Abstract
Acute myeloid leukemia (AML) is a heterogeneous malignancy characterized by recurrent genetic, epigenetic, and metabolic abnormalities. As a result of our increasing knowledge of the underlying biology of AML leading to rational drug development, several new targeted agents have been recently added to our therapeutic arsenal. The BCL2 inhibitor venetoclax in combination with low-dose cytarabine (LDAC) or hypomethylating agents (HMAs) is safe and effective in older patients with newly diagnosed AML ineligible for intensive chemotherapy. Glasdegib, a hedgehog pathway inhibitor, may be used in combination with LDAC for the same indication and improves survival compared with LDAC alone. In newly diagnosed, fit, older patients with therapy-related AML or AML with myelodysplasia-related changes, the liposome-encapsulated combination of daunorubicin and cytarabine (CPX-351) has shown superiority over the 7 + 3 regimen. The presence of an IDH1 or IDH2 mutation can be effectively targeted by ivosidenib or enasidenib, respectively. Gemtuzumab ozogamicin improves event-free survival in CD33+ patients with favorable or intermediate-risk cytogenetics. With new targeted agents available, comprehensive genomic characterization of AML at diagnosis and relapse is increasingly necessary to select optimal treatment. Herein, we review the new single-agent and combination biologics (omitting FLT3 inhibitors, which are discussed separately) and provide recommendations on how to best use and manage patients on these regimens in clinical practice.
Learning Objectives
Understand the importance of genomic profiling of acute myeloid leukemia at diagnosis and at relapse to select the optimal treatment of patients
Review how to best use and incorporate the new single-agent and combination therapies for acute myeloid leukemia in routine clinical practice
Introduction
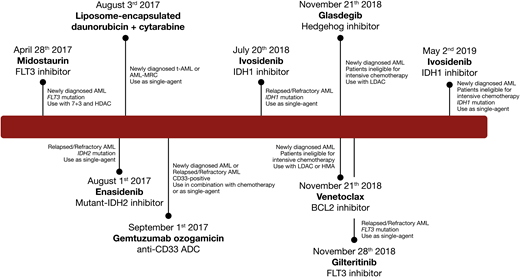
Acute myeloid leukemia (AML) is a heterogeneous hematopoietic malignancy characterized by genetic abnormalities in myeloid stem cells leading to differentiation arrest and accumulation of clonal myeloblasts in the bone marrow.1 The multiple genetic alterations identified in leukemic cells at diagnosis are the mainstay of the World Health Organization classification for AML and have important prognostic implications.1,2 Although several subtypes confer a favorable outcome with intensive chemotherapy, the prognosis of AML is globally poor, with a 5-year overall survival (OS) of 28%.3 The outcome is especially dismal for older patients deemed ineligible to receive intensive chemotherapy, for whom the median expected survival has remained under 1 year, underscoring the critical need for novel effective therapies.4 For decades, since the development of the 7 + 3 regimen, the standard treatment of AML has remained essentially unchanged, and although prognostic, the different genetic subtypes of AML provided limited therapeutic relevance apart for acute promyelocytic leukemia (APL)5 and core binding factor AML. Over the past decade, our understanding of the complex cellular and genomic heterogeneity of AML has expanded due to evolving gene sequencing technologies.2,6,7 Critical studies of the functional consequences of individual genetic alterations on cell signaling, epigenetics, or metabolism and a tremendous drug development effort have finally accelerated advances for therapy of AML. With 8 new Food and Drug Administration (FDA) approvals for the treatment of AML in the past 2 years, the field of AML therapeutics is rapidly evolving, renewing hope for improved outcomes in patients affected by this disease (Figure 1). Most of these newly FDA-approved drugs are targeted agents, confirming that AML therapeutics has officially entered the era of precision medicine in which characterization of morphologic, immunophenotypic, and genetic features of AML is not merely of diagnostic and prognostic relevance but now provides direct therapeutic implications. In this review, we will highlight through 2 clinical cases the recent progress in the treatment of AML with integration of novel single-agent and combination biologics into our therapeutic arsenal for this disease along with suggestions for optimal clinical practice while utilizing these novel agents.
Targeted agents recently approved by the FDA for the treatment of AML. ADC, antibody-drug conjugate; AML-MRC, acute myeloid leukemia with myelodysplasia-related changes; HDAC, high-dose cytarabine; HMA, hypomethylating agent; LDAC, low-dose cytarabine.
Targeted agents recently approved by the FDA for the treatment of AML. ADC, antibody-drug conjugate; AML-MRC, acute myeloid leukemia with myelodysplasia-related changes; HDAC, high-dose cytarabine; HMA, hypomethylating agent; LDAC, low-dose cytarabine.
Clinical case 1
A 72-year-old female patient was referred to our clinic for pancytopenia with peripheral blasts, progressive fatigue, and shortness of breath over the past month. She has a medical history of congestive heart failure, hypercholesterolemia, and poorly controlled diabetes; no previous exposure to chemotherapy or radiotherapy; and no known antecedent of hematological disorder. A bone marrow aspiration and biopsy confirmed the diagnosis of AML with 54% myeloblasts. Her karyotype was normal, and a next generation sequencing (NGS) panel revealed NPM1 and DNMT3A mutations with a wild-type FLT3 gene. Because of her age and comorbidities, she was not considered a suitable candidate for intensive chemotherapy. Treatment with azacitidine 75 mg/m2 on days 1 to 7 in combination with venetoclax continuously at a dose of 400 mg daily every 28 days was initiated. After her first cycle of therapy, she achieved a morphologic leukemia-free state (MLFS) with persistent neutropenia (absolute neutrophil count, 0.3 × 109/L) and thrombocytopenia (platelet count, 53 × 109/L). After interruption of venetoclax for 12 days and a dose of granulocyte colony-stimulating factor (G-CSF), the patient recovered her neutrophil count to 0.8 × 109/L and platelets to 76 × 109/L, and she resumed the planned treatment, with venetoclax duration modified to days 1 to 21 per cycle. She achieved complete remission (CR) with full hematological recovery after her second cycle of therapy and had negative measurable residual disease (MRD) as assessed by flow cytometry after cycle 4 of therapy. Venetoclax duration was reduced to 14 days per cycle after cycle 8 due to recurrent episodes of neutropenia after repeat bone marrow evaluations confirmed sustained remission in a mild to moderately hypocellular marrow. She is now receiving her 22th cycle of therapy, and she remains in prolonged MRD-negative CR with occasional G-CSF administration, no transfusion requirements, excellent quality of life, and tolerable side effects from her treatment.
