Abstract
Treating unfit patients with aggressive B-cell lymphoma poses the dilemma of balancing potential cure while minimizing toxicity because of frailty and comorbidities. Age greater than 80 years and common comorbidities such as cardiovascular disease and poorly controlled diabetes mellitus often preclude the use of full-dose anthracyclines and steroids, the backbones of standard regimens for aggressive B-cell lymphomas. Assessing patient fitness remains subjective, with no consensus on best practice or how to integrate assessment tools into decision making. Incorporation of prephase steroids for all unfit patients may markedly improve performance status with consideration of standard dose therapy, especially in patients less than age 80. Although randomized studies are lacking, current data suggest patients age ≥ 80 years are considered unfit a priori and should receive dose-reduced anthracycline regimens or anthracycline-free regimens. Severe toxicity is highest after the first cycle of chemotherapy. Dose reductions for cycle 1 in unfit patients with plans to escalate as tolerated is often an effective strategy. Unfit patients often benefit from comanagement with gerontologists, cardio-oncologists, and endocrinologists depending on age and the nature of comorbidities. Palliative therapy for patients with newly diagnosed aggressive B-cell lymphoma results in median survivals of less than 3 months, and in general, should only be considered in patients with untreatable comorbidities such as advanced dementia or refractory metastatic solid tumors. Incorporating new, potentially less toxic agents such as novel antibodies, antibody–drug conjugates, and bispecific antibodies into first-line therapy is an exciting future direction with potential for substantial benefit in less fit patients.
Learning Objectives
Compare the benefit of maintaining dose intensity in unfit patients with DLBCL aged <80 and ≥80
Describe the outcomes with anthracycline-free regimens for unfit patients with DLBCL
Clinical case
An 84-year-old woman with a history of diabetes mellitus (DM), chronic kidney disease, hypertension, atrial fibrillation, and diastolic dysfunction with preserved left ventricular ejection fraction (74%) presented with epigastric pain, night sweats, early satiety, and a 5-lb weight loss. Computed tomography scan revealed an 8.6-cm liver mass, and a biopsy was consistent with diffuse large B-cell lymphoma (DLBCL), germinal center B-cell (GCB) phenotype, with no evidence of MYC rearrangement. International prognostic index (IPI) was 4, performance status (PS) was 2, lactic dehydrogenase level was 415 U/L, hemoglobin level was 9.7 g/dL, creatinine level was 1.48 mg/dL, and brain natriuretic peptide level was 2700 pg/mL. Before her diagnosis, the patient was the full-time caregiver for her husband, who has Alzheimer disease. The patient and her family were considering palliative treatment options. Would you offer potentially curative therapy? If so, what are the chemotherapy options and what information can you provide the patient regarding prognosis, possible complications, and treatment-related mortality (TRM)?
Introduction
Patients with aggressive B-cell lymphoma who are unfit represent a unique challenge, framed by the common dilemma of whether to administer intensive therapy with the potential for cure or to de-escalate therapy, thereby reducing toxicity.1 The aging population has led to a substantial increase in the number of older patients with DLBCL, with 40% greater than 70 years of age, which is a group for whom frailty and comorbidities limit options.2 Age greater than 80 and common comorbidities such as cardiovascular disease and DM often preclude the use of the standard R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), with prednisone, vincristine, and doxorubicin each posing special risks to vulnerable patients.3 Although many comorbidities may be manageable during chemotherapy, especially with the support of endocrinologists, cardio-oncologists, and gerontologists, others such as advanced dementia or concurrent metastatic solid tumor may prohibit curative intervention for lymphoma. Guidelines for best practices for unfit patients continue to rely on single arm phase 2 studies, as well as retrospective and population-based data. The European Society for Medical Oncology recently released recommendations for the clinical management of elderly patients with aggressive lymphoma that provide general guidance applicable to less fit patients.4 Decisions about whether to treat unfit patients with an anthracycline-based vs anthracycline-free regimen, and when to dose reduce, are complex and driven by concerns that comorbidities, impaired marrow function, poor PS, and impaired nutritional status will contribute to more frequent treatment-related complications.5 Clinical trials often exclude the oldest and least fit patients, and no prospective randomized studies have addressed the appropriate regimen for this population. Additional challenges include the complexity and often labor-intensive nature of formal comprehensive assessments needed to categorize fitness accurately, as well as the lack of data to support use of these objective tools in medical decision making. This article will summarize treatment options for unfit patients with aggressive B-cell lymphoma including the use of prephase steroids and other supportive care measures, review data on the effect of dose intensity in older and less fit patients, and discuss strategies for choosing a regimen that optimizes efficacy while minimizing toxicity.
