Abstract
Nowadays a donor can be found for virtually all patients in need of an allogeneic stem cell transplantation, and the decision whether to use a matched or mismatched unrelated donor, an unrelated donor for umbilical cord blood transplantation (UCBT), or a haploidentical donor depends not only on the availability of the donor but also on patient-, disease-, and center-related factors. This paper summarizes the recent criteria in the selection of cord blood unit, including the cell dose requirement and the HLA typing for the optimal donor choice. The main strategies to optimize the results of UCBT, the conditioning regimens, and the use of antithymocyte globulin and the other platforms of graft-versus-host disease prophylaxis are discussed. The paper describes the results of UCBT in children and adults with malignant and nonmalignant diseases and the comparative analysis with other donor type and stem cell sources. Emerging strategies, focusing on the different platforms of ex vivo expansion and the new applications using cord blood stem cell, are also examined.
Learning Objectives
Understand the indications for UCBT and the main criteria for the optimal donor choice (cell dose, HLA matching, donor-specific antibodies) and transplantation procedures (conditioning regimen, graft-versus-host disease prophylaxis)
Describe the current most effective approaches addressed in clinical trials for optimizing outcomes of UCBT in children and adults
Review the results of comparative studies on UCBT and other donor and graft sources
Introduction
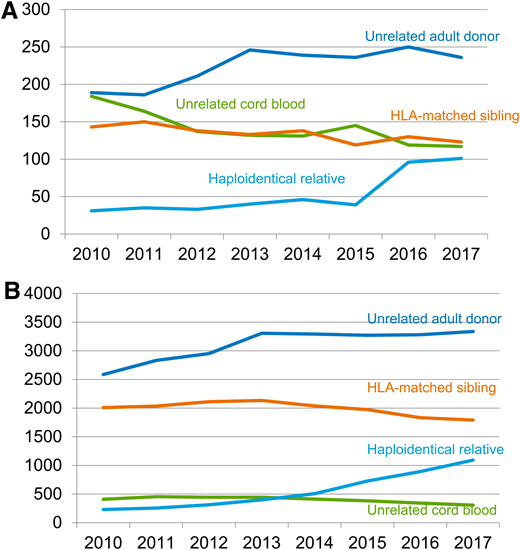
Hematopoietic stem cell transplantation (HSCT) has significantly improved and changed over time. Nowadays, when an HLA-matched sibling is lacking, matched unrelated donors (MUD) or mismatched unrelated donors, umbilical cord blood (UCB) units, and full-haplotype mismatched family members (haploidentical donors) are largely used (Figures 1A and 1B).1 Cord blood has been widely adopted for the treatment of both nonmalignant and malignant hematological diseases.2 Owing to the immaturity of the immune system at birth, fewer and less alloreactive T cells are present in the graft. Consequently, after umbilical cord blood transplantation (UCBT), the incidence and severity of acute and chronic graft-versus-host disease (GVHD) are decreased in comparison with other graft sources3 with, however, a delayed immune recovery and an increased risk of infections. UCB allows for less stringent donor-recipient HLA matching criteria for donor selection and extends the access to transplantation to patients for whom a MUD cannot be identified, especially those who are members of racial and ethnic minority groups, who are still underrepresented in international registries (Figures 2A–2C).4 To date, the global inventory of UCB units available for transplantation in public cord blood banks (CBBs) is more than 730 000 (Bone Marrow Donors Worldwide; www.bmdw.org), and more than 35 000 UCBT procedures have been performed worldwide (World Marrow Donor Association; www.wmda.info). New applications of cord blood–derived stem cells, also for immunotherapy using chimeric antigen receptors, are currently under investigation in clinical trials, opening new horizons for the use of UCB units.
Allogeneic transplantation activity in the United States from 2010 to 2017. Allogeneic transplantation activity in (A) children and (B) adults with hematologic malignancies is shown as the numbers of HSCTs per year from HLA-matched related sibling donors, unrelated donors, umbilical cord blood, and mismatched family donors (ie, mainly haploidentical donors) performed in the United States between 2010 and 2017. From the Center for International Blood and Marrow Transplant Research with permission from Dr. M. Eapen.
