Abstract
Recurrent venous thromboembolism (VTE, or deep vein thrombosis and pulmonary embolism) is associated with mortality and long-term morbidity. The circumstances in which an index VTE event occurred are crucial when personalized VTE recurrence risk is assessed. Patients who experience a VTE event in the setting of a transient major risk factor (such as surgery associated with general anesthesia for >30 minutes) are predicted to have a low VTE recurrence risk following discontinuation of anticoagulation, and limited-duration anticoagulation is generally recommended. In contrast, those patients whose VTE event occurred in the absence of risk factors or who have persistent risk factors have a higher VTE recurrence risk. Here, we review the literature surrounding VTE recurrence risk in a range of clinical conditions. We describe gender-specific risks, including VTE recurrence risk following hormone- and pregnancy-associated VTE events. Finally, we discuss how the competing impacts of VTE recurrence and bleeding have shaped international guideline recommendations.
Learning Objectives
Understand why an evaluation of risk factors that were present at the time of the index venous thromboembolism (VTE) event (and whether these risk factors were transient or persistent) is essential when assessing a patient's future VTE recurrence risk
Review data regarding additional predictors of VTE recurrence risk
Clinical case 1
A 45-year-old man who was diagnosed with an acute proximal deep vein thrombosis (DVT) and who has received therapeutic anticoagulation for 3 months attends your clinic. He has had no bleeding complications and has no identifiable risk factors for bleeding. Should he continue anticoagulation or stop?
Introduction
Recurrent venous thromboembolism (VTE; or DVT and pulmonary embolism [PE]) is associated with mortality and long-term morbidity. Conversely, anticoagulation confers potentially devastating bleeding risks. It is important to understand which patients are at highest VTE recurrence risk so that we can target awareness and prevention strategies to the right patients. Of critical importance are the circumstances in which a VTE event occurred: were risk factors present, and if so, what was their nature and are they persistent? These risk factors are central to decision making surrounding duration of anticoagulation.
In this review, we discuss which patients have the highest predicted VTE recurrence risk, including an update on emerging personalized strategies. Finally, we will take a deep-dive into VTE recurrence risk in women, with a focus on gender-specific challenges and knowledge gaps.
Predicting VTE recurrence risk: go back to the beginning!
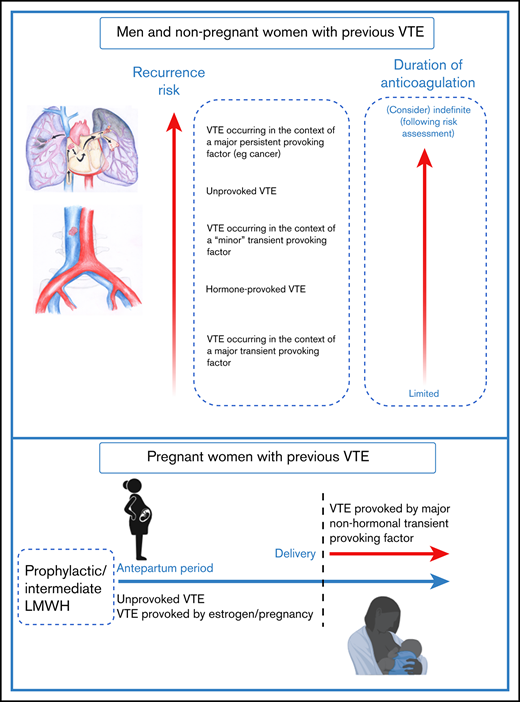
VTE recurrence risk is determined by risk factors that were present at the time of the initial VTE event1,2 (Figure 1). The predicted annual recurrence risk after an unprovoked VTE event is higher than that after a VTE provoked by a major transient risk factor (Table 1).1-4 (Although the term unprovoked is no longer encouraged by European guidelines,2 we do use it in this review to refer to “an index VTE episode that occurred in the absence of any identifiable risk factor” for simplicity).
VTE recurrence risk and duration of anticoagulation. For men and nonpregnant women with an index VTE event, VTE recurrence risk is driven by risk factors that were present at the time of the initial VTE event.1,2 The predicted annual recurrence risk after an unprovoked VTE event is higher than that after a VTE provoked by a major transient risk factor. VTE occurring in the context of minor risk factors are associated with higher predicted recurrence risk than those occurring in the context of major transient risk factors.6 Recurrence risks for patients with major persistent risk factors (most notably active cancer) are among the highest of all.1,2 Guideline recommendations on duration of anticoagulation are guided by data including VTE recurrence risk and bleeding risk on anticoagulation, with limited and indefinite duration anticoagulation being recommended for patients with low and high recurrence risks respectively.2,43 For some patients including “cis” and transgender women whose VTE occurred in the context of hormone use, estimation of recurrence risk may be particularly challenging because of knowledge gaps, and these areas are important research priorities.27 For pregnant women with prior VTE (especially those with an unprovoked or a hormone-provoked VTE,21 predicted recurrence risk during pregnancy is sufficiently high to warrant both antenatal and postnatal thromboprophylaxis, whereas postpartum thromboprophylaxis only is recommended for women with lower predicted recurrence risks.25 The optimal LMWH dose for women with prior VTE is currently being investigated in the ongoing Highlow study (NCT 01828697; highlowstudy.org).
VTE recurrence risk and duration of anticoagulation. For men and nonpregnant women with an index VTE event, VTE recurrence risk is driven by risk factors that were present at the time of the initial VTE event.1,2 The predicted annual recurrence risk after an unprovoked VTE event is higher than that after a VTE provoked by a major transient risk factor. VTE occurring in the context of minor risk factors are associated with higher predicted recurrence risk than those occurring in the context of major transient risk factors.6 Recurrence risks for patients with major persistent risk factors (most notably active cancer) are among the highest of all.1,2 Guideline recommendations on duration of anticoagulation are guided by data including VTE recurrence risk and bleeding risk on anticoagulation, with limited and indefinite duration anticoagulation being recommended for patients with low and high recurrence risks respectively.2,43 For some patients including “cis” and transgender women whose VTE occurred in the context of hormone use, estimation of recurrence risk may be particularly challenging because of knowledge gaps, and these areas are important research priorities.27 For pregnant women with prior VTE (especially those with an unprovoked or a hormone-provoked VTE,21 predicted recurrence risk during pregnancy is sufficiently high to warrant both antenatal and postnatal thromboprophylaxis, whereas postpartum thromboprophylaxis only is recommended for women with lower predicted recurrence risks.25 The optimal LMWH dose for women with prior VTE is currently being investigated in the ongoing Highlow study (NCT 01828697; highlowstudy.org).