Assessment of patient fitness
Despite several proposed tools to assess patient’s baseline status as fit, unfit, or frail, there is no uniform consensus on the optimal tool, how to integrate tools into decision making, and the impact of frailty assessments on patient outcomes. Traditionally, comprehensive geriatric assessments are time-consuming and often require consultation with a geriatrician. This may be unrealistic for many patients with aggressive lymphomas given the need to start treatment expeditiously and the complexity of obtaining expedited referrals. The International Society of Geriatric Oncology task force reviewed several screening tools to assess fitness and identified the G8 tool, which includes only 8 questions and age, as one of the simplest and most predictive assessments.6,7 The Charlson comorbidity index, a weighted index that takes into account the number and seriousness (scale of 0-5) of comorbid diseases, is commonly used in assessing the extent and severity of comorbidities.8 More recently, the Fondazione Italiana Linfomi group defined and validated a new Elderly Prognostic Index integrating geriatric and clinical assessment in 1353 patients age ≥ 65 years with DLBCL using a simplified Comprehensive Geriatric Assessment (sCGA) together with age to classify patients as fit, unfit, or frail (Table 1).9 sCGA incorporates the activities of daily living (ADL) score (1 point for bathing, dressing, toileting, transferring, feeding, and continence), instrumental activities of daily living (IADL) score (1 point for ability to use the phone, shop, prepare food, keep house, do laundry, travel on public transportation, handle money, and take own medication), and Comorbidity Index Rating Scale.1,9,10 Multivariate analysis confirmed the 3 sCGA groups, IPI, and hemoglobin < 12 g/dL were independent prognostic factors. Based on these independent factors, the Elderly Prognostic Index defined 3 groups of patients with a 3-year overall survival (OS) of 87% in the low-risk (0-1) group, 69% in the intermediate-risk group (2-4), and 42% in the high-risk group (5-7). Importantly, this model categorizes all patients age ≥ 80 as unfit or frail. Efforts are ongoing to develop an even simpler but meaningful geriatric assessment tool, sometimes referred to as a frailty vital sign, such as gait speed or grip strength that can be easily incorporated into routine physical examination.11,12
Categorization of fitness and integration of age and sCGA to define prognostic groups by the Fondazione Italiana Linfomi
| . | Fit . | Unfit . | Frail . | ||
|---|---|---|---|---|---|
| ADL | 6 | 5 | ≤4 | ||
| IADL | 8 | 7-6 | ≤5 | ||
| CIRS-G |
|
|
| ||
| Age | — | ≥80 fit | ≥80 unfit | ||
| . | Fit . | Unfit . | Frail . | ||
|---|---|---|---|---|---|
| ADL | 6 | 5 | ≤4 | ||
| IADL | 8 | 7-6 | ≤5 | ||
| CIRS-G |
|
|
| ||
| Age | — | ≥80 fit | ≥80 unfit | ||
| . | Fit . | Unfit . | Frail . | ||
|---|---|---|---|---|---|
| Age | <80 | ≥80 | <80 | ≥80 | |
| sCGA group | 1 | 2 | 3 | ||
| . | Fit . | Unfit . | Frail . | ||
|---|---|---|---|---|---|
| Age | <80 | ≥80 | <80 | ≥80 | |
| sCGA group | 1 | 2 | 3 | ||
CIRS-G, comorbidity index rating scale for geriatrics.
Reprinted from Spina et al9 with permission from the American Society of Hematology.
Use of even these simplified tools is uncommon in routine practice, because of time constraints and lack of data on how to incorporate the results into decision making. In a prospective trial of 100 patients age ≥ 70 years with DLBCL, Spina et al1 prescribed modulated chemotherapy based on the sCGA. Patients scoring less than 5 on either the ADL or iADL scale had a 50% dose reduction and those scoring 5 on the ADL scale or 5 to 6 on the iADL scale had a 25% dose reduction, with excellent outcomes and low toxicity rates reported. Despite the lack of randomized or confirmatory trials, these guidelines represent a reasonable quantitative approach to dosing for unfit patients. Quantifying ADL and IADL scores, as well as a mini-mental status examination score as standard practice, both at baseline and intermittently on therapy, would potentially allow objective guidance for dose escalation or de-escalation during subsequent cycles.
