Abstract
Targeting CD20 with the monoclonal antibody rituximab has improved survival in patients with aggressive B-cell lymphomas, the majority of which are cured with chemoimmunotherapy. Patients progressing through or relapsing after their treatment have a poor prognosis. Despite a number of promising novel agents with efficacy in relapsed disease, randomized trials building on the chemoimmunotherapy backbone have failed to show further survival benefit. Significant progress has been made in the last few years in relapsed or refractory disease with the emergence of therapies that harness the patient’s immune system to fight disease. The approval of 2 chimeric antigen receptor T-cell products has provided potential for curative therapy, although challenges remain with toxicities and access. The approval of the antibody drug conjugate polatuzumab in combination with chemoimmunotherapy has offered survival benefit to patients who are not candidates for more aggressive approaches and has the potential to change the standard of care for initial management. Several targeted agents have proven effective, but the majority do not produce durable responses, requiring development in combination with other targeted or conventional therapies. Herein, promising targets in aggressive lymphoma with the greatest potential for improving outcomes in these patients are discussed. Novel therapies, their toxicities, and their potential role in initial or subsequent treatment are highlighted.
Learning Objectives
Identify promising therapeutic targets in aggressive B-cell lymphomas and the different strategies to develop treatments directed at these targets
Recognize emerging therapies and discuss results of the most promising clinical trials evaluating these therapies
Understand further development of these therapies as single agents or in combination in the relapsed and frontline setting
Clinical case
A 55-year-old man presented with a 4-week history of left cervical lymphadenopathy (LN), drenching night sweats, and a 12-pound unintentional weight loss. Excisional LN biopsy confirmed an aggressive B-cell lymphoma. Biopsy showed diffuse infiltrate of medium to large atypical lymphocytes. Neoplastic cells were positive by immunohistochemistry for CD10, BCL6 (>70%), BCL2 (>90%), c-MYC (>90%), MUM1 (90%), and Ki67 ∼70%. Fluorescence in situ hybridization was negative for rearrangements in BCL2, BCL6, and MYC. The lymphoma was classified as diffuse large B-cell lymphoma (DLBCL), not otherwise specified with a nongerminal center (non-GC) B-cell phenotype by Hans algorithm with coexpression of BCL2 and c-MYC, called double expressor lymphoma. Positron emission tomography/computed tomography (PET/CT) was remarkable for hypermetabolic lymphadenopathy above and below the diaphragm, with multiple hypermetabolic splenic and osseous lesions. He received 6 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). His interim and end-of-therapy PET/CT were consistent with a complete metabolic response, Deauville 2. He noted enlarging right cervical LN just before 3-month follow-up, and biopsy confirmed relapsed disease. He enrolled in a clinical trial randomly assigning patients to standard of care (SOC) with salvage chemotherapy followed by autologous stem cell transplant (ASCT) versus an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy. He was randomly assigned to SOC and achieved a complete remission (CR) after 2 cycles of rituximab, ifosfamide, etoposide, and carboplatin. He received carmustine, etoposide, cytarabine, and melphalan conditioning and proceeded to ASCT with day +30 PET/CT showing complete metabolic response. Unfortunately, his day +100 PET/CT showed disease progression. He was initiated on ibrutinib bridging therapy, achieving a rapid CR, but he quickly progressed. He received his CAR-T cells without cytokine release syndrome (CRS) or neurotoxicity; however, his day +60 PET/CT showed progression. He enrolled in a clinical trial evaluating combination lenalidomide and nivolumab.