VTE risk factor classification
| ESC2 . | ISTH1 . | ||||
|---|---|---|---|---|---|
| RF category . | Recurrence risk . | Examples . | RF category . | Recurrence risk or risk of index event . | Examples . |
| . | . | . | VTE provoked by a transient risk factor . | ||
| Major transient or reversible RF associated with >10-fold increased risk for the index VTE event (compared with patients without the risk factor) | Low recurrence risk* (<3%/y) | Surgery with GA for >30 min | Major transient RF during the 3 mo before diagnosis of VTE | Half the risk of recurrent VTE (compared with if there was no transient risk factor), when the risk factor occurred up to 3 mo before the VTE or RF was associated with >10-fold increased risk of having a first VTE | Surgery with GA for >30 min |
| Confined to bed in hospital (only bathroom privileges) for ≥3 days because of an acute illness or acute exacerbation of a chronic illness | Confined to bed in hospital (only bathroom privileges) for ≥3 days because of an acute illness | ||||
| Cesarean section | |||||
| Trauma with fractures | |||||
| Transient or reversible factors associated with ≤10-fold increased risk for (index) VTE or | Intermediate recurrence risk (3-8%/y) | Minor surgery (GA for <30 min) | Minor (yet important) transient RF during the 2 mo before diagnosis of VTE | Half the risk of recurrent VTE after stopping anticoagulant therapy (compared with if there was no transient RF), when the RF occurred up to 2 mo before the VTE or RF was associated with a 3- to 10-fold increase in the risk of having a first VTE | Surgery with GA for <30 min |
| Admission to hospital for <3 days with an acute illness | Admission to hospital for <3 days with an acute illness | ||||
| Estrogen therapy/contraception | Estrogen therapy | ||||
| Pregnancy or puerperium | Pregnancy or puerperium | ||||
| Confined to bed out of hospital for ≥3 days with an acute illness | Confined to bed out of hospital for ≥3 days with an acute illness | ||||
| Leg injury (without fracture) associated with reduced mobility for ≥3 days | Leg injury associated with reduced mobility for at least 3 days | ||||
| Long haul flight | |||||
| Nonmalignant persistent risk factors Or | Inflammatory bowel disease | ||||
| Active autoimmune disease | |||||
| No identifiable risk factor | No identifiable risk factor | Unprovoked VTE | |||
| Recurrence risk individualized/stratified using other assessments† | No provoking factors (transient or persistent) | ||||
| VTE provoked by a persistent risk factor | |||||
| High recurrence risk (>8%/y) | Active cancer | Active cancer | Cancer is considered active if any of the following apply: (1) has not received potentially curative treatment; or (2) there is evidence that treatment has not been curative (eg, recurrent or progressive disease); or (3) treatment is ongoing | ||
| At least 1 previous episode of VTE in the absence of a major transient or reversible factor | |||||
| Antiphospholipid antibody syndrome | |||||
| Ongoing nonmalignant condition associated with at least a 2-fold risk of recurrent VTE after stopping anticoagulant therapy | Inflammatory bowel disease. | ||||
| ESC2 . | ISTH1 . | ||||
|---|---|---|---|---|---|
| RF category . | Recurrence risk . | Examples . | RF category . | Recurrence risk or risk of index event . | Examples . |
| . | . | . | VTE provoked by a transient risk factor . | ||
| Major transient or reversible RF associated with >10-fold increased risk for the index VTE event (compared with patients without the risk factor) | Low recurrence risk* (<3%/y) | Surgery with GA for >30 min | Major transient RF during the 3 mo before diagnosis of VTE | Half the risk of recurrent VTE (compared with if there was no transient risk factor), when the risk factor occurred up to 3 mo before the VTE or RF was associated with >10-fold increased risk of having a first VTE | Surgery with GA for >30 min |
| Confined to bed in hospital (only bathroom privileges) for ≥3 days because of an acute illness or acute exacerbation of a chronic illness | Confined to bed in hospital (only bathroom privileges) for ≥3 days because of an acute illness | ||||
| Cesarean section | |||||
| Trauma with fractures | |||||
| Transient or reversible factors associated with ≤10-fold increased risk for (index) VTE or | Intermediate recurrence risk (3-8%/y) | Minor surgery (GA for <30 min) | Minor (yet important) transient RF during the 2 mo before diagnosis of VTE | Half the risk of recurrent VTE after stopping anticoagulant therapy (compared with if there was no transient RF), when the RF occurred up to 2 mo before the VTE or RF was associated with a 3- to 10-fold increase in the risk of having a first VTE | Surgery with GA for <30 min |
| Admission to hospital for <3 days with an acute illness | Admission to hospital for <3 days with an acute illness | ||||
| Estrogen therapy/contraception | Estrogen therapy | ||||
| Pregnancy or puerperium | Pregnancy or puerperium | ||||
| Confined to bed out of hospital for ≥3 days with an acute illness | Confined to bed out of hospital for ≥3 days with an acute illness | ||||
| Leg injury (without fracture) associated with reduced mobility for ≥3 days | Leg injury associated with reduced mobility for at least 3 days | ||||
| Long haul flight | |||||
| Nonmalignant persistent risk factors Or | Inflammatory bowel disease | ||||
| Active autoimmune disease | |||||
| No identifiable risk factor | No identifiable risk factor | Unprovoked VTE | |||
| Recurrence risk individualized/stratified using other assessments† | No provoking factors (transient or persistent) | ||||
| VTE provoked by a persistent risk factor | |||||
| High recurrence risk (>8%/y) | Active cancer | Active cancer | Cancer is considered active if any of the following apply: (1) has not received potentially curative treatment; or (2) there is evidence that treatment has not been curative (eg, recurrent or progressive disease); or (3) treatment is ongoing | ||
| At least 1 previous episode of VTE in the absence of a major transient or reversible factor | |||||
| Antiphospholipid antibody syndrome | |||||
| Ongoing nonmalignant condition associated with at least a 2-fold risk of recurrent VTE after stopping anticoagulant therapy | Inflammatory bowel disease. | ||||
GA, general anesthesia; RF, risk factor.
If anticoagulation is discontinued after the first 3 months.
Described in text of guideline and in this current review.
In a recent systematic review, VTE recurrence rates at 24 months were reported to be 3.3% (95% confidence interval [CI], 2.8-3.9), 0.7% (95% CI, 0-1.5), 4.2% (95% CI, 2.8-5.6), and 7.4% (95% CI, 6.5-8.2) per patient year in patients with a transient risk factor, a surgical risk factor, a nonsurgical risk factor, and unprovoked VTE, respectively.5 Guidelines from the European Society of Cardiology (ESC)2 and the International Society on Thrombosis and Haemostasis (ISTH)1 provide a framework for categorization of VTE risk factors (Table 1). The categorization of risk factors for the index VTE event in both guidelines are broadly similar, with the exception of differing terminology (the ESC avoids terms such as provoked, unprovoked, or idiopathic VTE2 ; and a focus on the presence of risk factors [categorizing these as transient or persistent] in the ISTH guideline as the key definition of risk category).1
Attention to the initial provoking factor is also essential; in a recent study incorporating data from 2 randomized trials, recurrence rates in patients whose initial VTE event occurred in the context of nonmajor (transient or persistent) risk factors were similar to those of patients whose initial event was unprovoked (hazard ratio [HR], 0.81; 95% CI, 0.56-1.16). In the placebo arms, recurrence rates during the study period for patients with minor (as judged by the authors) transient, minor persistent, and unprovoked VTE were reported to be 7.1%, 10.7%, and 10%, respectively.6 Given the relatively small numbers of patients, firm conclusions on VTE recurrence risks associated with individual nonmajor risk factors will require future prospective clinical management studies.
Recurrence risks for patients with major persistent risk factors (most notably active cancer) are among the highest of all (Figure 1; Table 1).1,2 Conversely, predicting recurrence risk and making decisions on duration of anticoagulation in patients whose initial VTE event occurred in the context of an intermediate risk factor, such as hormonal therapy or pregnancy, is particularly challenging, especially as anticoagulation poses additional, gender-specific risks.