Prephase treatment
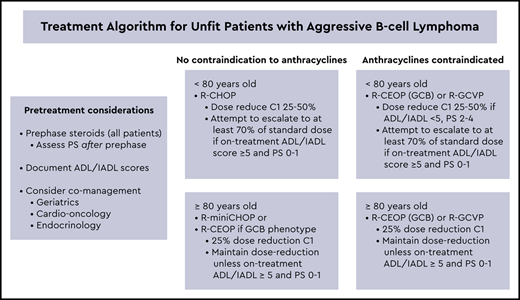
Treatment-related deaths in older patients undergoing chemotherapy for DLBCL occur most frequently after cycle 1. In a retrospective study of 530 veterans age 80 and older treated for DLBCL, there was an 18% TRM, with 67% (32 of 48) of deaths associated with the first cycle of chemotherapy; most of these were related to infection.13 PS at diagnosis was the most significant predictor of TRM: 27% in patients with a PS of 2 to 4 vs 8% in patients with a PS of 0 to 1. Prephase treatment was first introduced by the German High-Grade NHL Study Group in the NHL-B2 trial when a high rate of infection and death after the first cycle of CHOP chemotherapy was noted in older patients on the study.14 A trial amendment required a single injection of 1 mg vincristine and 5 to 7 days of prednisone, 100 mg daily, before initiating CHOP. As shown in Figure 1, introduction of prephase treatment significantly decreased the incidence of TRM in the first 2 cycles.15 Although not quantified, Pfreundschuh15 also describes a lower incidence of tumor lysis after the addition of prephase treatment. In subsequent studies, the German High-Grade NHL Study Group eliminated vincristine and prescribed only prephase prednisone. Peyrade et al16 incorporated vincristine 1 mg on day 7 and prednisone 60 mg on days −7 to −4 as prephase treatment in the ofatumumab miniCHOP study for patients age ≥ 80 with DLBCL, favoring a lower dose of steroids because of the increased risk of mania, psychosis, and hyperglycemia in patients over age 80. In this study, 5% of patients died on treatment, but none were treatment related, compared with the prior rituximab plus reduced-dose doxorubicin, cyclophosphamide, vincristine, and prednisone (R-miniCHOP) study, in which 27 of 150 (18%) of patients died on treatment, with 8% (12 of 150) due to treatment-related toxicity, mostly during cycles 1 and 2.16,17 Other small series, not limited to older patients, have confirmed an improvement in PS and decrease in first cycle admissions with prephase treatment.18,19 In patients with gastrointestinal involvement by aggressive lymphoma, prephase steroids may also decrease the risk of perforation and improve outcomes.20 Despite the lack of randomized trials, there seems little downside to prescribing 5 to 7 days of prednisone, 60 to 100 mg/day for all unfit or older patients before initiating chemotherapy. Delaying decisions regarding dose reductions until re-evaluation of PS after prephase steroids may allow a subset of previously unfit patients to transition to standard dosing.
Therapy-associated deaths in the NHL-B2 trial of CHOP in DLBCL before and after the introduction of prephase treatment. Before ( ) and after (
) and after ( ) the introduction of prephase treatment. Reprinted with permission. Reprinted from Pfreundschuh14 with permission of the American Society of Hematology.
) the introduction of prephase treatment. Reprinted with permission. Reprinted from Pfreundschuh14 with permission of the American Society of Hematology.
Therapy-associated deaths in the NHL-B2 trial of CHOP in DLBCL before and after the introduction of prephase treatment. Before ( ) and after (
) and after ( ) the introduction of prephase treatment. Reprinted with permission. Reprinted from Pfreundschuh14 with permission of the American Society of Hematology.
) the introduction of prephase treatment. Reprinted with permission. Reprinted from Pfreundschuh14 with permission of the American Society of Hematology.
Anthracycline-based chemotherapy
For patients without cardiac contraindications, anthracycline-containing regimens remain the standard of care.21 Lin et al21 identified 9 retrospective cohort studies and 2 SEER-database derived analyses comparing chemotherapy regimens with and without anthracyclines in more than 11 000 elderly patients with DLBCL. With the limitations of inherent selection bias and the lack of objective assessment of fitness, collectively, these studies support an association between use of anthracycline-containing regimens and improved OS (3-year OS, 63% vs 44%) with acceptable toxicities.21 Several other retrospective studies have tried to evaluate outcomes with and without anthracyclines based on measures of fitness. In a retrospective analysis of 135 patients with DLBCL ages 60 to 84, 53 (38%) were classified as unfit using CGA criteria.22 Among patients treated with curative intent, 1-year progression free survival (PFS) was 83.7% for fit vs 66.5% for unfit patients (P = .011), with unfit patients having higher IPI scores. Outcomes for patients treated with palliative intent were dismal.