Venetoclax combinations
Venetoclax is a selective inhibitor of BCL2, an antiapoptotic protein conferring enhanced survival and chemoresistance in multiple hematologic malignancies. Preclinical studies have shown that AML leukemic stem cells are dependent on oxidative phosphorylation and overexpress BCL2 for their survival, which renders them vulnerable to venetoclax and other BCL2 inhibitors.8,9
Based on preclinical data suggesting synergistic activity of venetoclax with hypomethylating agents (HMAs) or cytarabine, venetoclax was evaluated with either low-dose cytarabine (LDAC) or HMA in 2 independent multicenter clinical trials for older patients with untreated AML ineligible for intensive chemotherapy.10-12 In the phase 1b dose escalation and dose expansion study by DiNardo et al,10,11 145 treatment-naïve AML patients aged 65 years old or older received venetoclax 400, 800, or 1200 mg daily in combination with either decitabine 20 mg/m2 IV daily for 5 days or azacitidine 75 mg/m2 daily for 7 days. Patients were excluded if they had received HMA therapy for an antecedent hematologic neoplasm. Combination therapy was well tolerated, with most common side effects being gastrointestinal symptoms, fatigue, and neutropenic fever. The composite CR or complete remission with incomplete hematological recovery (CRi) rate was 67% and did not differ significantly between doses of venetoclax or between azacitidine or decitabine. Exemplified by our clinical case, patients with NPM1 mutation had a very high CR/CRi rate of 91%. Patients with adverse risk cytogenetics and/or TP53 mutation(s) had CR/CRi rates of 60% and 47%, respectively. Among patients achieving CR/CRi, 29% (28 of 97) also achieved MRD negativity as in our clinical case. The median duration of response (DOR) was 11.3 months (95% confidence interval [95% CI], 8.9 months to not reached [NR]), and median OS was 17.5 months (95% CI, 12.3 months to NR) at a median follow-up of 15.1 months, comparing favorably with historical data of HMA monotherapy associated with a median OS of <12 months.13,14 Although representing a small proportion of patients, the DOR and OS have not been reached yet in those patients who achieved MRD negativity with HMA plus venetoclax.
In a similar study by Wei et al,12 venetoclax in combination with LDAC was evaluated in patients with untreated newly diagnosed AML aged 60 years old or older and ineligible for intensive chemotherapy. In contrast to the study with HMA combination, patients in this study could have received prior HMA therapy for an antecedent hematological neoplasm. In the dose expansion cohort, patients received LDAC 20 mg/m2 once daily ×10 days in combination with venetoclax 600 mg daily, which was the recommended phase 2 dose. Adverse events were similar to those reported in the venetoclax plus HMA combination. Dose interruptions of venetoclax for adverse events were required in 55% of patients, and administration was reduced to 21 or 14 days in approximately one-half of patients. The CR/CRi rate was 54% in the global cohort; patients with de novo AML or intermediate cytogenetic risk had the best response rates, with CR/CRi of 63% and 71%, respectively. The CR/CRi rate was lower in patients with adverse risk cytogenetics (42%), secondary AML (35%), or prior HMA treatment (33%). Responses were higher in patients with NPM1 or IDH1/2 mutations (CR/CRi rate, 89% and 72%, respectively) and lower in patients with TP53 or FLT3 mutations (CR/CRi rate, 30% and 44%, respectively). The median DOR was 8.1 months (95% CI, 5.3-14.9 months), and the median OS was 10.1 months (95% CI, 5.7-14.2 months). The outcomes were especially favorable in patients with IDH1/2 mutations, with a median OS of 19.4 months (95% CI, 5.1 to NR).
In contrast to its use in chronic lymphocytic leukemia, no clinically significant tumor lysis syndrome (TLS) has been observed in the clinical trials with venetoclax in AML, acknowledging that precautions to prevent TLS were applied.11,12,15,16 All patients required hospitalization for the dose ramp up with appropriate IV hydration, uric acid-lowering agent (allopurinol or rasburicase), and careful monitoring of laboratory parameters of TLS. Venetoclax can be safely started at a dose of 100 mg on the first day, with doubling doses on subsequent days until achievement of the target dose. In the study of venetoclax plus HMA, patients were required to have a white blood cell (WBC) count <25 × 109/L before starting therapy, which could be achieved with hydroxyurea.10,11 With these precautious, only 2 cases of laboratory TLS without clinical significance were reported in the study of venetoclax plus LDAC.12 This suggests that, in an appropriate setting with close laboratory and clinical monitoring, selected future patients without risk factors for TLS could safely be treated without requiring hospitalization.