Introduction
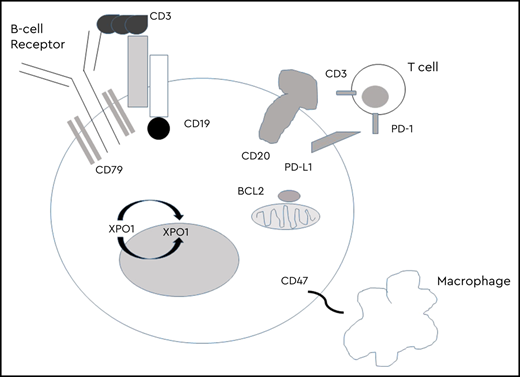
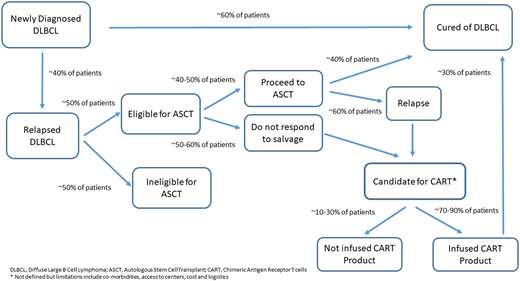
The addition of the anti-CD20 antibody rituximab to CHOP results in a cure for ∼60% of patients and, despite multiple trials, represents the last treatment breakthrough for untreated DLBCL.1 Outcomes are driven by biological heterogeneity including distinct cell of origin (COO),2 molecular clusters,3,4 translocations of MYC, BCL2, or BCL6 (high-grade B-cell lymphoma), and BCL2 and c-MYC protein overexpression without translocation.5 For patients who relapse, there is curative potential with intensive treatment including ASCT, but this occurs in a minority of patients, with outcomes significantly worse for patients receiving previous rituximab-based therapy or progressing within 1 year of initial therapy.6 CAR-T therapy targeting CD19 has shown promising results in relapsed or refractory (r/r) aggressive B-cell non-Hodgkin lymphoma (NHL), improving outcomes for patients not responding to salvage or relapsing after ASCT, where median overall survival (OS) is 6 months.7 Two second-generation CAR-T therapies (axicabtagene ciloleucel and tisagenlecleucel) are approved, with overall response rates (ORRs) of 52% to 83% and 40% to 58% CR.8,9 Despite impressive response rates, the majority of patients progress, and treatment is associated with significant toxicities including CRS and neurotoxicity (Figure 1). Accessibility to CAR-T centers, central manufacturing of cells, time to infusion, and financial burden remain challenges to broad access.
DLBCL. *Not defined, but limitations include comorbidities, access to centers, cost, and logistics.
DLBCL. *Not defined, but limitations include comorbidities, access to centers, cost, and logistics.
Improving frontline treatment for high-risk patients and identifying effective therapies for patients not candidates for or progressing after ASCT or CAR-T are of great importance. Herein, I highlight select recent and ongoing therapeutic approaches with promise to improve outcomes in r/r DLBCL.
Immunotherapy
Targeting CD19
The B-lymphocyte antigen C19 is expressed throughout B-cell development until terminal plasma cell differentiation, with high expression on most malignant B cells.10 Expression is preserved throughout lymphoma treatment, making CD19 an ideal target.
Tafasitamab is a novel Fc-engineered, humanized, CD19 monoclonal antibody. A phase 2a trial of single-agent tafasitamab included 35 patients with r/r DLBCL with a 26% ORR. The median duration of response (DOR) for 9 responders was 20 months, including 5 patients with responses ≥12 months.11 A phase 2 study evaluating tafasitamab and lenalidomide in 80 patients with r/r DLBCL considered ineligible for ASCT reported an impressive 60% ORR with 43% CR, median DOR 21.7 months with median follow-up 17.3 months, and median progression-free survival (PFS) 12.1 months.12 Responses occurred irrespective of COO, with activity seen in patients with both GC and non-GC DLBCL and in patients with poor prognostic features including similar responses seen in patients with or without refractory disease. With an additional year of follow-up, the median DOR was 34.6 months, confirming durability of this immunologic combination.13 The most frequent toxicities were hematologic toxicity, diarrhea, and fatigue. The US Food and Drug Administration (FDA) granted accelerated approval in late July 2019 for this combination in r/r DLBCL. Tafasitamab is being evaluated in a randomized phase 2/3 study in combination with bendamustine compared with bendamustine + rituximab (BR) in r/r DLBCL (NCT02763319) and in a frontline phase 1 study in combination with R-CHOP and R-CHOP + lenalidomide (NCT04134936).