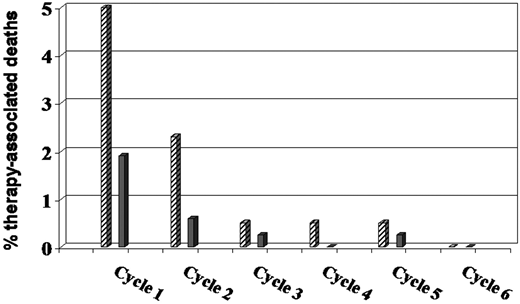
In an attempt to improve the tolerability of anthracycline-based regimens in the oldest patients, a large single-arm phase 2 study tested R-miniCHOP in 149 patients over age 80 with previously untreated DLBCL.17 R-CHOP dose modifications included doxorubicin 25 mg/m2, cyclophosphamide 400 mg/m2, vincristine 1 mg, and prednisone 40 mg/m2, but no prophylactic growth factors. The 2-year OS was 59% and 2-year PFS was 47%, with 12 (8%) treatment-related deaths (Figure 2). An IADL score less than 4 was predictive of outcome in univariate but not multivariate analysis. A follow-up study by the same group explored ofatumumab-miniCHOP in a similar patient group and reported a 2-year OS of 64.7% and PFS of 57.2% with no treatment-related deaths.16 In addition to adding prephase steroids, this study incorporated documentation of a simplified 4-question IADL, Buzby nutrition index, and Charlson Comorbidity score; however, the only factor predictive of outcome was the IPI score. These regimens have not been tested in patients age less than 80.
PFS in patients older than 80 years treated with R-miniCHOP (n = 150). Reprinted from Peyrade17 with permission of Elsevier.
PFS in patients older than 80 years treated with R-miniCHOP (n = 150). Reprinted from Peyrade17 with permission of Elsevier.
Impact of dose intensity based on age and fitness
Several retrospective series have attempted to address the impact of dose intensity in older, unfit, and frail patients with DLBCL. In a prospective study incorporating the CGA tool in 173 consecutive newly diagnosed patients age ≥ 70 treated during a 1-year period, curative treatment defined as >70% of the full-dose intent to treat resulted in improved outcomes in the 16% of patients categorized as unfit with a 2-year OS of 75% vs 45%, (P = .32). This was not the case in frail patients.10 In an Asian population of 192 patients greater than age 60 with DLBCL, a PS of 2, age ≥ 75 years, and doxorubicin and cyclophosphamide relative dose intensity < 60% were all independent prognostic factors for survival.23
In 690 consecutive patients with age > 70 years with newly diagnosed DLBCL treated between 2009 and 2018 across 8 UK centers, the intended dose intensity <80% vs ≥80% in patients with age 70 to 79 years was highly predictive of PFS and OS (P < .001) but had no effect on outcomes in patients 80 or older (P = .88; Figure 3).5,24 Comorbidities were associated with worse OS, but not lymphoma-specific survival. Similar results were reported in another retrospective study of 142 patients treated at a single medical center, with a marked reduction in OS for patients with age <80 years receiving <90% of the recommended dose intensity of doxorubicin (P = .005) or cyclophosphamide (P = .03), but not in patients with age ≥ 80 years after controlling for IPI and albumin.25
Impact of intended and relative dose intensity of R-CHOP in patients ≥ 70 years with DLBCL in a representative, consecutive cohort across 8 UK centers (2009-2018) treated with curative intent. (A) OS and (B) PFS by age and intended dose intensity. Reprinted from Eyre24 with permission of the Journal of Internal Medicine.
Impact of intended and relative dose intensity of R-CHOP in patients ≥ 70 years with DLBCL in a representative, consecutive cohort across 8 UK centers (2009-2018) treated with curative intent. (A) OS and (B) PFS by age and intended dose intensity. Reprinted from Eyre24 with permission of the Journal of Internal Medicine.