The addition of venetoclax to LDAC or HMA is associated with myelosuppression, most specifically on-target neutropenia, which can increase the risk of infectious complications.10-12 In patients who achieve a morphologic remission with <5% bone marrow blasts but delayed hematological recovery and/or hypocellular marrow, venetoclax should be interrupted up to 14 days to allow neutrophils and platelet to recover to at least 0.5 and 25 × 109/L, respectively, before starting the next cycle. Once in remission, G-CSF administration may help augment neutrophil recovery, particularly after MRD negative remission is obtained. In the setting of persistent neutropenia, a decrease in venetoclax duration (to 21 days initially and then, 14 days if persistent, etc) should be considered, and in the setting of a hypocellular marrow, the HMA may also require dose reduction according to standard practice. Because venetoclax is a substrate of CYP3A4, significant dose adjustments are necessary with the concomitant administration of prophylactic azole antifungals and/or ciprofloxacin, which inhibit this cytochrome and may increase venetoclax levels and thus, toxicity. Based on pharmacokinetics studies, the dose of venetoclax should be reduced by 50% with concomitant administration of moderate CYP3A4 inhibitors, such as fluconazole or isavuconazole, and by at least 75% for strong inhibitors, such as posaconazole or voriconazole.17
Clinical case 2
A 61-year-old male patient with no significant medical history with progressive thrombocytopenia and neutropenia was diagnosed outside our institution with myelodysplastic syndrome (MDS) with excess blasts. The bone marrow aspiration showed a hypercellular marrow with multilineage dysplasia and 7% clonal myeloblasts. The karyotype demonstrated trisomy 8, and a limited NGS panel revealed mutations in ASXL1 and IDH1 without mutation in NPM1, FLT3, and CEBPA. He was classified with high-risk disease according to the Revised International Prognostic Scoring System (IPSS-R), and he started therapy with azacitidine at the standard dosing. After 6 months of therapy without any hematological improvement, a bone marrow aspiration was performed and revealed progression to AML, with 24% blasts, sustained trisomy 8, ASXL1 and IDH1 mutations, and wild-type status for NPM1 and FLT3 genes. He received induction therapy with liposome-encapsulated daunorubicin and cytarabine (CPX-351) at a dose of 100 mg/m2 on days 1, 3, and 5, and his blast count reduced to 11% after a first cycle, which was complicated by mucositis and neutropenic fever. He successfully achieved CRi with 3% blasts and prolonged/persistent thrombocytopenia after a second course of CPX-351, and he was subsequently referred to our institution to undergo an allogeneic hematopoietic stem cell transplantation (HSCT) from a matched unrelated donor.
CPX-351
CPX-351 is a liposome-encapsulated combination of cytarabine and daunorubicin with an optimal 5:1 synergistic drug ratio and increased/selective uptake by leukemic cells. This novel formulation was initially compared with the 7 + 3 regimen in a randomized phase 2 study including patients with newly diagnosed AML aged 60 to 75 years old.18 CPX-351 was administered at a dose of 100 U/m2 on days 1, 3, and 5, which contains 100 mg/m2 cytarabine and 44 mg/m2 daunorubicin per dose. If applicable, the second induction consisted of the same dose on days 1 and 3. When remission was achieved, the patient received up to 3 consolidation cycles with CPX-351 65 U/m2 on days 1 and 3. In the overall cohort, the CR/CRi rate was higher with CPX-351 compared with the 7 + 3 regimen (66.7% vs 51.2%, P = .07), but differences were most striking among patients with adverse cytogenetics (77.3% vs 38.5%, P = .03) and secondary AML (57.6% vs 31.6%, P = .06). The median OS was also improved in the subgroup of secondary AML (12.1 vs 6.1 months, P = .01), whereas there was no significant difference in the overall cohort. These findings led to the design of a confirmatory phase 3 randomized trial of CPX-351 vs 7 + 3 in the restricted population of older patients with newly diagnosed secondary AML, including therapy-related AML and acute myeloid leukemia with myelodysplasia-related changes (AML-MRC).19 In this trial, patients receiving CPX-351 experienced an improved CR/CRi rate compared with 7 + 3 (47.7% vs 33.3%, P = .016) and improved OS (median, 9.56 vs 5.95 months, P = .003), with a median follow-up of 20.7 months. This improvement in OS was partly explained by a higher proportion of patients undergoing HSCT after CPX-351 treatment (34% vs 25%), with especially favorable outcome in these patients (12-month OS > 60%). Although MRD assessment was not centrally performed, the improved post-HSCT outcome after CPX-351 may suggest that deeper remissions were achieved with this liposomal formulation. In subgroup analyses, patients with therapy-related AML, secondary AML evolving from chronic myelomonocytic leukemia, or secondary AML without prior exposure to HMA therapy seemed to derive the greatest benefit over the conventional 7 + 3 regimen. Even if our clinical case achieved CR and underwent HSCT after receiving CPX-351, it is worth mentioning that only 36.0% of patients with antecedent MDS and prior HMA exposure achieved CR/CRi compared with 32.7% with the 7 + 3 regimen, and the median OSs were similar between the 2 arms (5.65 vs 7.34 months, hazard ratio = 0.98). Hence, MDS progressing to AML after HMA failure still represent an unmet clinical need. Regarding safety, the adverse events profile of CPX-351 is similar to that of the conventional 7 + 3 regimen. Although the time to recovery of blood counts in patients who achieve a marrow remission is longer with CPX-351 compared with 7 + 3, infection rates were similar between both treatments.19
Limitations with generalizability include the absence of high-dose cytarabine in the 7 + 3 arm, which would normally be administered during consolidation. Additionally, because younger patients (<60 years old) were excluded from the registrational study, it is unknown how CPX-351 would compare with other intensive regimens, such as fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin (FLAG-Ida), for the younger AML patient population.
Clinical case 2 (continued)
The patient did not experience any major complication in his early posttransplant period, with mild steroid-responsive skin graft-versus-host disease. However, on a routine follow-up at 9 months post-HSCT, the patient presented with relapsed disease, including circulating blasts on the peripheral blood smear. A bone marrow aspiration confirmed relapsed disease with 17% blasts and recurrent trisomy 8. The NGS panel revealed the same IDH1 R132H and ASXL1 mutations in the leukemic cells found at the diagnosis of MDS without mutations in NPM1 and FLT3. He was initiated on the mutant IDH1 inhibitor ivosidenib at 500 mg orally once daily. During the first cycle of therapy, a rise in his WBC count up to 34 × 109/L (predominantly neutrophils) was noted without any obvious signs or symptoms of differentiation syndrome (DS). Hydroxyurea was administered and successfully reduced his WBC to <10 × 109/L. The bone marrow aspiration at the end of cycle 1 showed mild reduction in bone marrow blasts to 12%. On day 46, the patient was admitted for fever, shortness of breath, and diffuse pulmonary infiltrates. Infection, relapse, or DS was considered, and the patient was promptly treated with dexamethasone 10 mg IV twice daily, diuretics, and broad spectrum antibiotics to cover possible infection. His WBC remained <25 × 109/L during this episode, but creatinine increased to 1.6 mg/dL. The patient improved rapidly with steroids without interruption of ivosidenib. At the end of cycle 2, the bone marrow aspiration confirmed CR, with trisomy 8 and IDH1 and ASXL1 mutations still detectable.