Antibody–drug conjugates (ADCs) carry a cytotoxic payload directed against tumor-associated antigens in an effort to maximize efficacy while limiting off-target toxicity. Loncastuximab tesirine (lonca) is a humanized anti-CD19 antibody conjugated to a pyrrolobenzodiazepine dimer. A phase 1 study included 61 patients with r/r DLBCL with a 49% ORR, 32% CR, and median DOR, PFS, and OS of 4.8, 2.9, and 10.9 months, respectively.14 There was no maximum tolerated dose (MTD) in this trial; the most common toxicities were hematologic toxicity, fatigue, edema, liver test abnormalities, nausea, rash, and dyspnea, generally reversible and manageable with dosage delays or reductions. A phase 2 study of 145 patients with r/r aggressive B-NHL reported a 45.5% ORR and 20% CR, including activity in patients with refractory disease and a small number of patients with high grade B-cell lymphoma.15 Lonca is being investigated in combination with ibrutinib (NCT03684694) and durvalumab (NCT03685344) with a randomized phase 3 trial of rituximab + lonca versus gemcitabine and oxaliplatin (NCT04384484) planned. A phase 2 study of the ADC coltuximab ravtansine, conjugated to a cytotoxic maytansinoid DM4, showed similar responses but is not being further developed in DLBCL.16
Bispecific T-cell engagers bring together T cells and tumor cells to trigger T-cell cytotoxicity and cytokine production when both binding sites are occupied. Blinatumomab, a bispecific T-cell engager targeting CD19 and CD3, was evaluated in a phase 1 study in r/r NHL with a 55% ORR in 14 patients with DLBCL.17 A phase 2 study in 25 patients with r/r DLBCL demonstrated a 43% ORR, 19% CR, median DOR 11.6 months, and median PFS 3.7 months at a median follow-up of 15 months. Stepped-up dosing was required for tolerability, which limited activity for patients with aggressive tumors. Although serious neurologic toxicity occurred in the first 2 patients treated with flat-rate dosing at the target dosage, even with stepped-up dosing, 22% grade 3 neurologic toxicity occurred. Optimization of the dosing schedule and toxicity has limited development in DLBCL.
Adoptive cellular therapy using CAR-T cells has had a significant impact on our ability to treat r/r aggressive lymphomas. Although axicabtagene ciloleucel and tisagenlecleucel are highly active, a significant number of patients receiving these therapies progress, and their broad use has been limited by toxicity, with 13% to 22% grade ≥3 CRS and 12% to 28% grade ≥3 neurologic dysfunction.8,9 A third product under priority review by the FDA, lisocabtagene maraleucel,18 reported a 73% ORR and 55% CR with 1% grade ≥3 CRS and 15% grade ≥3 neurologic toxicity. The encouraging activity for high-risk patients has led to investigation of CAR-T earlier in the course of treatment, including at first relapse in both transplant-eligible and non–transplant-eligible patients. A plethora of ongoing studies are being conducted with CAR-T, including novel constructs and rational combinations (Table 1), with ongoing efforts aimed at improving durable remissions and decreasing toxicity. CAR T-cell exhaustion and immune evasion, CD19 antigen loss, and lack of persistence are all potential mechanisms of resistance. The development of CARs targeting multiple antigens, or bispecific CARs, are under investigation with both CD19/CD22 and CD19/CD20 bispecific CARs.19,20 “Armored” CAR constructs have additional genetic modifications designed to secrete cytokines or express ligands that enhance or interact with endogenous immune cells.21
Select ongoing novel chimeric antigen receptor T-cell trials
| Target . | Phase . | Identifier . | Additional agents . |
|---|---|---|---|
| CD19/CD20 | 1 | NCT04215016 NCT04007029 | |
| CD19/CD22 | 1 | NCT03233854 | |
| CD19/CD22 | 1/2 | NCT03287817 | Followed by PD-1 antibody pembrolizumab |
| CD19 | 1 | NCT02706405 | Followed by PD-1 antibody durvalumab |
| CD19 | 1/2 | NCT04257578 | BTK inhibitor acalabrutinib prior |
| CD20 | 1/2 | NCT03277729 | |
| CD22 | 1 | NCT04088890 |
| Target . | Phase . | Identifier . | Additional agents . |
|---|---|---|---|
| CD19/CD20 | 1 | NCT04215016 NCT04007029 | |
| CD19/CD22 | 1 | NCT03233854 | |
| CD19/CD22 | 1/2 | NCT03287817 | Followed by PD-1 antibody pembrolizumab |
| CD19 | 1 | NCT02706405 | Followed by PD-1 antibody durvalumab |
| CD19 | 1/2 | NCT04257578 | BTK inhibitor acalabrutinib prior |
| CD20 | 1/2 | NCT03277729 | |
| CD22 | 1 | NCT04088890 |
BTK, Bruton’s tyrosine kinase inhibitor; PD-1, programmed death 1.
One of the challenges moving forward will be sequencing of CD19-directed therapies, because it remains to be seen whether efficacy of these agents will be hindered by previous use of others.