In a population based Danish cohort study of 1011 patients with age ≥ 75 years with DLBCL, the importance of dose intensity was age dependent.2 Patients with ages 75 to 79, with or without comorbidities, were better served by standard treatment with R-CHOP than low-intensity (LI) treatment without anthracyclines. In patients with ages 80 to 84 years with no comorbidities, standard treatment also resulted in better PFS and OS than LI treatment. However, after adjusting for baseline characteristics, patients with age ≥ 80 years with comorbidities or patients with age ≥ 85 years regardless of fitness did not benefit from standard treatment over LI treatment. In all 3 age groups, palliative treatment resulted in a median OS of 0.2 years.
In another population-based study, in 530 veterans age 80 and older with newly diagnosed DLBCL treated between 1998 and 2008, only 285 received systemic treatment, including 193 with an anthracycline-based regimen.13 Dose intensity ≥ 85% was associated with worse outcomes compared with dose intensity < 85%. Particularly striking was a first cycle mortality of 11% in patients given full-dose doxorubicin compared with 2% in those not treated with full dose. At 1 year, 59% of those treated at full dose intensity were alive compared with 70% treated with dose intensity < 85%; however, by 2 years, the OS rates were 53% vs 48%, perhaps suggesting higher relapse rates with lower dose intensity. After controlling for other variables, anthracycline use was not associated with OS. The 40% of patients who received no treatment had a median OS of 1.9 months.
If an anthracycline-based regimen is selected, a multidisciplinary approach including a cardiologist should be considered for all unfit and older patients, with cardiovascular profiling and risk stratification before treatment if feasible.26 Following cardiac biomarker measurements (troponin and brain natriuretic peptide) on treatment may ameliorate cardiotoxicity by allowing earlier intervention.26
Anthracycline-free regimens
Although regimens that do not include anthracyclines are considered most commonly for patients with cardiovascular comorbidities, a group of patients commonly excluded from trials, this approach may also be appropriate for patients greater than 80 years of age who are unfit. Anthracycline-free regimens have been associated with a higher risk of death in patients with age 75 to 79 regardless of comorbidities and in fit patients with age 80 to 84.2 However, in patients with ages 80 to 84 years who were unfit, or patients with age 85 years or older regardless of comorbidities, outcomes without anthracyclines were not inferior to standard treatment.1
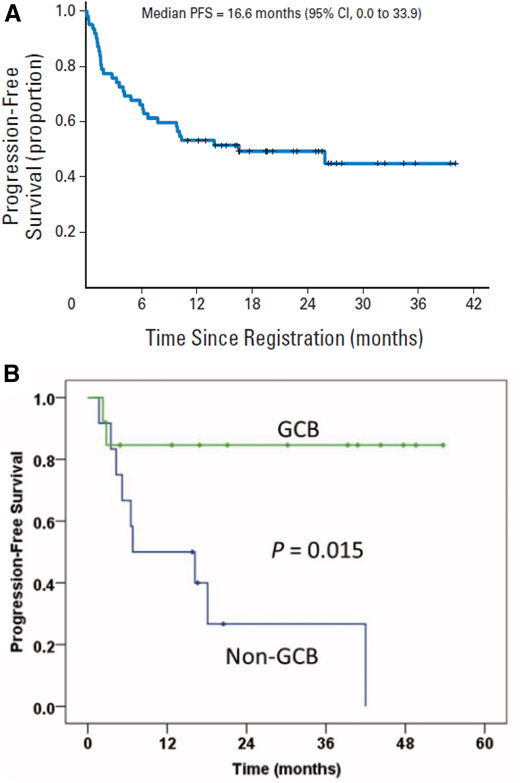
When anthracyclines are contraindicated, one approach is to simply omit the doxorubicin and administer R-CVP (rituximab, cyclophosphamide, vincristine, and prednisone). In multiple retrospective series of patients ≥ 80 years of age, results with R-CVP are generally inferior to R-CHOP, in large part likely because of selection bias.27-29 Replacing doxorubicin with an alternative active, but less toxic agent, such as etoposide or gemcitabine, is also an option. A phase 2 multicenter trial of R-GCVP (rituximab, cyclophosphamide, vincristine, gemcitabine, and prednisolone), accrued 61 patients (median age, 76.5 years) with newly diagnosed DLBCL and cardiac comorbidities.30 Overall response rate was 61.3%, 2-year PFS was 49.8%, and 2-year OS was 55.8% with no significant difference in outcomes for patients with left ventricular ejection fraction >50% vs ≤50% (Figure 4A).30 Common grade ≥3 toxicities included hematologic (55%), infection (27%), and cardiac (16%), with 3 fatal cardiac events on treatment. In the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, and prednisone) regimen, doxorubicin is replaced by etoposide (50 mg/m2 intravenously on day 1 and 100 mg/m2 orally on days 2 and 3).31,32 One retrospective study of 81 patients (median age, 73 years) ineligible for R-CHOP had a 5-year time to progression of 57% after treatment with R-CEOP.31 A single institution report of R-CEOP in 26 patients with a median age of 83 years reported 2-year event-free survival and OS rates of 49% and 59%, respectively.32 An unexpected finding in this small series was a 2-year PFS of 32% in the 13 patients with a non-GCB phenotype compared with 80% in the 12 patients with GCB phenotype (Figure 4B).