Ivosidenib and enasidenib
Recurrent mutations in isocitrate dehydrogenase (IDH) enzymes IDH1 and IDH2 are identified in ∼15% to 25% of cases of AML.7,20 The normal wild-type IDH enzymes catalyze the conversion of isocitrate to α-ketoglutarate (αKG) in the citric acid cycle, whereas mutated IDH1/2 acquires a neomorphic enzymatic activity that transforms αKG into 2-hydroxyglutarate (2HG).21,22 The latter acts as an oncometabolite, blocking αKG-mediated reactions, leading to impaired histone demethylation, and resulting in differentiation blockade involved in leukemogenesis.23,24 Mutations in IDH1/2 occur more frequently in association with intermediate-risk karyotypes, NPM1 and DNMT3A mutations, and older age as highlighted by our clinical case.20 The prognostic impact of IDH1/2 alterations is variable depending on the location of the mutation and co-occurring genetic abnormalities; for instance, IDH1/2 mutations in combination with NPM1 mutation are associated with a particularly favorable outcome.20
Enasidenib is an oral selective mutant IDH2 enzyme inhibitor. It was evaluated in 239 patients with IDH2-mutated myeloid malignancy, including 176 (73%) patients with relapsed or refractory AML in a first-in-human phase 1/2 study.25,26 The overall response rate (including CR, CRi, and MLFS) with the dose of 100 mg daily was 38.8%, with a CR rate of 19.6%.26 Although most patients had significant reduction in 2HG serum level, the extent of reduction was not correlated with response to enasidenib. The median time to response was 1.9 months (range, 0.5-9.4 months), and median DOR was 5.6 months (DOR, 8.8 months in CR patients).25 About one-half of patients had stable disease with a median time on treatment of 4 months, which in some patients, has been associated with hematological improvement and reduction in transfusional burden. At a median follow-up of 7.8 months, the median OS was 8.8 months (95% CI, 7.7-9.6 months), which compares favorably with historical data with any salvage chemotherapy.27
The safety and efficacy of ivosidenib, an oral IDH1 inhibitor, were evaluated in a similar phase 1 study including 258 patients with IDH1-mutated myeloid neoplasms.28 The selected dose for the expansion cohort was 500 mg daily based on safety, efficacy, and favorable pharmacokinetics data at this dose. The overall response rate (CR, CRi, and MLFS) with ivosidenib was 41.6%, with a CR rate of 21.6%. The median time to response was 1.9 (range, 0.8-4.7), the median DOR was 6.5 months (95% CI, 4.6-09.3), and DOR was 9.3 months in patients achieving CR. At a median follow-up of 14.8 months, the median OS was 8.8 months (95% CI, 6.7-10.2) in the global population, and it was longer in patients achieving CR/CRi (median NR with a median follow-up of 14.8 months), particularly in the 21% of responding patients who had clearance of their IDH1 mutation. Future research will be required to understand the prognostic value of mutation clearance with IDH inhibitors, although available data so far suggest improved outcomes in patients who achieve deeper remission.
Both IDH1/2 inhibitors are well tolerated, and they function via differentiation and not myelosuppression, which allows recovery of blood counts without an intervening period of aplasia, resulting in reduced rates of infections and febrile neutropenia compared with expectations with most salvage chemotherapy options.25,27,28 The most common nonhematological adverse events with these drugs are grade 1 to 2 gastrointestinal symptoms. Enasidenib, through UGT1A1 metabolism, causes an indirect hyperbilirubinemia in 38% of patients, which is typically not clinically significant. Ivosidenib is associated with QTc prolongation (12%). Because these agents induce remission by differentiation, IDH inhibitors can be associated with DS, which was reported in ∼12% of patients on the original clinical trials.25,28 IDH-DS manifests with variable signs and symptoms, including fever, peripheral edema, weight gain, dyspnea, hypoxemia, bilateral pulmonary infiltrates, renal insufficiency, and skin rash. Although leukocytosis can co-occur, DS is not necessarily associated with a rise in the WBC and remains a clinical diagnosis. Contrasting with DS with all-trans retinoic acid (ATRA) in APL occurring during induction, the IDH-DS tends to occur later at medians of 48 days (range, 10-340 days) and 29 days (range, 5-59 days) with enasidenib and ivosidenib, respectively. Patients treated with these agents need to be monitored closely and educated on this late-onset adverse event, because most patients will be outpatient during this time period. IDH-DS should be treated promptly as soon as it is suspected with dexamethasone 10 mg IV twice daily for 3 days or until symptomatic improvement, which can then be tapered when patient improves. Diuretics should be added in the case of peripheral edema and weight gain. The IDH inhibitors have a long half-life (>4 days), and thus, holding therapy will not lead to rapid resolution, although it should be interrupted in the case of severe pulmonary involvement, oxygen requirement, hemodynamic instability, or increase in creatinine; in such cases, it can be resumed when clinical manifestations improve. As demonstrated by our case, leukocytosis was observed in 17% and 36% of patients treated with enasidenib and ivosidenib, respectively, and it was not always related to IDH-DS. In most cases, leukocytosis occurs in the first 30 days of treatment, and the WBCs can be managed as needed with hydroxyurea. The time to response with ivosidenib and enasidenib is ∼2 months, and late responses are possible. Also, some patients with stable disease may experience improved quality of life, including transfusion independence and decreased infections. Therefore, it is recommended to treat patients for 4 to 6 cycles before considering the disease refractory, unless AML is clearly progressing.
The IDH1 inhibitor ivosidenib was also recently approved as monotherapy for older patients ineligible for intensive chemotherapy with IDH1-mutated untreated AML. This approval was based on the clinical data obtained from a subset of patients (n = 28) with newly diagnosed AML included in the aforementioned trial. In this patient population, the rate of CR or complete remission with partial hematological recovery (CRh) was 42.9% (95% CI, 24.5%-62.8%). Promising avenues of combination therapies, including ivosidenib, are now currently under investigation for IDH1-mutated AML (NCT03471260).