CD20 bispecific antibodies
Though initially promising, newer anti-CD20 monoclonal antibodies combined with CHOP have failed to show improvement over rituximab. Bispecific antibodies combine the specificity of two antibodies to simultaneously bind to different antigens, an antigen on the cancer cell and T-cell. Bispecific antibodies targeting CD20 and CD3 are in development, with mosunetuzumab and REGN1970 reporting promising activity. In a phase 1/2 study (NCT03677154), mosunetuzumab produced a 37.1% ORR with 19.4% CR in 124 patients with aggressive B-cell lymphoma with 1.1% grade ≥3 CRS and 3.7% neurotoxicity.22 A phase 1 study of REGN1970 (NCT03888105) produced a 33.3% ORR and 17.8% CR, with no DLTs observed and 7.4% grade ≥3 CRS and no neurologic toxicity.23 Both of these agents induced responses in patients progressing after CAR-T, which has been a difficult-to-treat population.
Immune checkpoint inhibition
Targeting programmed death 1 (PD-1) and programmed death-ligand 1 has proven disappointing in r/r DLBCL, with low ORRs to single-agent therapy.24 Checkpoint inhibition of the innate immune system through antibodies to CD47 has shown promise. CD47 is the dominant macrophage checkpoint overexpressed on most cancers, acting as a “do not eat me” signal that enables macrophage immune evasion. Magrolimab is a humanized, anti-CD47 monoclonal antibody that induces macrophage phagocytosis of cancer cells by blocking the “do not eat me” signal. A phase 1b/2 study of magrolimab and rituximab (NCT02953509) included 59 r/r DLBCL with 36% ORR, 15% CR, and median DOR not reached (NR).25 Therapy was well tolerated, with no MTD reached; the most common adverse events were on-target anemia and infusion reactions, with opportunities to safely incorporate this therapy into combination approaches.
Targeting CD79b
The ADC polatuzumab (Pola) targets CD79b, a component of the B-cell receptor (BCR). Pola was approved in combination with BR in r/r DLBCL based on a randomized phase 2 trial showing significant improvements in end-of-treatment ORR and CR compared with BR, with a median DOR, PFS, and OS of 12.6, 9.5, and 12.4 months, respectively.26 Efficacy was seen across all risk groups, with activity independent of COO and patients benefiting regardless of refractory disease or number of previous lines of therapy. The addition of pola resulted in higher rates of grade 3-4 neutropenia without higher rates of infection and 44% grade 1-2 peripheral neuropathy with improvement or resolution in most. Other novel combinations are being investigated (Table 2). A phase 3 frontline trial of 875 patients evaluating R-CHOP versus R-CHOP + polatuzumab (NCT03274492) recently completed accrual, with potential to change the SOC for frontline treatment.
Select ongoing clinical trials with novel combinations
| Agent . | Combination . | Phase . | Line of therapy . | Identifier . |
|---|---|---|---|---|
| Polatuzumab | Rituximab and lenalidomide | 1 | Relapsed | NCT02600897 |
| Polatuzumab | Obinutuzumab, rituximab, venetoclax | 1 | Relapsed | NCT02611323 |
| Polatuzumab | R-EPOCH | 1 | Frontline | NCT04231877 |
| Polatuzumab | ± RGemOx | 3 | Relapsed | NCT04182204 |
| Polatuzumab | + R-CHOP vs + R-CHP | 3 | Frontline | NCT04332822 |
| Venetoclax | Obinutuzumab, lenalidomide, ibrutinib, prednisone | 1 | Relapsed | NCT03223610 |
| Venetoclax | Rituximab and ibrutinib | 1 | Relapsed | NCT03136497 |
| Venetoclax | Obinutuzumab and lenalidomide | 1 | Relapsed | NCT02992522 |
| Venetoclax | Obinutuzumab | 2 | Relapsed | NCT02987400 |
| Venetoclax | Rituximab and bortezomib | 2 | Relapsed | NCT02987400 |