Unprovoked VTE
Up to 50% of all people with a first VTE episode have no identifiable cause for this event (Table 2).2 A recent systematic review and meta-analysis estimated that the risk of recurrent VTE in patients whose initial event was unprovoked was 10%, 25%, and 36% at 1, 5, and 10 years after treatment, respectively.8
VTE recurrence risks in selected studies evaluating risk factors for recurrence
| Study . | Type of study/nature of initial VTE* . | Cumulative recurrence risk for entire group . | Predictors of VTE recurrence . | VTE recurrence risk RR or HR (95% CI) . |
|---|---|---|---|---|
| Heit et al 2000 (45) | Prospective cohort study: provoked and unprovoked first VTE | 1 y: 12.9% | Male sex | Male vs. Female: HR 1.29 (1.06-1.57) |
| 10 y: 30.4% | Definite/probable VTE: 2.07 (1.60-2.67) | |||
| Kyrle et al 2004 (46) | Prospective cohort study: first unprovoked VTE | 5 y: | Male sex | Male vs. Female: RR 3.6 (2.3-5.5) |
| Men: 30.7% (23.8-37.6) | ||||
| Women: 8.5% (5.0-12.0) | ||||
| Baglin et al 2004 (47) | Prospective single-center cohort study: provoked/unprovoked VTE | 2 y: | Male sex | Male vs. Female: HR 2.66 (1.49-4.77) |
| Men: 19.2% | ||||
| Women: 7.7% | ||||
| Rodger et al 2008 (42) | Multicenter prospective cohort study: First unprovoked VTE | N/R | Male sex | Annual recurrence risk: |
| Men 13.7% (10.8-17.0%) | ||||
| Women: 5.5% (3.7-7.8%); P < .001 | ||||
| Lijfering et al 2009 (48) | Post hoc analysis of pooled data from family cohort studies: Provoked and unprovoked VTE | N/R | Male sex | Male vs. Female: RR 1.6 (95% CI, 1.3-2.0) |
| Christiansen et al 2010 (33) | Prospective follow-up of case-control study; provoked and unprovoked first VTE | N/R | Male sex | Men: IR 41.2/1000 patient-years |
| Women: IR 14.2/1000 patient -years | ||||
| HR 2.8 (1.4-5.7) (unprovoked VTE) | ||||
| Douketis et al 2011 (49) | Patient-level meta-analysis (provoked and unprovoked VTE) | 1 y: | Male sex | Male vs. Female: HR 2.2 (1.7-2.8) |
| Men: 9.5% (7.9%-11.4%) | ||||
| Women: 5.3% (4.1%-6.7%) | ||||
| 3 y: | ||||
| Men: 19.7% (16.5%-23.4%) | ||||
| Women: 9.1% (7.3% −11.3%) | ||||
| Khan et al 2019 (8) | Meta-analysis first unprovoked VTE | 2 y: 16% (13-19%) | Male sex | First year: |
| 5 y: 25% (21- 29%) | Men: 11.9/100 patient-years (9.6-14.4) Women: 8.9/100 patient-years (6.8-11.3) | |||
| 10 y: 36% (28-45%) | Rate ratio 1.4 (1.3-1.6) | |||
| Palareti et al 2006 (50) | RCT first unprovoked VTE | N/R | D-dimer 1 mo after D/C AC | HR (abnormal vs normal D-dimer without AC) 2.49 (1.35–4.59) |
| Verhovsek et al 2008 (51) | Meta-analysis (first unprovoked VTE) | N/R | Abnormal D-dimer levels after anticoagulation completion | Positive D-dimer results: |
| −8.9%/year (5.8%-11.9%) | ||||
| Negative D-dimer results: | ||||
| −3.5%/year (2.7%-4.3%) | ||||
| Cosmi et al 2010 (52) | Prospective multicenter study (first unprovoked VTE) | N/R | D-dimer 1 mo after D/C AC | Persistently abnormal D-dimer: 27%/person-years (12-48) |
| Persistently normal D-dimer: 2.9%/person-years (1-7) | ||||
| Adjusted HR 7.9 (2.1-30) | ||||
| Palaretti et al 2014 (53) | Prospective clinical management study (VTE associated with no/weak risk factors) | N/R | D-dimers after AC | 1. Persistently negative D-dimer after D/C AC: |
| 3.0 per 100 person-years (2.0-4.4) | ||||
| 2. Positive D-dimers, refused to resume AC: | ||||
| 8.8 per 100 person-years (5-14.1) | ||||
| HR 1 vs 2: 2.92 (1.87-9.72) | ||||
| Kearon et al 2015 (29) | Prospective clinical management study (first unprovoked VTE) | N/R | D-dimers at end of AC: if second test negative >1 mo, AC not restarted in men/women | Persistently negative D-dimers: |
| Overall: 6.7% (4.8-9.0%)/person-year | ||||
| Men: 9.7% (6.7-13.7%)/person-year | ||||
| Nonestrogen women: 5.4% | ||||
| (2.5-10.2%)/person-year | ||||
| Estrogen women: 0.0% (0.0-3.0%)/person-year | ||||
| Tan et al 2011 (54) | Systematic review: studies including provoked and unprovoked first VTE | N/R | Residual vein thrombosis | Residual vein thrombosis vs. none: Overall: OR 2.02 (1.62-2.5), unprovoked VTE: OR 1.5 (1.12-2.01) |
| Carrier et al 2011 (55) | Systematic review (unprovoked and provoked first VTE) | N/R | Residual vein occlusion | Residual vein occlusion vs. none: Any VTE: OR 1.5 (1.1-2.0) unprovoked VTE: OR 1.24 (0.9-1.7) |
| Study . | Type of study/nature of initial VTE* . | Cumulative recurrence risk for entire group . | Predictors of VTE recurrence . | VTE recurrence risk RR or HR (95% CI) . |
|---|---|---|---|---|
| Heit et al 2000 (45) | Prospective cohort study: provoked and unprovoked first VTE | 1 y: 12.9% | Male sex | Male vs. Female: HR 1.29 (1.06-1.57) |
| 10 y: 30.4% | Definite/probable VTE: 2.07 (1.60-2.67) | |||
| Kyrle et al 2004 (46) | Prospective cohort study: first unprovoked VTE | 5 y: | Male sex | Male vs. Female: RR 3.6 (2.3-5.5) |
| Men: 30.7% (23.8-37.6) | ||||
| Women: 8.5% (5.0-12.0) | ||||
| Baglin et al 2004 (47) | Prospective single-center cohort study: provoked/unprovoked VTE | 2 y: | Male sex | Male vs. Female: HR 2.66 (1.49-4.77) |
| Men: 19.2% | ||||
| Women: 7.7% | ||||
| Rodger et al 2008 (42) | Multicenter prospective cohort study: First unprovoked VTE | N/R | Male sex | Annual recurrence risk: |
| Men 13.7% (10.8-17.0%) | ||||
| Women: 5.5% (3.7-7.8%); P < .001 | ||||
| Lijfering et al 2009 (48) | Post hoc analysis of pooled data from family cohort studies: Provoked and unprovoked VTE | N/R | Male sex | Male vs. Female: RR 1.6 (95% CI, 1.3-2.0) |
| Christiansen et al 2010 (33) | Prospective follow-up of case-control study; provoked and unprovoked first VTE | N/R | Male sex | Men: IR 41.2/1000 patient-years |
| Women: IR 14.2/1000 patient -years | ||||
| HR 2.8 (1.4-5.7) (unprovoked VTE) | ||||
| Douketis et al 2011 (49) | Patient-level meta-analysis (provoked and unprovoked VTE) | 1 y: | Male sex | Male vs. Female: HR 2.2 (1.7-2.8) |
| Men: 9.5% (7.9%-11.4%) | ||||
| Women: 5.3% (4.1%-6.7%) | ||||
| 3 y: | ||||
| Men: 19.7% (16.5%-23.4%) | ||||
| Women: 9.1% (7.3% −11.3%) | ||||
| Khan et al 2019 (8) | Meta-analysis first unprovoked VTE | 2 y: 16% (13-19%) | Male sex | First year: |
| 5 y: 25% (21- 29%) | Men: 11.9/100 patient-years (9.6-14.4) Women: 8.9/100 patient-years (6.8-11.3) | |||
| 10 y: 36% (28-45%) | Rate ratio 1.4 (1.3-1.6) | |||
| Palareti et al 2006 (50) | RCT first unprovoked VTE | N/R | D-dimer 1 mo after D/C AC | HR (abnormal vs normal D-dimer without AC) 2.49 (1.35–4.59) |
| Verhovsek et al 2008 (51) | Meta-analysis (first unprovoked VTE) | N/R | Abnormal D-dimer levels after anticoagulation completion | Positive D-dimer results: |
| −8.9%/year (5.8%-11.9%) | ||||
| Negative D-dimer results: | ||||
| −3.5%/year (2.7%-4.3%) | ||||
| Cosmi et al 2010 (52) | Prospective multicenter study (first unprovoked VTE) | N/R | D-dimer 1 mo after D/C AC | Persistently abnormal D-dimer: 27%/person-years (12-48) |
| Persistently normal D-dimer: 2.9%/person-years (1-7) | ||||
| Adjusted HR 7.9 (2.1-30) | ||||
| Palaretti et al 2014 (53) | Prospective clinical management study (VTE associated with no/weak risk factors) | N/R | D-dimers after AC | 1. Persistently negative D-dimer after D/C AC: |
| 3.0 per 100 person-years (2.0-4.4) | ||||
| 2. Positive D-dimers, refused to resume AC: | ||||
| 8.8 per 100 person-years (5-14.1) | ||||
| HR 1 vs 2: 2.92 (1.87-9.72) | ||||
| Kearon et al 2015 (29) | Prospective clinical management study (first unprovoked VTE) | N/R | D-dimers at end of AC: if second test negative >1 mo, AC not restarted in men/women | Persistently negative D-dimers: |
| Overall: 6.7% (4.8-9.0%)/person-year | ||||
| Men: 9.7% (6.7-13.7%)/person-year | ||||
| Nonestrogen women: 5.4% | ||||
| (2.5-10.2%)/person-year | ||||
| Estrogen women: 0.0% (0.0-3.0%)/person-year | ||||
| Tan et al 2011 (54) | Systematic review: studies including provoked and unprovoked first VTE | N/R | Residual vein thrombosis | Residual vein thrombosis vs. none: Overall: OR 2.02 (1.62-2.5), unprovoked VTE: OR 1.5 (1.12-2.01) |
| Carrier et al 2011 (55) | Systematic review (unprovoked and provoked first VTE) | N/R | Residual vein occlusion | Residual vein occlusion vs. none: Any VTE: OR 1.5 (1.1-2.0) unprovoked VTE: OR 1.24 (0.9-1.7) |
AC, anticoagulation; D/C, discontinued; HR, hazard ratio; N/R, not reported; OR: odds ratio; RR, relative risk.