Allogeneic transplantation activity in the United States from 2010 to 2017. Allogeneic transplantation activity in (A) children and (B) adults with hematologic malignancies is shown as the numbers of HSCTs per year from HLA-matched related sibling donors, unrelated donors, umbilical cord blood, and mismatched family donors (ie, mainly haploidentical donors) performed in the United States between 2010 and 2017. From the Center for International Blood and Marrow Transplant Research with permission from Dr. M. Eapen.
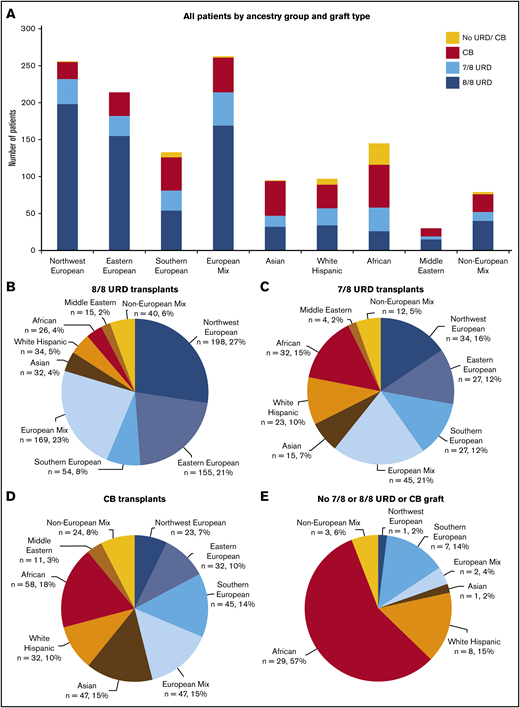
Patient ancestry distribution by graft type. (A) All patients (n = 1312), divided by ancestry group and graft type: 8/8 unrelated donors (URDs; n = 723), 7/8 URDs (n = 219), cord blood (CB; n = 319), or no 7/8 or 8/8 URD or CB (n = 51). (B) 8/8 URD transplantation patients, divided by ancestry group (n = 723). In all, 576 (80%) of 723 patients were European, and 147 (20%) of 723 were non-European. (C) 7/8 URD transplantation patients, divided by ancestry group (n = 219). In all, 133 (61%) of 219 patients were European, and 86 (39%) of 219 were non-European. (D) CB transplantation patients, divided by ancestry group (n = 319). In all, 147 (46%) of 319 patients were European, and 172 (54%) of 319 were non-European. (E) Patients without a 7/8 or 8/8 URD or CB graft, divided by ancestry group (n = 51). In all, 10 (20%) of 51 patients were European, and 41 (80%) of 51 were non-European. Reprinted from Barker et al.11
Patient ancestry distribution by graft type. (A) All patients (n = 1312), divided by ancestry group and graft type: 8/8 unrelated donors (URDs; n = 723), 7/8 URDs (n = 219), cord blood (CB; n = 319), or no 7/8 or 8/8 URD or CB (n = 51). (B) 8/8 URD transplantation patients, divided by ancestry group (n = 723). In all, 576 (80%) of 723 patients were European, and 147 (20%) of 723 were non-European. (C) 7/8 URD transplantation patients, divided by ancestry group (n = 219). In all, 133 (61%) of 219 patients were European, and 86 (39%) of 219 were non-European. (D) CB transplantation patients, divided by ancestry group (n = 319). In all, 147 (46%) of 319 patients were European, and 172 (54%) of 319 were non-European. (E) Patients without a 7/8 or 8/8 URD or CB graft, divided by ancestry group (n = 51). In all, 10 (20%) of 51 patients were European, and 41 (80%) of 51 were non-European. Reprinted from Barker et al.11
Clinical case 1
A 37-year-old African American woman presented at the emergency hospital complaining of asthenia, fever, and subsequent appearance of leg hematomas. Her blood counts revealed hyperleukocytosis, anemia, and thrombocytopenia. A diagnosis of high-risk acute myeloid leukemia with monosomy 7 and Fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) was retained. Nucleophosmin 1 was negative. The patient was started on a standard induction therapy with anthracycline and cytosine arabinoside, and first complete remission was achieved. She then received 2 consolidation courses with anthracycline and cytosine arabinoside.