Glasdegib
Glasdegib is an oral selective inhibitor of the sonic hedgehog receptor smoothened involved in the hedgehog pathway, which is aberrantly expressed in various hematological malignancies, including AML. It is now approved by the FDA in combination with LDAC for treatment of newly diagnosed untreated AML in patients older than 75 years old or those ineligible for intensive chemotherapy. In a randomized phase 1b/2 clinical trial by Cortes et al,29 patients aged 55 years old or older and considered unsuitable candidates for intensive chemotherapy received LDAC 20 mg twice daily for 10 days every 28 days either alone or in combination with glasdegib at a dose of 100 mg daily. Eighty-eight patients received LDAC plus glasdegib, and 44 patients received LDAC alone; the median follow-up times for OS were 21.7 and 20.1 months, respectively. The median OS was 8.8 months (80% CI, 6.9-9.9 months) with glasdegib plus LDAC compared with 4.9 months (80% CI, 3.5-6.0 months) with LDAC alone, and the 12-month estimated OS values were 39.5% (80% CI, 32.5%-46.3%) and 9.5% (80% CI, 4.8%-16.3%), respectively. The rate of overall response rate (including CR, CRi, and MLFS) was 26.9% with glasdegib plus LDAC and 5.3% with LDAC alone, including CR rates of 17.0% and 2.3%, respectively. The addition of glasdegib to LDAC was associated with increased gastrointestinal symptoms, dysgeusia, muscle spasms, and fatigue, although most of these symptoms were grade 1 to 2 and manageable. Additional studies of glasdegib in combination with azacitidine or intensive chemotherapy are currently ongoing (NCT03416179).
Gemtuzumab ozogamicin
Gemtuzumab ozogamicin (GO) is an antibody-drug conjugate targeted against CD33 and coupled to calicheamicin. It was initially approved by the FDA in 2000 for the treatment of CD33+ relapsed AML in patients ≥60 years old ineligible for intensive chemotherapy. In the Southwest Oncology Group (SWOG) S0106 confirmatory trial required for the full approval of GO, the addition of gemtuzumab to intensive chemotherapy (6 mg/m2 single dose with 7 + 3 and 5 mg/m2 single dose with consolidation) did not improve CR rates and disease-free survival, and it was associated with an increased induction death rate, consequently leading to its market withdrawal in 2010.30 However, other clinical trials have evaluated gemtuzumab at lower doses (3 mg/m2) or fractionated lower doses in combination with intensive chemotherapy while demonstrating superiority.31-35 In a meta-analysis of individual patient data from 5 randomized trials, Hills et al36 demonstrated that GO in addition to intensive chemotherapy significantly reduced the risk of relapse and improved OS, but this benefit is limited to patients without adverse-risk cytogenetics. GO is particularly beneficial for patients with favorable-risk cytogenetics (absolute increase in estimated 6-year OS of 20.7%) and provides modest benefit for patients with intermediate-risk cytogenetics (absolute increase in estimated 6-year OS of 5.7%). GO was reapproved by the FDA in 2017 based on the ALFA-0701 clinical trial, which demonstrated an improvement in event-free survival, OS, and relapse-free survival with the addition of GO at a dose of 3 mg/m2 on days 1, 4, and 7 of induction chemotherapy and 3 mg/m2 on day 1 of consolidation chemotherapy.31 Persistent thrombocytopenia and bleeding complications are more frequent with GO, but lower doses of 3 mg/m2 are not associated with an increased risk of treatment-related mortality.
GO has also been studied in older patients not eligible for intensive chemotherapy either as single agent or in combination with LDAC.37,38 Although GO single agent or in combination with LDAC may achieve remission in 27% to 30% of patients, it does not improve OS compared with LDAC alone. Additional studies are warranted to evaluate how to best incorporate GO into combination therapies for AML.
Future perspectives
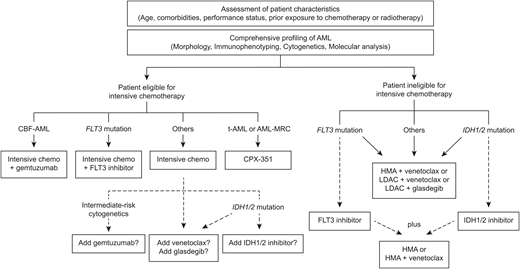
With the approval of 8 novel therapies in the past 2 years, our treatment algorithm for AML is evolving rapidly, and it is more than ever embracing the concept of precision medicine (Figure 2). In addition to the patients’ age, medical history, and assessment of comorbidities and performance status, the comprehensive analysis of the disease biology, including morphology, immunophenotyping, cytogenetics, and gene mutation screening, is now critical to select the best available therapy, which may include targeted agents. In older patients with newly diagnosed AML ineligible to receive intensive chemotherapy, glasdegib in combination with LDAC and venetoclax in combination with LDAC or HMA are valuable new options improving outcomes compared with conventional care, although results are awaiting confirmation in ongoing phase 3 trials (Table 1). In fit patients with therapy-related AML or AML-MRC, CPX-351 improves OS over the standard 7 + 3 regimen and may be of particular benefit for patients who were not previously treated with HMA and who will undergo HSCT in first CR. GO should be considered in newly diagnosed or relapsed/refractory CD33+ AML with favorable- or intermediate-risk cytogenetics. Screening for FLT3, IDH1, and IDH2 mutations is recommended both at diagnosis and at relapse, because patients with any of these mutations will benefit from incorporation of targeted FLT3 inhibitors (reviewed in the work by Smith39 ), ivosidenib, or enasidenib, respectively. Furthermore, the utilization of an NGS panel at diagnosis and relapse to assess for presence of recurrent mutations in AML is increasingly recognized as a useful tool to refine the prognosis of patients, identify biomarkers of treatment response, and select patients who may benefit from novel targeted therapies under investigation.
Treatment algorithm of newly diagnosed AML incorporating novel single-agent and combination therapies. Dotted lines represent therapeutic options of unclear benefit and/or warranting additional research. This algorithm does not encompass the treatment of acute promyelocytic leukemia. CBF-AML, core binding factor acute myeloid leukemia; chemo, chemotherapy; HMA, hypomethylating agent; t-AML, therapy-related acute myeloid leukemia.
Treatment algorithm of newly diagnosed AML incorporating novel single-agent and combination therapies. Dotted lines represent therapeutic options of unclear benefit and/or warranting additional research. This algorithm does not encompass the treatment of acute promyelocytic leukemia. CBF-AML, core binding factor acute myeloid leukemia; chemo, chemotherapy; HMA, hypomethylating agent; t-AML, therapy-related acute myeloid leukemia.