In these studies, VTE events included DVT and PE.
Cancer
People with active cancer are among those with the highest VTE recurrence risk, with a 2-9 fold increased risk compared with noncancer patients.7,9,10 A recent meta-analysis reported recurrent VTE and fatal recurrent VTE rates of 23.7 (95% CI, 20.1-27.8) and 1.9 (95% CI, 0.8-4.0) per 100 patient-years, respectively.10 In a cohort study including 543 patients, validated in an independent set of 819 patients, a proposed recurrent VTE prediction score was developed that included breast cancer (−1 point), tumor node metastasis stage I or II (−1 point), and female sex, lung cancer, and previous VTE (+1 point each).11 Patients with a score of ≤0 and ≥1, respectively, had low (≤4.5%) and high (≥19%) recurrence rates during 6 months. A population-based cohort study also identified predictors for VTE recurrence in cancer patients including cancer type and the presence of metastases.9
Inherited thrombophilia/family history
Inherited thrombophilia is a recognized predisposing factor for an index VTE event.3 However, the impact of inherited thrombophilia on VTE recurrence risk is less clear. In a prospective cohort study following unselected patients with a first VTE, 85% of whom underwent inherited thrombophilia screening, VTE recurrence rates were not significantly different in those with or without inherited thrombophilia (HR, 1.50; 95% CI, 0.82-2.77).12 The risk of recurrent thrombosis was also determined in a prospective follow-up study of 474 patients who had participated in the Leiden Thrombophilia Study, a large population-based case-control study of risk factors for a first DVT.3 There was no significance increase in thrombosis recurrence risk among 319 patients with ≥1 thrombophilic abnormality compared with those with none (HR, 1.4; 95% CI, 0.9-2.2). In particular, there was no increase in thrombosis recurrence risk in patients who were heterozygous for either factor V Leiden polymorphism or the prothrombin gene mutation, including after adjustment for age, sex, and duration of anticoagulation. In patients with anticoagulant protein deficiency (protein C, protein S, or antithrombin, the corresponding HR was 1.8 (95% CI, 0.9-3.7).
Antiphospholipid syndrome
Patients with antiphospholipid syndrome (APS) who have experienced a VTE event seem to have a high predicted recurrence risk.13 An observational cohort study including 55% VTE patients, 55% of whom remained on therapeutic anticoagulation after their first event, reported a thrombotic recurrence rate of 49% after 10 years.14 In a very recently published systematic review, the 2-year recurrent thromboembolism rate in patients with VTE who were and were not taking anticoagulant therapy was 0.054 (95% CI, 0.037-0.079) and 0.178 (95% CI, 0.150-0.209), respectively.15 Given this high recurrence risk, international guidelines recommend indefinite anticoagulation for many VTE patients with a diagnosis of APS.2,16 However, as in all clinical scenarios, individual risk assessment with careful attention to the nature of risk factors is essential to guide decision making. Current international guidelines do not recommend universal APS screening for all patients with unprovoked VTE.17
Toward personalization of recurrence risk?
Emerging data suggest that additional risk factors may modulate predicted VTE recurrence risk including male sex, D-dimer levels, and postthrombotic syndrome (Table 2). Knowledge of these determinants may be useful during shared decision making with patients, especially in circumstances where the optimal duration of anticoagulation is not clear, taking into account patient preferences (Figure 2). For example, studies have consistently reported an approximately twofold higher VTE recurrence risk for men compared with women (Table 2).
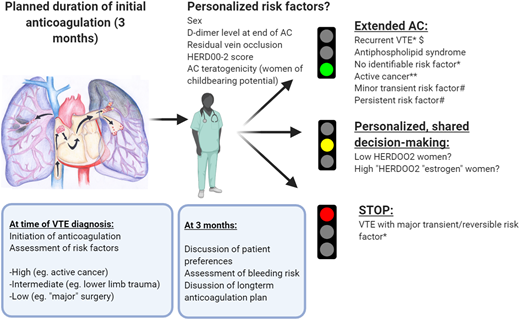
How we approach duration of anticoagulation in patients with a first VTE event. VTE recurrence risk is primarily informed by an initial evaluation of risk factors. We discuss an initial plan with the patient at the time of VTE diagnosis regarding the likely duration of anticoagulation, based on an evaluation of the circumstances in which the VTE occurred and bleeding risk. After a limited period of anticoagulation (typically 3 months), we schedule a consultation44 to confirm whether extended anticoagulation is warranted based on initial risk factors, ongoing risk factors, bleeding risk, gender-specific considerations, comorbidities, potential additional personalized risk factors, and patient preferences, particularly in situations where the optimal duration of anticoagulation is less clear. This discussion guides our evidence-based shared decision making on duration of anticoagulation. Patients whose initial event occurred in the context of a major, transient/reversible provoking factor (such as major surgery or surgery with general anesthesia for >30 minutes) are recommended by international guidelines to receive limited-duration anticoagulation (red light).2,43 In contrast, those with an unprovoked VTE event and other VTE events associated with a high predicted recurrence risk (and who have a low bleeding risk) are recommended by guidelines to receive extended or indefinite-duration anticoagulation (green light). Emerging data may in the future guide optimal management of patients with higher or lower personalized risk (orange light): for simplicity, in this figure the strength of recommendation, where indicated, is in accordance with the ESC 2019 Guideline on Acute PE.2 *Class I B (ESC ‘is recommended’; data derived from a single randomized clinical trial or large nonrandomized studies). **Class IIa level A (ESC ‘should be considered’; data derived from multiple randomized clinical trials or meta-analyses). #Class IIa level C (ESC should be considered; consensus of opinion of experts and/or small studies, retrospective studies, registries), although the relative strengths of recommendations are in line with other similar guidelines. AC, anticoagulation. $At least 1 previous episode of VTE not related to a major transient or reversible risk factor. Low HERDOO2 risk: ≤1 HERDOO2 criteria (hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age ≥ 65 years).18
How we approach duration of anticoagulation in patients with a first VTE event. VTE recurrence risk is primarily informed by an initial evaluation of risk factors. We discuss an initial plan with the patient at the time of VTE diagnosis regarding the likely duration of anticoagulation, based on an evaluation of the circumstances in which the VTE occurred and bleeding risk. After a limited period of anticoagulation (typically 3 months), we schedule a consultation44 to confirm whether extended anticoagulation is warranted based on initial risk factors, ongoing risk factors, bleeding risk, gender-specific considerations, comorbidities, potential additional personalized risk factors, and patient preferences, particularly in situations where the optimal duration of anticoagulation is less clear. This discussion guides our evidence-based shared decision making on duration of anticoagulation. Patients whose initial event occurred in the context of a major, transient/reversible provoking factor (such as major surgery or surgery with general anesthesia for >30 minutes) are recommended by international guidelines to receive limited-duration anticoagulation (red light).2,43 In contrast, those with an unprovoked VTE event and other VTE events associated with a high predicted recurrence risk (and who have a low bleeding risk) are recommended by guidelines to receive extended or indefinite-duration anticoagulation (green light). Emerging data may in the future guide optimal management of patients with higher or lower personalized risk (orange light): for simplicity, in this figure the strength of recommendation, where indicated, is in accordance with the ESC 2019 Guideline on Acute PE.2 *Class I B (ESC ‘is recommended’; data derived from a single randomized clinical trial or large nonrandomized studies). **Class IIa level A (ESC ‘should be considered’; data derived from multiple randomized clinical trials or meta-analyses). #Class IIa level C (ESC should be considered; consensus of opinion of experts and/or small studies, retrospective studies, registries), although the relative strengths of recommendations are in line with other similar guidelines. AC, anticoagulation. $At least 1 previous episode of VTE not related to a major transient or reversible risk factor. Low HERDOO2 risk: ≤1 HERDOO2 criteria (hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age ≥ 65 years).18
Risk prediction models for patients with unprovoked VTE: can we identify low-risk groups who may safely discontinue anticoagulation?