PFS for R-GCVP and R-CEOP in patients not eligible for R-CHOP. (A) PFS in patients (n = 63) considered unfit for anthracycline-containing chemoimmunotherapy because of cardiac comorbidity treated with R-GCVP. Reprinted from Fields30 with permission of the American Society of Clinical Oncology. (B) Median PFS from a single institution report of R-CEOP in patients with DLBCL (n = 26) separated into GCB and non-GCB phenotype. Reprinted from Rashidi32 with permission of Taylor & Francis.
PFS for R-GCVP and R-CEOP in patients not eligible for R-CHOP. (A) PFS in patients (n = 63) considered unfit for anthracycline-containing chemoimmunotherapy because of cardiac comorbidity treated with R-GCVP. Reprinted from Fields30 with permission of the American Society of Clinical Oncology. (B) Median PFS from a single institution report of R-CEOP in patients with DLBCL (n = 26) separated into GCB and non-GCB phenotype. Reprinted from Rashidi32 with permission of Taylor & Francis.
Other non–anthracycline-based approaches tested in the front-line setting for unfit patients are regimens that are currently used in the DLBCL salvage setting. A phase 2 study of R-GemOx (rituximab, gemcitabine, and oxaliplatin) was conducted in 60 patients with previously untreated DLBCL age 70 or older, or age 60 to 69 with PS 2.33 At a median follow-up of 45 months, the 3-year PFS and OS were 49% and 65%, respectively. In multivariate analysis, only IPI score was a poor prognostic factor, with 2-year OS of 38% in patients with IPI 3 to 5 compared with 85% for those with 0 to 2 (hazard ratio, 3.7). Febrile neutropenia occurred in 8% of patients with no TRM. A randomized trial is ongoing in China comparing R-miniCHOP to R-GemOx in the frontline setting (NCT02767674).
Tolerability and effectiveness of the bendamustine rituximab (BR) regimen in indolent lymphoma, stimulated evaluation of this regimen, initially in relapsed DLBCL but more recently as first line in older, less fit patients. In a phase 2 trial of 49 patients greater than 70 years of age with DLBCL and significant comorbidities (unfit and frail), the 2-year overall response rate was 62%, with 53% complete responses, but a disappointing 2-year PFS of 38%.34 In a retrospective comparison of BR vs R-CHOP in 140 patients ≥ 75 years of age, or >65 years of age with PS ≥ 2, BR was associated with marked inferior median OS (16.3 vs 75.4 mo, P = .006).35 BR patients were older and had higher-risk disease but no difference in comorbidities or PS. In the subset of patients with Charlson comorbidity index ≥ 6, there was no difference in outcomes between BR and R-CHOP. Although the BR regimen is well tolerated and has activity in DLBCL, the duration of remission in several reports appears significantly shorter that other regimens in this setting.36,37 Table 2 summarizes outcomes with the anthracycline- and non–anthracycline-based regimens discussed above in unfit and older patients.