Ongoing clinical trials with novel single-agent or combination therapies in AML
| Population/indication . | Treatment . | Phase . | Identifier . |
|---|---|---|---|
| Venetoclax | |||
| Untreated AML older patients unfit for IC | Azacitidine ± venetoclax | Phase 3 | NCT02993523 |
| Untreated AML older patients unfit for IC | LDAC ± venetoclax | Phase 3 | NCT03069352 |
| Untreated AML older patients unfit for IC | Azacitidine + venetoclax | Phase 2 | NCT03466294 |
| Untreated AML older patients unfit for IC | Decitabine 10 d + venetoclax | Phase 2 | NCT03404193 |
| Untreated AML older patients unfit for IC | LDAC + cladribine + azacitidine + venetoclax | Phase 2 | NCT03586609 |
| Untreated or R/R AML patients fit for IC | FLAG-Ida + venetoclax | Phase 2 | NCT03214562 |
| Untreated or R/R AML patients fit for IC | Fludarabine + cytarabine + idarubicin + venetoclax | Phase 2 | NCT03455504 |
| Untreated or R/R AML patients fit for IC | CPX-351 + venetoclax | Phase 2 | NCT03629171 |
| Untreated AML patients fit for IC | 7 + 3 + venetoclax | Phase 1b | NCT03709758 |
| Untreated AML older patients unfit for IC | Azacitidine + venetoclax + pevonedistat | Phase 1/2 | NCT03862157 |
| Untreated AML patients 18 to 59 y old | Azacitidine + venetoclax | Phase 2 | NCT03573024 |
| R/R AML | Venetoclax + dinaciclib | Phase 1b | NCT03484520 |
| R/R AML | Venetoclax + alvocidib | Phase 1b | NCT03441555 |
| R/R AML | Venetoclax + ruxolitinib | Phase 1 | NCT03874052 |
| R/R AML | Venetoclax + gilteritinib | Phase 1 | NCT03625505 |
| R/R AML | Venetoclax + AMG-176 (MCL1 inhibitor) | Phase 1b | NCT03797261 |
| R/R AML | Venetoclax + S64315 (MCL1 inhibitor) | Phase 1 | NCT03672695 |
| R/R AML | Venetoclax + cobimetinib or venetoclax + idasanutlin | Phase 1b/2 | NCT02670044 |
| R/R AML | Venetoclax + lintuzumab-225Ac | Phase 1/2 | NCT03867682 |
| R/R AML | CPX-351 + venetoclax | Phase 1 | NCT03826992 |
| R/R AML, FLT3 mutated | Venetoclax + quizartinib | Phase 1b/2 | NCT03735875 |
| Ivosidenib or enasidenib | |||
| Untreated AML patients fit for IC | Intensive chemotherapy + ivosidenib or enasidenib | Phase 1 | NCT02632708 |
| Untreated AML patients fit for IC | Intensive chemotherapy + ivosidenib or enasidenib | Phase 3 | NCT03839771 |
| Untreated AML older patients unfit for IC | Azacitidine + ivosidenib or azacitidine + enasidenib | Phase 1/2 | NCT02677922 |
| Untreated AML older patients unfit for IC | Enasidenib vs azacitidine, LDAC, or intermediate-dose cytarabine | Phase 3 | NCT02577406 |
| Untreated AML older patients unfit for IC | Azacitidine + ivosidenib or placebo | Phase 3 | NCT03173248 |
| Untreated or R/R AML | Venetoclax + ivosidenib ± azacitidine | Phase 1/2 | NCT03471260 |
| R/R AML | Azacitidine + enasidenib | Phase 2 | NCT03683433 |
| R/R AML | CPX-351 + enasidenib | Phase 2 | NCT03825796 |
| Post-HSCT maintenance | Enasidenib | Phase 1 | NCT03515512 |
| Post-HSCT maintenance | Enasidenib | Phase 1 | NCT03728335 |
| Glasdegib | |||
| Untreated AML patients fit for IC | IC ± glasdegib | Phase 3 | NCT03416179 |
| Untreated AML older patients unfit for IC | Azacitidine ± glasdegib | Phase 3 | NCT03416179 |
| Untreated AML older patients unfit for IC | Azacitidine + glasdegib | Phase 1b | NCT02367456 |
| Untreated or R/R AML | Glasdegib + GO | Phase 1 | NCT03390296 |
| Untreated AML patients fit for IC | Intensive chemotherapy + glasdegib | Phase 1 | NCT02038777 |
| Untreated AML older patients unfit for IC | LDAC + glasdegib | Phase 1 | NCT02038777 |
| Untreated AML older patients unfit for IC | Azacitidine or decitabine + glasdegib | Phase 2 | NCT03226418 |
| Untreated AML older patients unfit for IC | Decitabine + glasdegib | Phase 2 | NCT01546038 |
| Liposome-encapsulated cytarabine plus daunorubicin combination (CPX-351) | |||
| Untreated AML patients fit for IC | CPX-351 vs standard IC | Phase 3 | NCT03897127 |
| Untreated AML older patients fit for IC | CPX-351 vs DA + GO | Phase 3 | NCT02272478 |
| Untreated t-AML or AML-MRC | CPX-351 + palbociclib | Phase 1/2 | NCT03844997 |
| Untreated AML secondary to MPN | CPX-351 + ruxolitinib | Phase 1/2 | NCT03878199 |
| Untreated AML older patients fit for IC | CPX-351 + GO | Phase 1 | NCT03878927 |
| R/R AML | CPX-351 + GO | Phase 1 | NCT03672539 |
| R/R AML | CPX-351 + GO | Phase 1 | NCT03904251 |
| R/R AML | CPX-351 | Phase 2 | NCT00822094 |
| GO | |||
| Untreated AML patients fit for IC | GCLAM + GO | Phase 1/2 | NCT03531918 |
| Untreated AML, FLT3 mutated, patients fit for IC | 7 + 3 + GO + midostaurin | Phase 1 | NCT03900949 |
| Untreated CBF AML patients fit for IC | FLAG-Ida + GO | Phase 2 | NCT00801489 |
| MRD positivity | GO single agent | Phase 2 | NCT03737955 |
| R/R AML | Mitoxantrone + etoposide + GO | Phase 2 | NCT03839446 |
| R/R AML | Pracinostat + GO | Phase 1 | NCT03848754 |
| Population/indication . | Treatment . | Phase . | Identifier . |
|---|---|---|---|