“Personalization” of risk profile could optimize benefit-to risk ratios by reliably identifying patients with lower and higher VTE recurrence risk, who could perhaps more safely discontinue or continue anticoagulation (Table 3). The recently published REVERSE II multinational management study aimed to determine whether patients with a low risk of recurrence can safely stop anticoagulant therapy after 5 to 7 months of treatment.18 The authors evaluated a previously derived HERDOO2 rule (hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥65 years) in 2785 patients with a first unprovoked proximal DVT or PE who had completed 5 to 12 months of anticoagulation. The primary outcome was independently and blindly adjudicated recurrent VTE during 1 year and occurred in low-risk women at a rate of 3.0%/patient-year (95% CI, 1.8-4.8%) and in high-risk women and men who discontinued and continued anticoagulation at a rate of 8.1%, (95% CI, 5.2-11.9) and 1.6% (95% CI, 1.1-2.3), respectively.
Recurrence risk prediction models after a first unprovoked VTE
| Study . | Type of study . | Predictors of VTE recurrence (vs no risk factor) . | VTE recurrence risk or RR, HR (95% CI), or score in model . |
|---|---|---|---|
| Rodger et al 2008 (HERDOO2 derivation study)42 | Prospective cohort study | (1) Postthrombotic syndrome (HER) | (1) Men: RR 2.54 (1.48-4.38) |
| (2) Elevated D-dimer on anticoagulation | (1) Women: RR 3.04 (1.40-6.60) | ||
| (3) Obesity | (2) Women: RR 3.02 (1.41-6.51) | ||
| (4) Older age | (3) Women: RR 2.33 (1.14-4.74) | ||
| (4) Women: RR 2.26 (1.12-4.56) | |||
| Eichinger et al 2010 (Vienna prediction model)19 | Prospective cohort study | Male sex | HR 1.91 (1.37-2.67) |
| Proximal vs distal DVT | HR 2.76 (1.57-4.84) | ||
| PE vs distal DVT | HR 3.15 (1.83-5.44) | ||
| Elevated D-dimer | HR 1.24 (1.05-1.45) | ||
| Tosetto et al 2012 (DASH score)20 | Patient-level meta-analysis | Elevated D-dimer | Score 2 |
| Young age* | Score 1 | ||
| Male sex | Score 1 | ||
| Hormone use | Score −2 | ||
| Rodger et al 2017 (REVERSE; HERDOO2 rule)18 | Prospective cohort management study | Low-HERDOO2-risk women | 3% per 100 patient years (1.8-4.8) |
| Men and high-risk women: | 1.6% per 100 patient years (1.1-2.3) | ||
| Continued AC | 8.1% per 100 patient years (5.2-11.9) | ||
| D/C AC | 7.4% per 100 patient years (3.0-15.2) | ||
| High-HERDOO-risk women who D/C AC |
| Study . | Type of study . | Predictors of VTE recurrence (vs no risk factor) . | VTE recurrence risk or RR, HR (95% CI), or score in model . |
|---|---|---|---|
| Rodger et al 2008 (HERDOO2 derivation study)42 | Prospective cohort study | (1) Postthrombotic syndrome (HER) | (1) Men: RR 2.54 (1.48-4.38) |
| (2) Elevated D-dimer on anticoagulation | (1) Women: RR 3.04 (1.40-6.60) | ||
| (3) Obesity | (2) Women: RR 3.02 (1.41-6.51) | ||
| (4) Older age | (3) Women: RR 2.33 (1.14-4.74) | ||
| (4) Women: RR 2.26 (1.12-4.56) | |||
| Eichinger et al 2010 (Vienna prediction model)19 | Prospective cohort study | Male sex | HR 1.91 (1.37-2.67) |
| Proximal vs distal DVT | HR 2.76 (1.57-4.84) | ||
| PE vs distal DVT | HR 3.15 (1.83-5.44) | ||
| Elevated D-dimer | HR 1.24 (1.05-1.45) | ||
| Tosetto et al 2012 (DASH score)20 | Patient-level meta-analysis | Elevated D-dimer | Score 2 |
| Young age* | Score 1 | ||
| Male sex | Score 1 | ||
| Hormone use | Score −2 | ||
| Rodger et al 2017 (REVERSE; HERDOO2 rule)18 | Prospective cohort management study | Low-HERDOO2-risk women | 3% per 100 patient years (1.8-4.8) |
| Men and high-risk women: | 1.6% per 100 patient years (1.1-2.3) | ||
| Continued AC | 8.1% per 100 patient years (5.2-11.9) | ||
| D/C AC | 7.4% per 100 patient years (3.0-15.2) | ||
| High-HERDOO-risk women who D/C AC |
Low HERDOO2 risk, ≤1 HERDOO2 criteria (hyperpigmentation, edema, or redness [HER] in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65 years).
AC, anticoagulation; D/C, discontinued; HR, hazard ratio; RR, relative risk.
First quartile (14-47 years) vs fourth quartile (>72 years).
Another model, the Vienna prediction model, was derived in a prospective cohort study including people with a first unprovoked VTE. Predictors included sex, the location and type of the VTE event, and D-dimer level.19 Finally, a score termed DASH (D-dimer, Age, Sex, Hormonal therapy) was derived in a meta-analysis of individual patient data from prospective studies.20 In this model, an abnormal D-dimer measured 3 to 5 weeks after discontinuation of anticoagulation, younger age, male sex, and hormone therapy were assigned scores of 2, 1, 1, and −2, respectively. Neither the Vienna prediction model nor the DASH score have been prospectively validated in a clinical management study.
As noted by the recently published ESC guidelines on acute PE, the therapeutic implications of risk prediction models such as the HERDOO-2 rule and the Vienna prediction and DASH scores may be less certain in the direct oral anticoagulant (DOAC) era,2 and future studies will no doubt shed more light on the optimal risk assessment strategy.
Returning to the individual whose case was described in the opening paragraph, indefinite anticoagulation (with regular follow-up) was recommended and jointly agreed with the patient, given his predicted VTE recurrence risk and low bleeding risk, according to the strategy outlined in Figure 2.