Clinical trials with elderly/unfit patients with DLBCL
| . | Phase . | Patients, n . | Median age, y . | Patients age ≥ 80, % . | ORR, % . | CR, % . | PFS, % . | OS, % . | TRM, % . | Grade 3-4 F/N, %, . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R-miniCHOP | II | 150 | 83 | 100 | 73 | 62 | 47 (2-y PFS) | 59 (2 y) | 8 | 6 | 17 |
| Ofa-miniCHOP | II | 120 | 83 | 100 | 68 | 56 | 57 (2-y PFS) | 65 (2 y) | 0 | 21 | 16 |
| R-GCVP | II | 61 | 76 | 26 | 61 | 29 | 50 (2-y PFS) | 56 (2 y) | 7 | 0 | 30 |
| R-CEOP | Retro | 81 | 73 | NR | NR | NR | 57 (5-y PFS) | 49 (5 y) | 4 | NR | 31 |
| Retro | 26 | 83 | 65 | 75 | 58 | 49 (2-y PFS) | 59 (2 y) | 4 | 19 | 32 | |
| R-Benda | II | 45 | 81 | NR | 62 | 53 | 38 (2-y PFS) | 51 (2 y) | 0 | 2 | 34 |
| R-GemOx | II | 60 | 75 | 27 | 75 | 47 | 49 (3-y PFS) | 65 (3 y) | 0 | 5 | 33 |
| . | Phase . | Patients, n . | Median age, y . | Patients age ≥ 80, % . | ORR, % . | CR, % . | PFS, % . | OS, % . | TRM, % . | Grade 3-4 F/N, %, . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R-miniCHOP | II | 150 | 83 | 100 | 73 | 62 | 47 (2-y PFS) | 59 (2 y) | 8 | 6 | 17 |
| Ofa-miniCHOP | II | 120 | 83 | 100 | 68 | 56 | 57 (2-y PFS) | 65 (2 y) | 0 | 21 | 16 |
| R-GCVP | II | 61 | 76 | 26 | 61 | 29 | 50 (2-y PFS) | 56 (2 y) | 7 | 0 | 30 |
| R-CEOP | Retro | 81 | 73 | NR | NR | NR | 57 (5-y PFS) | 49 (5 y) | 4 | NR | 31 |
| Retro | 26 | 83 | 65 | 75 | 58 | 49 (2-y PFS) | 59 (2 y) | 4 | 19 | 32 | |
| R-Benda | II | 45 | 81 | NR | 62 | 53 | 38 (2-y PFS) | 51 (2 y) | 0 | 2 | 34 |
| R-GemOx | II | 60 | 75 | 27 | 75 | 47 | 49 (3-y PFS) | 65 (3 y) | 0 | 5 | 33 |
CR, complete response; F/N, febrile neutropenia; NR, not reported; ORR, overall response rate; Retro, retrospective study.
Supportive care measures
Supportive care measures are essential in older patients undergoing aggressive chemotherapy. In addition to specific recommendations described below, there should be consideration of more frequent follow-up of older patients, especially during the first 2 cycles of treatment. For example, assessing patients weekly with blood counts and the need for hydration or calorie supplementation may allow earlier intervention and avoid life-threatening complications. For patients with DM, engaging the primary physician or an endocrinologist to follow blood sugar control closely during treatment may lessen the risk of severe hyperglycemia related to prednisone.
Prophylactic antibiotics
As described above, increased rates of infection were observed in a number of R-CHOP–based trials in elderly patients. The German NHL study group noted an increased risk of grade 3 and 4 infections in the DENSE-R-CHOP14 trial, which enrolled patients 61 to 80 years of age.38 After the first 20 patients, acyclovir (daily) and cotrimoxazole (twice a week) were added to the regimen, and the serious infection rate dropped from 35% to 18%. This rate was also substantially lower than the 28% reported in a similar patient population treated with standard R-CHOP-14, leading the German NHL study group to recommend this prophylaxis in all older patients receiving an R-CHOP regimen.15,38 Although no consensus guidelines exist for antibiotic prophylaxis in less fit patients with aggressive lymphoma, it is reasonable to consider acyclovir and cotrimoxazole.
Myeloid and erythroid growth factors
Prophylactic myeloid growth factors should be considered for all patients 80 years or older and those with significant comorbidities receiving cytotoxic chemotherapy for aggressive lymphomas, regardless of the regimen. Growth factors were not administered prophylactically in the R-miniCHOP regimen, and the incidence of febrile neutropenia was only 7%; however, 3 of 149 (2%) of patients died of neutropenic sepsis during cycle 1.17 The American Society of Clinical Oncology guidelines recommend the use of myeloid growth factors for regimens with a ≥20% incidence of febrile neutropenia; however, data used to develop these guidelines would have included few patients age 80 and older and few unfit or frail patients.39 I favor erring on the side of administering growth factors, particularly for cycle 1, in this patient population, even if administering a less intensive regimen.