| Venetoclax | |||
| Untreated AML older patients unfit for IC | Azacitidine ± venetoclax | Phase 3 | NCT02993523 |
| Untreated AML older patients unfit for IC | LDAC ± venetoclax | Phase 3 | NCT03069352 |
| Untreated AML older patients unfit for IC | Azacitidine + venetoclax | Phase 2 | NCT03466294 |
| Untreated AML older patients unfit for IC | Decitabine 10 d + venetoclax | Phase 2 | NCT03404193 |
| Untreated AML older patients unfit for IC | LDAC + cladribine + azacitidine + venetoclax | Phase 2 | NCT03586609 |
| Untreated or R/R AML patients fit for IC | FLAG-Ida + venetoclax | Phase 2 | NCT03214562 |
| Untreated or R/R AML patients fit for IC | Fludarabine + cytarabine + idarubicin + venetoclax | Phase 2 | NCT03455504 |
| Untreated or R/R AML patients fit for IC | CPX-351 + venetoclax | Phase 2 | NCT03629171 |
| Untreated AML patients fit for IC | 7 + 3 + venetoclax | Phase 1b | NCT03709758 |
| Untreated AML older patients unfit for IC | Azacitidine + venetoclax + pevonedistat | Phase 1/2 | NCT03862157 |
| Untreated AML patients 18 to 59 y old | Azacitidine + venetoclax | Phase 2 | NCT03573024 |
| R/R AML | Venetoclax + dinaciclib | Phase 1b | NCT03484520 |
| R/R AML | Venetoclax + alvocidib | Phase 1b | NCT03441555 |
| R/R AML | Venetoclax + ruxolitinib | Phase 1 | NCT03874052 |
| R/R AML | Venetoclax + gilteritinib | Phase 1 | NCT03625505 |
| R/R AML | Venetoclax + AMG-176 (MCL1 inhibitor) | Phase 1b | NCT03797261 |
| R/R AML | Venetoclax + S64315 (MCL1 inhibitor) | Phase 1 | NCT03672695 |
| R/R AML | Venetoclax + cobimetinib or venetoclax + idasanutlin | Phase 1b/2 | NCT02670044 |
| R/R AML | Venetoclax + lintuzumab-225Ac | Phase 1/2 | NCT03867682 |
| R/R AML | CPX-351 + venetoclax | Phase 1 | NCT03826992 |
| R/R AML, FLT3 mutated | Venetoclax + quizartinib | Phase 1b/2 | NCT03735875 |
| Ivosidenib or enasidenib | |||
| Untreated AML patients fit for IC | Intensive chemotherapy + ivosidenib or enasidenib | Phase 1 | NCT02632708 |
| Untreated AML patients fit for IC | Intensive chemotherapy + ivosidenib or enasidenib | Phase 3 | NCT03839771 |
| Untreated AML older patients unfit for IC | Azacitidine + ivosidenib or azacitidine + enasidenib | Phase 1/2 | NCT02677922 |
| Untreated AML older patients unfit for IC | Enasidenib vs azacitidine, LDAC, or intermediate-dose cytarabine | Phase 3 | NCT02577406 |
| Untreated AML older patients unfit for IC | Azacitidine + ivosidenib or placebo | Phase 3 | NCT03173248 |
| Untreated or R/R AML | Venetoclax + ivosidenib ± azacitidine | Phase 1/2 | NCT03471260 |
| R/R AML | Azacitidine + enasidenib | Phase 2 | NCT03683433 |
| R/R AML | CPX-351 + enasidenib | Phase 2 | NCT03825796 |
| Post-HSCT maintenance | Enasidenib | Phase 1 | NCT03515512 |
| Post-HSCT maintenance | Enasidenib | Phase 1 | NCT03728335 |
| Glasdegib | |||
| Untreated AML patients fit for IC | IC ± glasdegib | Phase 3 | NCT03416179 |
| Untreated AML older patients unfit for IC | Azacitidine ± glasdegib | Phase 3 | NCT03416179 |
| Untreated AML older patients unfit for IC | Azacitidine + glasdegib | Phase 1b | NCT02367456 |
| Untreated or R/R AML | Glasdegib + GO | Phase 1 | NCT03390296 |
| Untreated AML patients fit for IC | Intensive chemotherapy + glasdegib | Phase 1 | NCT02038777 |
| Untreated AML older patients unfit for IC | LDAC + glasdegib | Phase 1 | NCT02038777 |
| Untreated AML older patients unfit for IC | Azacitidine or decitabine + glasdegib | Phase 2 | NCT03226418 |
| Untreated AML older patients unfit for IC | Decitabine + glasdegib | Phase 2 | NCT01546038 |
| Liposome-encapsulated cytarabine plus daunorubicin combination (CPX-351) | |||
| Untreated AML patients fit for IC | CPX-351 vs standard IC | Phase 3 | NCT03897127 |
| Untreated AML older patients fit for IC | CPX-351 vs DA + GO | Phase 3 | NCT02272478 |
| Untreated t-AML or AML-MRC | CPX-351 + palbociclib | Phase 1/2 | NCT03844997 |
| Untreated AML secondary to MPN | CPX-351 + ruxolitinib | Phase 1/2 | NCT03878199 |
| Untreated AML older patients fit for IC | CPX-351 + GO | Phase 1 | NCT03878927 |
| R/R AML | CPX-351 + GO | Phase 1 | NCT03672539 |
| R/R AML | CPX-351 + GO | Phase 1 | NCT03904251 |
| R/R AML | CPX-351 | Phase 2 | NCT00822094 |
| GO | |||
| Untreated AML patients fit for IC | GCLAM + GO | Phase 1/2 | NCT03531918 |
| Untreated AML, FLT3 mutated, patients fit for IC | 7 + 3 + GO + midostaurin | Phase 1 | NCT03900949 |
| Untreated CBF AML patients fit for IC | FLAG-Ida + GO | Phase 2 | NCT00801489 |
| MRD positivity | GO single agent | Phase 2 | NCT03737955 |
| R/R AML | Mitoxantrone + etoposide + GO | Phase 2 | NCT03839446 |
| R/R AML | Pracinostat + GO | Phase 1 | NCT03848754 |
CBF, core binding factor; DA, daunorubicin plus cytarabine; GCLAM, G-CSF, cladribine, cytarabine, mitoxantrone; IC, intensive chemotherapy; MPN, myeloproliferative neoplasm; R/R, relapsed or refractory; t-AML, therapy-related acute myeloid leukemia.