VTE recurrence risk in women
Pregnant women
Case 2: A 25-year-old woman presents at 8 weeks of gestation. She experienced a PE while on a combined oral contraceptive pill 2 years ago and completed a limited duration of anticoagulation. Should she receive prophylactic anticoagulation to prevent VTE recurrence during pregnancy?
Women with a personal VTE history have a high VTE recurrence risk during pregnancy21,22 and require special attention, because VTE remains a leading cause of maternal death as a direct consequence of pregnancy.2 Pregnant women who are at highest risk are those with a prior history of an unprovoked or a hormone-provoked VTE.21 In a pooled analysis of 4 cohort studies, major antenatal VTE recurrence rates during pregnancy without prophylaxis were 1.1% (95% CI, 0.2-5.8) 6.4% (95% CI, 3.9-10.4), and 3.6% (95% CI, 1.4-8.9) for provoked (nonhormonal), estrogen-related, and unprovoked VTE, respectively.23 It appears that this risk is reduced with low-molecular-weight heparin (LMWH).23 Previous guidelines have suggested various approaches to VTE prevention in these women including a low prophylactic or an intermediate LMWH dose24,25 (Figure 1). The optimal LMWH dose for women with prior VTE is currently being investigated in the ongoing Highlow study (NCT01828697; highlowstudy.org), a multinational randomized controlled trial (RCT) evaluating efficacy and safety of a fixed low dose of LMWH compared with an intermediate weight-adjusted dose in the prevention of VTE recurrence during pregnancy.
VTE recurrence risk in women whose initial VTE event was provoked by hormone use
Case 3: A 35-year-old woman was diagnosed with a proximal right DVT. She started a DOAC, and you now meet her after 3 months of anticoagulation. She complains of persistent right lower limb pain and swelling with erythema. She does not describe DOAC-related abnormal uterine bleeding or any other bleeding complications. Should she discontinue anticoagulation?
For women, VTE events occurring in the context of hormone use have traditionally been regarded as provoked events for the purposes of decision making surrounding duration of anticoagulation.22 Studies evaluating the impact of hormone use on VTE recurrence risk have reported conflicting results (Table 4). Absolute reported recurrence risks in hormonal contraceptive users also vary widely between studies (Table 4). Hormone use is categorized as an intermediate VTE-provoking factor, with a 3% to 8% predicted associated recurrence risk, by ESC guidelines,2 and as a minor (yet important) transient risk factor (with a 50% predicted recurrence risk compared with unprovoked VTE) by the ISTH.1 Many current guidelines do not provide clear guidance on duration of anticoagulation in women experiencing a VTE in the context of contraceptive use, with the exception of the 2012 ISTH guidelines on duration of anticoagulation, which suggest that a duration of 3 months is sufficient, provided that hormone use is discontinued.26 An ESC consensus statement addressing this topic will be published in 2020.
VTE recurrence risks in women with hormone exposure at the time of index VTE
| Reference . | Study type . | Recurrence rates of hormone VTE . | Groups compared (for HR, RR, or IRR) . | HR, RR, or IRR (95% CI) . |
|---|---|---|---|---|
| Heit et al 2000 (45) | Retrospective cohort | N/R for ♀ separately | COC- VTE ♀* vs all VTE | HR 0.3 (0.12-0.73) |
| HRT-related VTE vs all VTE | HR 0.7 (0.31-1.59) | |||
| Kyrle et al 2004 (46) | Prospective cohort | OC-VTE ♀: 5.9% at 5 y | OC- VTE vs idiopathic-VTE ♀ | RR 0.8 (0.1-4.0) |
| Non-OC ♀: 4.3% at 5 y | HRT-VTE vs idiopathic-VTE ♀ | RR 1.6 (0.4-6.0) | ||
| Baglin et al 2004 (47) | Prospective cohort | Estrogen-VTE: 8.7% at 2 y | Estrogen ♀ vs ♀ with other risk factors | HR 1.181 (0.35-4.03) |
| Other ♀: 8.7% at 2 y | ||||
| Cushman et al 2006 (56) | Data from PREVENT RCT | Hormone-VTE: 5% at 3 y | 1.Hormone-VTE vs idiopathic | 1.aHR 0.54 (0.19-1.54) |
| Other ♀: 15% at 3 y | 2.OC-VTE vs idiopathic | 2.aHR 0.43 (0.07-2.46) | ||
| 3.HRT-VTE vs idiopathic | 3.aHR 0.62 (0.2-1.91) | |||
| Rodger et al 2008 (42) | Prospective cohort | N/R | 1.OC-VTE vs non-OC-VTE ♀ | 1.RR 0.37 (0.11-1.21) |
| 2. HRT-VTE vs non-HRT-VTE ♀ | 2.RR 2.19 (0.86-5.55) | |||
| Le Gal et al 2010 (28) | Data from prospective cohort study | COC-VTE: 1.7% pa | 1.COC-VTE v snon-COC VTE ♀ | 1.aHR 0.6 (0.1-2.8) |
| HRT-VTE: 10% pa | 2.HRT-VTE vs non-HRT VTE ♀ | 2.aHR 1.8 (0.6-5.2) (adjusted for age) | ||
| Non-COC-VTE ♀ 5% pa | ||||
| High HERDOO2 OC-VTE 4% pa | ||||
| Douketis et al 2011 (49) | Patient-level meta-analysis | N/R | 1. Hormone-VTE vs unprovoked VTE ♀ | 1. HR 0.5 (0.3-0.8) |
| 2. OC-VTE vs unprovoked ♀ | 2. HR 0.39 (0.16-0.91) | |||
| 3. HRT-VTE vs unprovoked ♀ | 3. HR 0.76 (0.39-1.49) | |||
| Tosetto et al 2012 (20) | Individual patient data (prospective studies) | N/R | Hormone-VTE vs non-hormone-VTE ♀ | HR N/R (β coefficient −1.08; P = .002†) |
| Le Moigne et al 2013 (57) | Prospective single-center cohort | COC-VTE: IR 17.9/1000/y | COC-VTE vs non-COC VTE ♀ | IRR 0.7 (0.2-2.4) |
| Non-COC: 17.6/1000/y | ||||
| Eischer et al 2014 (58) | Prospective cohort (all women) | Estrogen-VTE: 6% at 5 y | 1.EC-VTE vs non-EC VTE ♀ | 1.RR 0.3 (0.1-0.5) |
| Non estrogen ♀: 17% at 5 y | 2.HRT-VTE vs non-HRT VTE ♀ | 2.RR 0.7 (0.3-1.5) | ||
| Ljungqvist et al 2014 (59) | Prospective follow-up of case control study | Estrogen-VTE: 2% pa | Hormone-VTE vs unprovoked ♀ | HR 0.57 (0.36-0.9) |
| Non estrogen ♀: 3.2% pa | aHR 0.7 (0.43-1.20) | |||
| Kearon et al 2015 (29) | Prospective clinical management | Non-estrogen ♀: 5.4% PPY | Men, nonestrogen ♀, estrogen ♀ | N/R (P = .001 for the 3–group comparison) |
| Estrogen ♀: 0.0% PPY | ||||
| All had persistent negative D-dimer at end of AC and off AC | ||||
| Rodger et al 2017 (18) | Prospective clinical management | Low HERDOO2 stopping AC; <50 y: | N/R | N/R |
| Estrogen-VTE: 1.4%/100 PY | ||||
| Nonestrogen ♀: 3.1%/100 PY | ||||
| Kiconco et al 2017 (60) | Retrospective population-based cohort study | Hormone-VTE: 37/1000 PY | 1.Hormone-VTE vs unprovoked ♀ | 1. aHR 0.72 (0.58-0.88) |
| Non hormone ♀: 51/1000 PY | 2. OC-VTE vs unprovoked ♀ | 2. aHR 0.71 (0.52-0.96) | ||
| 3. HRT-VTE vs unprovoked ♀ | 3. aHR 0.71 (0.53-0.95) |
| Reference . | Study type . | Recurrence rates of hormone VTE . | Groups compared (for HR, RR, or IRR) . | HR, RR, or IRR (95% CI) . |
|---|---|---|---|---|