| Venetoclax | Rituximab and idasanutlin | 1/2 | Relapsed | NCT03135262 |
| Venetoclax | RICE | 1/2 | Relapsed | NCT03064867 |
| Selinexor | RICE | 1 | Relapsed | NCT02471911 |
| Selinexor | RGDP or RDHAOx | 1b | Relapsed | NCT02741388 |
| Selinexor | Ibrutinib | 1 | Relapsed | NCT02303392 |
| Selinexor | Venetoclax | 1 | Relapsed | NCT03955783 |
| Selinexor | R-CHOP | 1b | Frontline | NCT031478850 |
| Agent . | Combination . | Phase . | Line of therapy . | Identifier . |
|---|---|---|---|---|
| Polatuzumab | Rituximab and lenalidomide | 1 | Relapsed | NCT02600897 |
| Polatuzumab | Obinutuzumab, rituximab, venetoclax | 1 | Relapsed | NCT02611323 |
| Polatuzumab | R-EPOCH | 1 | Frontline | NCT04231877 |
| Polatuzumab | ± RGemOx | 3 | Relapsed | NCT04182204 |
| Polatuzumab | + R-CHOP vs + R-CHP | 3 | Frontline | NCT04332822 |
| Venetoclax | Obinutuzumab, lenalidomide, ibrutinib, prednisone | 1 | Relapsed | NCT03223610 |
| Venetoclax | Rituximab and ibrutinib | 1 | Relapsed | NCT03136497 |
| Venetoclax | Obinutuzumab and lenalidomide | 1 | Relapsed | NCT02992522 |
| Venetoclax | Obinutuzumab | 2 | Relapsed | NCT02987400 |
| Venetoclax | Rituximab and bortezomib | 2 | Relapsed | NCT02987400 |
| Venetoclax | Rituximab and idasanutlin | 1/2 | Relapsed | NCT03135262 |
| Venetoclax | RICE | 1/2 | Relapsed | NCT03064867 |
| Selinexor | RICE | 1 | Relapsed | NCT02471911 |
| Selinexor | RGDP or RDHAOx | 1b | Relapsed | NCT02741388 |
| Selinexor | Ibrutinib | 1 | Relapsed | NCT02303392 |
| Selinexor | Venetoclax | 1 | Relapsed | NCT03955783 |
| Selinexor | R-CHOP | 1b | Frontline | NCT031478850 |
R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CHP, rituximab, cyclophosphamide, doxorubicin, and prednisone; RDHAOx, rituximab dexamethasone, cytarabine, and oxaliplatin; R-EPOCH, rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; RGDP, rituximab, gemcitabine, dexamethasone, and cisplatin; RGemOx, rituximab, gemcitabine, and oxaliplatin; RICE, rituximab, ifosfamide, etoposide, and carboplatin.
BCR signaling pathway
Targeting BCR signaling with oral kinase inhibitors has changed the treatment landscape in several B-cell lymphomas; however, these therapies have modest activity in limited subsets of patients with DLBCL. The highest single-agent activity is reported with ibrutinib, which is active and well tolerated in non-GC DLBCL but with short DOR.27 Multiple combinations have been evaluated, the most promising being rituximab, ibrutinib, and lenalidomide (IR2). A phase 1b trial of the combination reported an ORR of 65%, with 41% CR in 23 patients with non-GC DLBCL with no MTD reached.28 The phase 2 enrolled 89 patients with non-GC DLBCL at 2 dose levels (n = 55 and n = 34, lenalidomide 20 mg and 25 mg).29 The ORR was 47%, with 28% CR, with median DOR, PFS, and OS of 18, 5, and 14 months, respectively. Median DOR was NR in patients achieving CR, and median PFS and OS in responders were 21 months and NR, respectively. A single-arm phase 2 study of untreated patients evaluating 2 cycles of IR2 followed by 6 cycles of IR2 + CHOP/etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin (EPOCH) in non-GC DLBCL enrolled 60 high-risk patients.30 The ORR and CR were 84.6% and 38.5%, respectively, after 2 cycles of IR2, increasing to 98% and 92.3% after 6 additional cycles of IR2 + chemotherapy. The 1-year PFS and OS were 92.5% and 96.5%, respectively. Longer follow-up is needed, but this trial is the first to show that “chemo-free” combinations are active in untreated DLBCL and may have a role in frontline therapy with or without combination chemotherapy.