Many of the new single-agent and combination therapies are indicated for newly diagnosed patients ineligible for intensive chemotherapy. The evaluation of eligibility to receive intensive chemotherapy based on patients’ characteristics remains a difficult task for the clinician, and some experts advocate for model-based approaches, including comorbidity scores, whereas others rely on the more subjective global clinical judgment.40-42 With the favorable results obtained with the combination of venetoclax plus HMA, if confirmed with the phase 3 trial, one may question whether this regimen could be beneficial in older patients who would normally be considered suitable candidates for intensive chemotherapy. This could change our paradigm of selecting treatment primarily based on patients’ age and fitness, and future clinical trials are warranted to evaluate this hypothesis.
Several clinical trials are now underway to evaluate the safety and efficacy of targeted agents in combination with intensive chemotherapy in younger AML patients in the frontline setting (Table 1). Venetoclax is currently being evaluated in combination with the 7 + 3 regimen or the FLAG-Ida regimen. A phase 3 randomized clinical trial will evaluate the addition of glasdegib to intensive chemotherapy. Ivosidenib or enasidenib has been combined with the 7 + 3 regimen in a phase 1 trial, and interim results have been presented at the 2018 ASH annual meeting.43 Among 134 patients treated, the response rates (including CR, CRi, and CR with incomplete platelet recovery) were 78% and 69% with the addition of ivosidenib or enasidenib to intensive chemotherapy, respectively. Longer follow-up is needed for the survival data, but preliminary results were encouraging, with >75% estimated 1-year OS; however, we also acknowledge that OS may not be the best and only clinical end point necessary to lead to approval of a drug.44
An additional and important question that arises with new targeted therapies is how to address the presence of multiple mutations that can each be targeted by specific agents. FLT3 mutations are identified in ∼20% to 25% of patients with IDH1/2-mutated AML, although patients with these co-occurring mutations were underrepresented in the early-phase clinical trials evaluating ivosidenib and enasidenib.25,28,45 The co-occurrences of these mutations are not trivial, because the patients with mutations in the receptor tyrosine kinase pathways (eg, NRAS or FLT3-ITD) had lower response rates to both IDH inhibitors as monotherapy.26,28 Furthermore, evaluation of mechanisms of primary or secondary resistance to targeted therapies merits additional research, because none of these agents were demonstrated to be curative without additional intensive therapy, such as chemotherapy or HSCT. A study of paired samples at different time points from patients treated with enasidenib showed various patterns of clonal selection or evolution through acquisition of new mutations involving different biological pathways on relapse, confirming the complex clonal heterogeneity and adaptation potential of AML.46 Altogether, these observations strongly support the concept that the combination of targeted agents with different mechanisms of action or chemotherapy is likely to improve the rates and depths of response and reduce the risk of relapse compared with single agents. Studies are required to confirm the added benefit of combination therapies, and moving forward, many doublet and triplet combinations are already being studied in clinical trials (Table 1). For example, because IDH1/2-mutated AML is associated with a hypermethylated phenotype, is sensitive to venetoclax, and can be targeted by IDH inhibitors, a clinical trial evaluating the triple combination of ivosidenib and venetoclax with or without azacitidine for IDH1-mutated AML is currently underway at the MD Anderson Cancer Center (Table 1). With preliminary results available for 12 evaluable patients treated with ivosidenib and venetoclax, 9 (75%) achieved CR, CRh, or CRi, which is encouraging compared with response rates of ∼40% with ivosidenib monotherapy.47 The addition of FLT3 inhibitors to venetoclax plus LDAC or HMA is also likely to improve outcome in patients with FLT3 mutation as reported in a subset of patients treated with decitabine for 10 days plus venetoclax and FLT3 inhibitors at our institution,48 but this assumption remains to be rigorously proven in several ongoing clinical trials. Overexpression of MCL1, another member of the BCL2 family of antiapoptotic proteins, is a known mechanism of resistance to venetoclax, and MCL1 inhibitors (or MDM2 inhibitors, which inhibit MCL1 indirectly) are currently in clinical development to overcome this resistance mechanism.49-51 Furthermore, mutations in receptor kinase signaling pathways, such as NRAS, KRAS, and PTPN11 mutations, seem to confer primary or secondary resistance to both IDH inhibitors and venetoclax combination therapies. Inhibition of these pathways with effective kinase inhibitors in concomitant or sequential combinations might help achieve higher response rates and improve outcomes with small molecule-targeted therapeutics.48,51
Conclusion
The development and subsequent approval of several single-agent and combination biologic therapies for AML in the recent years are truly encouraging and bear witness to the rapidly evolving field of AML. With the addition of these targeted agents to our therapeutic arsenal, recurrent genomic features are not merely of diagnostic and prognostic relevance but now provide direct therapeutic implications at both diagnosis and relapse. Many challenges remain to further improve on outcomes of patients suffering from AML, most notably for those with very adverse features, such as secondary AML, complex karyotype, TP53 mutations, or MECOM-rearranged AML. However, the pace of scientific progress and the ongoing development of novel safe and effective therapies for AML confirm an increasingly bright future for our patients.
Correspondence
Courtney D. DiNardo, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Box 428, Houston, TX 77030; e-mail: cdinardo@mdanderson.org.
References
Competing Interests
Conflict-of-interest disclosure: G.R.-C. has no conflicts of interest to disclose. C.D.D. receives honoraria from Daiichi Sankyo, Jazz Pharmaceuticals, MedImmune, and Syros and is a member on advisory committees or board membership for AbbVie, Agios, Celgene, and Notable Labs.
Author notes
Off-label drug use: None disclosed.