| Heit et al 2000 (45) | Retrospective cohort | N/R for ♀ separately | COC- VTE ♀* vs all VTE | HR 0.3 (0.12-0.73) |
| HRT-related VTE vs all VTE | HR 0.7 (0.31-1.59) | |||
| Kyrle et al 2004 (46) | Prospective cohort | OC-VTE ♀: 5.9% at 5 y | OC- VTE vs idiopathic-VTE ♀ | RR 0.8 (0.1-4.0) |
| Non-OC ♀: 4.3% at 5 y | HRT-VTE vs idiopathic-VTE ♀ | RR 1.6 (0.4-6.0) | ||
| Baglin et al 2004 (47) | Prospective cohort | Estrogen-VTE: 8.7% at 2 y | Estrogen ♀ vs ♀ with other risk factors | HR 1.181 (0.35-4.03) |
| Other ♀: 8.7% at 2 y | ||||
| Cushman et al 2006 (56) | Data from PREVENT RCT | Hormone-VTE: 5% at 3 y | 1.Hormone-VTE vs idiopathic | 1.aHR 0.54 (0.19-1.54) |
| Other ♀: 15% at 3 y | 2.OC-VTE vs idiopathic | 2.aHR 0.43 (0.07-2.46) | ||
| 3.HRT-VTE vs idiopathic | 3.aHR 0.62 (0.2-1.91) | |||
| Rodger et al 2008 (42) | Prospective cohort | N/R | 1.OC-VTE vs non-OC-VTE ♀ | 1.RR 0.37 (0.11-1.21) |
| 2. HRT-VTE vs non-HRT-VTE ♀ | 2.RR 2.19 (0.86-5.55) | |||
| Le Gal et al 2010 (28) | Data from prospective cohort study | COC-VTE: 1.7% pa | 1.COC-VTE v snon-COC VTE ♀ | 1.aHR 0.6 (0.1-2.8) |
| HRT-VTE: 10% pa | 2.HRT-VTE vs non-HRT VTE ♀ | 2.aHR 1.8 (0.6-5.2) (adjusted for age) | ||
| Non-COC-VTE ♀ 5% pa | ||||
| High HERDOO2 OC-VTE 4% pa | ||||
| Douketis et al 2011 (49) | Patient-level meta-analysis | N/R | 1. Hormone-VTE vs unprovoked VTE ♀ | 1. HR 0.5 (0.3-0.8) |
| 2. OC-VTE vs unprovoked ♀ | 2. HR 0.39 (0.16-0.91) | |||
| 3. HRT-VTE vs unprovoked ♀ | 3. HR 0.76 (0.39-1.49) | |||
| Tosetto et al 2012 (20) | Individual patient data (prospective studies) | N/R | Hormone-VTE vs non-hormone-VTE ♀ | HR N/R (β coefficient −1.08; P = .002†) |
| Le Moigne et al 2013 (57) | Prospective single-center cohort | COC-VTE: IR 17.9/1000/y | COC-VTE vs non-COC VTE ♀ | IRR 0.7 (0.2-2.4) |
| Non-COC: 17.6/1000/y | ||||
| Eischer et al 2014 (58) | Prospective cohort (all women) | Estrogen-VTE: 6% at 5 y | 1.EC-VTE vs non-EC VTE ♀ | 1.RR 0.3 (0.1-0.5) |
| Non estrogen ♀: 17% at 5 y | 2.HRT-VTE vs non-HRT VTE ♀ | 2.RR 0.7 (0.3-1.5) | ||
| Ljungqvist et al 2014 (59) | Prospective follow-up of case control study | Estrogen-VTE: 2% pa | Hormone-VTE vs unprovoked ♀ | HR 0.57 (0.36-0.9) |
| Non estrogen ♀: 3.2% pa | aHR 0.7 (0.43-1.20) | |||
| Kearon et al 2015 (29) | Prospective clinical management | Non-estrogen ♀: 5.4% PPY | Men, nonestrogen ♀, estrogen ♀ | N/R (P = .001 for the 3–group comparison) |
| Estrogen ♀: 0.0% PPY | ||||
| All had persistent negative D-dimer at end of AC and off AC | ||||
| Rodger et al 2017 (18) | Prospective clinical management | Low HERDOO2 stopping AC; <50 y: | N/R | N/R |
| Estrogen-VTE: 1.4%/100 PY | ||||
| Nonestrogen ♀: 3.1%/100 PY | ||||
| Kiconco et al 2017 (60) | Retrospective population-based cohort study | Hormone-VTE: 37/1000 PY | 1.Hormone-VTE vs unprovoked ♀ | 1. aHR 0.72 (0.58-0.88) |
| Non hormone ♀: 51/1000 PY | 2. OC-VTE vs unprovoked ♀ | 2. aHR 0.71 (0.52-0.96) | ||
| 3. HRT-VTE vs unprovoked ♀ | 3. aHR 0.71 (0.53-0.95) |
Low HERDOO2 risk: ≤1 HERDOO2 criteria (hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65 years).
AC, anticoagulation; aHR, adjusted hazard ratio; CHC, combined hormonal contraceptives; COC, combined oral contraceptive; EC, estrogen contraceptives (including nonoral formulations); FVL, factor V Leiden polymorphism; Gen, generation; HC, hormonal contraceptive; HR, hazard ratio; HRT, hormone replacement therapy; IR, incidence rate; IRR, incidence rate ratio; N/R, not reported; N/S, not specified; OC, oral contraceptives; pa, per annum; PP, postpartum; PPY, per patient year; PY, person year; RR, risk ratio.
COC-VTE: initial VTE occurring in the context of COC.
Recurrence risk modeled using multivariable Cox regression; β coefficient and P value after backward elimination is reported.
Recent data suggest that recurrence risk for women with hormone-provoked VTE may be modulated by additional risk factors, thus potentially increasing or decreasing predicted recurrence risk. Highlighting the importance of individual risk assessment, the REVERSE investigators reported a high annual recurrence risk (6.6%; 95% CI, 0.0-15.9 and 4.1%; 95% CI, 0.0-12.2, respectively) among estrogen hormone replacement therapy and estrogen contraceptive users who were high risk by HERDOO2 criteria.28 Conversely, it may be possible to estimate a group of women with a potentially lower recurrence risk: a multinational prospective clinical management study reported VTE recurrence rates of 5.4% (95% CI, 2.5-10.2) per patient-year in nonestrogen women and a very low rate (0.0%; 95% CI, 0.0-3.0 per patient-year) in estrogen women who had, importantly, a persistently negative D-dimer after discontinuation of anticoagulation.29
Collectively, these data are hypothesis generating. Most women with contraceptive-provoked VTE may discontinue anticoagulation after 3 months, provided that alternative contraceptive options are provided that do not increase thrombotic risk. However, future studies may further explore personalized benefit to risk ratio in women with hormone-provoked VTE. In the meantime, the consultation process (Figure 2) should include discussion of risk factors for VTE recurrence, sex-specific bleeding risk (including abnormal uterine bleeding), adequate contraception, methods and preconceptual counseling.22
VTE recurrence risk in nonpregnant women whose initial event was provoked by pregnancy
For women with a prior VTE event provoked by pregnancy or the postpartum period, the incidence of recurrent VTE has reported to be lower than in the case of unprovoked VTE, prompting guideline categorization of this risk factor as associated with an intermediate recurrence risk.2 In a large retrospective study, the cumulative incidence of recurrent VTE up to 60 months after the index VTE event was significantly higher in women aged 18 to 46 years with unprovoked VTE compared with pregnancy (or postpartum)-associated VTE (10.4% and 5.8%, respectively; P < .02; adjusted HR, 0.6; 95% CI, 0.4-0.9).30 More recently, the Registro Informatizado de Enfermedad TromboEmbólica (Computerized Registry of Patients with Venous Thromboembolism) investigators reported a 2-year VTE recurrence rate of 3.3% for women whose initial event was pregnancy (or postpartum) associated.31 Collectively, these data suggest that limited duration anticoagulation may be appropriate for most women with prior pregnancy-associated VTE, after close follow-up and personalized assessment of risk factors as outlined in Figure 2.