Selective inhibitor of nuclear export
Selinexor is a first-in-class selective oral inhibitor of XPO1. Inhibition of XPO1 leads to nuclear accumulation and reactivation of tumor suppressor proteins such as p53 and p21 and reduction in oncoproteins including c-MYC, BCL2, and BCL6. A phase 1 study reported a 25.6% ORR in r/r DLBCL, with 4 patients achieving CR, 2 lasting ≥1 year. The most common gastrointestinal and constitutional adverse events (nausea, vomiting, anorexia, fatigue) were managed with antiemetics and appetite stimulators and grade 3-4 hematologic toxicity with dose delays or reductions.31 A multicenter phase 2 study in r/r DLBCL enrolled 129 patients with a 27.6% ORR, 10% CR, and median DOR of 9.2 months.32 Activity was seen in both GC (ORR 34%, CR 14%) and non-GC (ORR 21%, CR 10%) subtypes, and responses were consistent across risk groups. Patients were required to have a 60-day washout from previous therapy, suggesting some selection for less proliferative or aggressive disease. The FDA has granted priority review for selinexor for r/r DLBCL. A number of ongoing studies are evaluating selinexor combinations (Table 2).
BCL2 inhibition
BCL2 is a pro-apoptotic protein overexpressed in ∼30% of DLBCL.33 BCL2 overexpression correlates with resistance to R-CHOP and is associated with overall worse prognosis, with dismal outcomes when c-MYC is concomitantly overexpressed (double-expresser [DE] or double-hit [DH] lymphomas).34,35 Venetoclax (ven) is a highly selective BCL2 inhibitor with a potential role in overcoming resistance to chemotherapy. A phase 1 study including 34 patients with r/r DLBCL showed ven to be well tolerated, with an 18% ORR and 12% CR, with potential for rational combination therapy.36 A phase 1b/2 study in combination with R-CHOP established the recommended phase 2 dose with no MTD reached.37 Of the 8 patients with DE lymphoma, 7 achieved a CR. The phase 2 portion enrolled 211 patients, showing favorable PFS for patients with immunohistochemistry + BCL2 compared with historical controls.38 A phase 1 study in aggressive B-cell lymphomas in combination with dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin (R-EPOCH) reported a 93% ORR with 87% CR in DH lymphoma, with 10/13 patients with DH lymphoma maintaining CR at 11 months and 2/2 patients with DE lymphoma maintaining CR at 27 and 29 months.39 An ongoing randomized frontline phase 2/3 trial (NCT03984448) is evaluating the addition of ven to R-EPOCH in patients with DH lymphoma and R-CHOP in patients with DE lymphoma to improve outcomes in these patients with a poor prognosis. Several combinations in relapsed disease are being evaluated (Table 2).
Clinical case
The patient in the clinical case presented with non-GC double expressor lymphoma, which is associated with inferior prognosis if treated with R-CHOP. An ongoing randomized phase 2/3 study is evaluating whether R-CHOP + ven is able to improve outcomes for these patients by overcoming chemotherapy resistance. Patients with primary refractory DLBCL are often resistant to further chemotherapy; the role of CAR-T in lieu of SOC salvage and ASCT (NCT03391466) is under investigation. Although he did achieve a CR to SOC and proceeded to ASCT, he relapsed shortly after, where median OS is <6 months. He responded to ibrutinib, which is active in non-GC DLBCL, but responses are not durable. He proceed to CAR-T without concerning toxicity; however, he progressed soon after, representing a population of patients where clinical trials are priority. He enrolled in a trial with nivolumab and lenalidomide, which have potential activity after chimeric antigen receptor T cell therapy. He is responding to treatment and has an allogeneic donor.
Concluding remarks
SOC for DLBCL has remained the same for nearly 20 years, and improving upon R-CHOP will probably require targeted therapy active across COOs or novel trial designs incorporating targeted therapies for select patients. Remarkable progress has been made in treating r/r aggressive lymphomas with the approval of CAR-T, and efforts to improve the efficacy, safety, and accessibility of these therapies is ongoing. Targeting CD19 with other immunotherapies is attractive, with additional agents showing promising responses and favorable toxicity profiles. A number of novel therapies are active but have limited single-agent activity, and durable responses will require combination therapy. Novel combinations targeting biologic drivers of disease are of high interest.
Correspondence
Kami Maddocks, Division of Hematology, Department of Internal Medicine, Arthur G. James Comprehensive Cancer Center, The Ohio State University Wexner Medical Center, 320 W 10th St, A347C Starling Loving Hall, Columbus, OH 43210; e-mail: kami.maddocks@osumc.edu.
References
Competing Interests
Conflict-of-interest disclosure: K.M. has received research funding from Pharmacyclics, BMS, Merck, and Novartis. K.M. has consulted for Pharmacyclics, Janssen, Morphosys, Celgene, Karyopharm, and Seattle Genetics.
Author notes
Off-label drug use: None disclosed.