectious complications, particularly for adult patients who may not routinely receive care from a primary care physician. Furthermore, the timing and importance of vaccines may be most effectively conveyed by subspecialists who care for patients with complex or chronic illnesses. Dedicated teams or services for patients with asplenia are recommended to substantially improve adherence to preventive measures, including vaccination rates and antibiotic prophylaxis, and reduce occurrence of severe sepsis.24,45,46
Asplenia and coronavirus disease 2019 infection
In a large cohort of 17 million adult patients in England, there were 27 917 adults diagnosed with asplenia and 40 coronavirus disease 2019 (COVID-19)-associated deaths (0.14%).47 Similar to other reported studies, older age, male sex, non-White ethnic groups, obesity, diabetes, other comorbid conditions (respiratory, cardiovascular, renal, hepatic, neurologic disease), and immunosuppression (eg, hematologic malignancy, organ transplant) were associated with a higher hazard of death. Patients with asplenia had a mildly elevated (hazard ratio, 1.3), but not statistically significant, risk in multivariate models. Clinical characteristics of COVID-19 in patients with hemoglobinopathies appear to be similar in nature to those described for the overall population, considering the presence of comorbidities (ie, obesity, diabetes, chronic respiratory or cardiovascular disease, immunocompromise), though direct comparisons are not yet available.48 Until a safe and effective COVID-19 vaccine is recommended for use, patients with asplenia should follow guidance to minimize exposure to the virus through social distancing, masking, hand hygiene, and cleaning and disinfection.
Conclusions
Recommendations for patients with functional or anatomic asplenia include antibiotic prophylaxis, vaccination, and patient and family education to ensure prevention of infections and timely management of febrile illnesses. Adherence to best practices can be challenging without targeted efforts to provide care for this population and track key process measures such as vaccination rates. Given the lifelong risk associated with infection in these patients, future efforts should focus on improving the quality of care delivered to children and adults with asplenia.
Correspondence
Grace M. Lee, Stanford University School of Medicine, 300 Pasteur Dr, H306a, Stanford, CA 94305; e-mail: gmlee@stanford.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.