VTE recurrence risks in people identifying as transgender
People identifying as transgender who have experienced a VTE face particular challenges.32 Hormone therapy is a key component of the transition from male to female. In a cohort study, cis women (women genetically assigned female at birth who identify as female) whose initial event was hormone-associated and who continued oral contraceptives after discontinuation of anticoagulation had a fourfold increased risk of recurrent VTE compared with women who stopped oral contraceptives (incident rate ratio, 4.6; 95% CI, 1.9-11.5).33 Although the recurrence risk for a transgender women with hormone-provoked VTE is not known, similar recurrence risks if hormones are continued without anticoagulation may potentially apply. However, discontinuation of hormones is often unacceptable. In a recent insightful review on the topic, Connors and Middeldorp32 suggest a similar approach for transwomen who have experienced prior VTE to that taken for cis women during the phase of ongoing anticoagulation, with consideration given to extended anticoagulation if continued hormone therapy is chosen by the patient for quality-of-life reasons.34
What does this mean for decision making on duration of anticoagulation?
Anticoagulation reduces the risk of recurrent VTE; however, this benefit does not persist after discontinuation of anticoagulation.35 When balancing benefit and harm for an individual patient, the case-fatality rates of bleeding and recurrent VTE and the probability of each are weighed. Case fatality rates in recent meta-analyses were 3.8% (95% CI, 2.0-6.1) for recurrent VTE,8 10.4% (95% CI, 6.6-15.4) for major bleeding during the initial VTE treatment phase with vitamin K antagonists (VKA), and 6.1% (95% CI, 2.7-11.7) for DOAC therapy.36 Case fatality rates for major bleeding during the secondary prevention phase of anticoagulation appear lower, at 0% (95% CI, 0.0-15.4) and 6.8% (95% CI, 1.4-18.6) for DOACs and VKA, respectively.37 Although DOACs are associated with lower major bleeding rates than VKA therapy,2 it should be noted that studies evaluating 2 different DOAC dosing strategies in comparison with either placebo38 or aspirin39 were not powered to compare outcomes between the 2 DOAC dosing strategies used (apixaban 2.5 mg and 5 mg twice daily in the AMPLIFY-EXT RCT38 and rivaroxaban 10 mg and 20 mg once daily in the EINSTEIN CHOICE RCT39 ).
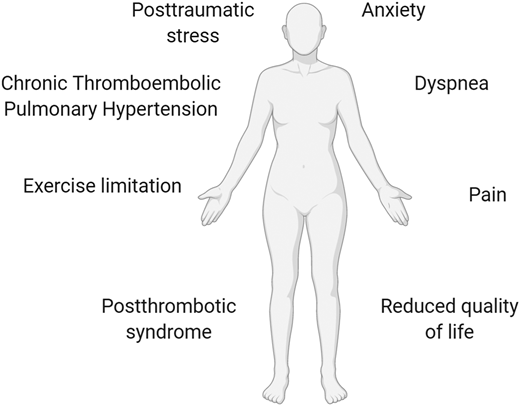
Nonfatal, life-altering complications of VTE should also be considered in this decision process, including postthrombotic syndrome and chronic thromboembolic pulmonary hypertension.2 There is a new emerging awareness that nonfatal consequences of PE (collectively termed the post-PE syndrome) are more varied and common than had previously been realized and include exercise intolerance, anxiety, and functional limitation.40 These symptoms can have a major impact on functional outcome, quality of life, and health care costs40 (Figure 3).
Long-term complications of recurrent VTE. Nonfatal, life-altering complications of VTE include postthrombotic syndrome (after DVT), chronic thromboembolic pulmonary hypertension (after PE), exercise intolerance, posttraumatic stress, dyspnea, anxiety, functional/exercise limitation, pain, reduced quality of life, and health care costs.2,40 These symptoms may have a major impact on functional outcome.40
Long-term complications of recurrent VTE. Nonfatal, life-altering complications of VTE include postthrombotic syndrome (after DVT), chronic thromboembolic pulmonary hypertension (after PE), exercise intolerance, posttraumatic stress, dyspnea, anxiety, functional/exercise limitation, pain, reduced quality of life, and health care costs.2,40 These symptoms may have a major impact on functional outcome.40
An ISTH consensus statement previously suggested that it may be safe to discontinue anticoagulation if the risk of recurrent VTE is predicted to be less than 5% (with an upper bound of the 95% CI of 8%) at 1 year after discontinuation,41 whereas studies aimed at deriving and validating clinical decision tools have sought to identify groups with predicted recurrence risks < 3%.18,42 However, these thresholds may in the future be revaluated in light of an emerging awareness of long-term complications40 and reported decreases in case-fatality rates of acute PE based on time-trend analyses in European, Asian, and North American populations.2 Moreover, assessment and awareness of individualized bleeding risk is crucial during decision making: American College of Chest Physicians recommendations provide a superb framework to guide this risk assessment, which includes validated risk factors for bleeding while on anticoagulation including older age, comorbidities (including previous stroke and diabetes), recent surgery, frequent falls, alcohol abuse, cancer, metastatic disease, chronic renal or hepatic failure, thrombocytopenia, requirement for antiplatelet therapy, and a history of bleeding without a reversible cause.43
Collectively, these data have shaped recent international guidelines,2,43 which recommend limited-duration anticoagulation for patients with a first VTE event that is provoked by a major transient/reversible risk factor (Figure 2). In contrast, indefinite anticoagulation is recommended for patients with recurrent unprovoked VTE or confirmed antiphospholipid syndrome. The strength of recommendation is somewhat weaker for those with a first unprovoked VTE, for those with persistent risk factors other than the antiphospholipid syndrome and for those with transient minor risk factors (as defined by Table 1), reflecting the lower level of certainty on the balance of benefit and harm in these patients: Consensus guidelines suggest that anticoagulation should be continued indefinitely in patients with unprovoked VTE who do not have a high bleeding risk. American College of Chest Physicians guidelines additionally note that personalized risk factors including “patient sex and D-dimer level measured a month after stopping anticoagulant therapy may influence the decision to stop or extend anticoagulant therapy.”
Conclusions
VTE recurrence risk is largely determined by personalized risk factors, most notably the circumstances in which the index VTE event occurred (Figures 1 and 2). International guidelines recommend limited-duration anticoagulation for patients with a first VTE event that is provoked by a major transient/reversible risk factor who have a low predicted VTE recurrence risk (Figure 2), and indefinite anticoagulation for patients with, for example, recurrent unprovoked VTE or antiphospholipid syndrome who have a high predicted VTE recurrence risk (provided that their bleeding risk does not preclude this). Individualized discussion and shared decision making is important for those whose personalized risk factors are not yet addressed by high-quality data, and prospective clinical management studies and RCTs to address these knowledge gaps should be prioritized, notably in the area of cis-women’s and transgender women’s health.
Acknowledgments
The authors thank Renata Donciu, who contributed the original artwork in Figures 1 to 3. This work was supported by a grant from the Health Research Board (KEDS-2018-004).
Correspondence
Fionnuala Ní Áinle, University College Dublin, Eccles Street, Dublin 7, Ireland; e-mail: fniainle@mater.ie.
References
Competing Interests
Conflict-of-interest disclosure: F.N.A. receives investigator-initiated research grant support paid to the university from Leo Pharma, Actelion, and Bayer. The remaining author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.
This article was selected by the Blood Advances and Hematology 2020 American Society of Hematology Education Program editors for concurrent submission to Blood Advances and Hematology 2020. It is reprinted from Blood Advances 2020, Volume 4.