Current guidelines do not recommend erythropoietin for patients with curable cancers because of concerns of increased thromboembolic complications and increased risk of progression in certain malignancies. A large randomized trial of darbopoeitin vs placebo in older patients with DLBCL receiving R-CHOP showed no detrimental effect of darbopoeitin on PFS or OS.40 A large meta-analysis also suggested the relative safety of these agents in lymphoma.41 Importantly, many centers limit transfusions to patients with hemoglobin less than 7 or 8 g/dL, which may lead to more symptoms in patients over age 80 or those with significant comorbidities. Even with less intense regimens such as ofatumumab-miniCHOP, 15% of patients required transfusions.16 Based on individual patient circumstances, erythropoietin could be considered in the very elderly or unfit who are experiencing significant treatment-related anemia.
Vitamin D supplementation
Although difficult to prove causality, several studies have shown worse outcomes for a number of cancers in patients with low vitamin D levels. A retrospective evaluation of pretreatment serum samples from 359 patients 61 to 80 years of age with DLBCL treated on the RICOVER-60 trial, showed that 54% were vitamin D deficient (<10 ng/mL) and 46% were insufficient (10-30 ng/mL).42 In contrast to the United States, there is no vitamin D fortification of milk in Germany, perhaps accounting in part for the remarkably high incidence of vitamin D deficiency in this patient population. In a multivariate analysis, both event-free survival and OS were significantly worse in patients with vitamin D levels ≤8 ng/mL compared with those with levels >8 ng/mL. Interestingly, there was no difference in patients who did not receive rituximab, supporting the hypothesis that vitamin D enhances rituximab-mediated cellular cytotoxicity.43 Vitamin D supplementation to maintain levels >30 ng/mL is recommended in this particularly vulnerable population.
Conclusions and future directions
Evidence-based guidance for treating less fit patients with aggressive B-cell lymphoma is limited. Available data suggest that patients ≥ 80 years of age and those with significant limitations of ADLs and IADLs do not benefit from full-dose chemotherapy and should be treated with R-miniCHOP or an anthracycline-free regimen. Prephase steroids should be considered for all unfit and older patients, and decisions regarding treatment should be reassessed based on PS after prephase. In unfit patients, I start with a 25% to 50% dose reduction for cycle 1, and in those younger than 80 years of age, I attempt to escalate to at least 75% of standard dose with subsequent cycles if tolerated.
Returning to the earlier case presentation, I recommended R-CEOP with prophylactic pegfilgrastim for this 84-year-old woman with high-risk, GCB phenotype DLBCL after describing the very poor survival with palliative approaches and an approximately 40% to 50% chance of cure with a 5% to 10% TRM using chemotherapy. Although I did not administer prephase steroids, in retrospect, I should have. After cycle 1, she was hospitalized for 2 weeks because of volume overload with marked lower extremity edema, hyperglycemia requiring initiation of neutral protamine Hagedorn and sliding scale insulin per endocrinology consult, and a gastrointestinal bleed. The edema and hyperglycemia continued to be problematic throughout treatment; however, she was able to complete 6 cycles at full dose. Although end-of-treatment positron emission tomography-computed tomography was interpreted as a partial response, she remains in remission 21 months after completion of treatment, living independently and caring for her disabled husband.
As new therapies emerge for aggressive B-cell lymphomas, especially targeted approaches, patients who are unfit and those >80 years of age are also likely to benefit because many of these agents, such as anti-CD19 antibodies, antibody drug conjugates, bispecific antibodies, and liposomal formulations have less toxicity than conventional chemotherapy. Combining new, less toxic agents with modified chemotherapy regimens either concurrently or as maintenance may lead to better outcomes. Trials designed specifically for the oldest and less fit patients are likely to have the greatest impact in improving treatment of this subgroup. Ongoing and recently completed trials such as R-miniCHOP vs R-miniCHOP/lenalidomide, R-miniCHOP vs R-miniCHOP/azacytadine, R-miniCHOP vs R-miniCHP/polatuzumab vedotin, single agent mosunutuzumab, R-lenalidomide, and R-lenalidomide plus a Bruton tyrosine kinase inhibitor may provide much needed evidence-based guidance on how to approach these challenging patients.
Acknowledgment
The author thanks the Barnes Jewish Hospital Foundation for support.
Correspondence
Nancy L. Bartlett, Washington University in St. Louis, 660 South Euclid Ave, St. Louis, MO 63110; e-mail: nbartlet@wustl.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.