A search for a suitable donor was promptly started at the time of diagnosis. Neither an HLA-identical sibling nor an unrelated donor (URD) was identified. The patient was an only child, and her parents had comorbidities, preventing a search for haploidentical donors. The only suitable UCB unit identified for this patient was a 6/8 HLA match (considering HLA loci A, B, C, and DRB1) with a class I HLA mismatch on locus B. The patient’s total nucleated cell (TNC) count at cryopreservation was 4.2 × 107/kg, and her CD34+ count was 1.8 × 105/kg (her body weight was 63 kg). There was a minor mismatch in the ABO group (donor 0+, recipient A+). The results of screening of the patient for anti-HLA donor-specific antibodies (DSAs) were positive, both by complement‐dependent cytotoxicity (>20% cell lysis) and by Luminex assay (mean fluorescence intensity level, 2600) crossmatches, but no other suitable cord blood unit (CBU) could be identified for this patient. The UCB unit was selected, and the patient underwent effective DSA desensitization, 1 week before starting her conditioning regimen, with therapeutic plasma exchange performed 3 times per week and intravenous immunoglobulin.
Criteria for CBU selection in patients: cell dose and HLA matching
TNCs, colony-forming units, and CD34+ cells are the most important prognostic factors for outcomes of UCBT, mainly engraftment, nonrelapse mortality (NRM), and overall survival, as demonstrated over the last several years.5 A minimum of 2.5-3 × 107/kg of TNCs at cryopreservation should be obtained in a single UCB unit for transplantation in patients with malignant diseases.6 The threshold for TNCs (Table 1) should be higher (5 × 107/kg at cryopreservation) in nonmalignant diseases to overcome the higher risk of associated graft failure.7 When selecting the UCB unit, TNC is the main requirement applied by CBB, in association with the CD34+ cell dose, which is not clearly standardized across the different cell therapy laboratories. However, the most often recommended threshold for CD34+ cell dose is 1-1.5 × 105/kg at cryopreservation, especially when more than one unit meeting the required TNC criteria is available.
Eurocord criteria for choice of cord blood unit, according to Eurocord
| Cryopreserved cell dose |
| Number of cells in the UCB unit for malignant disease |
| a. Single UCBT TNC/kg: ≥2.5-3 × 107 and/or CD34+/kg ≥1.5 × 105* |
| b. Double UCBT: TNC/kg: ≥1.5 × 107 for each unit and/or CD34+/kg ≥1 × 105 for each unit |
| HLA typing |
| a. High-resolution typing for HLA-A, -B, -C, and -DRB1 of patients and UCB units |
| b. Avoid UCB units with >2 HLA mismatches and avoid HLA-C mismatches |
| c. In double UCBT, unit-to-unit HLA match is not required |
| Adapt cell dose and HLA matching to graft indication |
| a. Nonmalignant diseases: increase cell dose (>5.0 × 107 TNC/kg) and find the best HLA match. If the criterion for the minimum number of cells in a single UCB unit is not achieved, a double UCBT should be considered also in nonmalignant diseases |
| b. Cell dose should be considered first over HLA match for high–body weight patients |
| Perform anti-HLA antibody screening in patients and avoid CBU against which patient has DSA, when possible, especially in those with nonmalignant diseases (due to the risk of rejection) |
| Cord blood bank accreditation status (favor units from accredited cord blood banks) |
| Selection of UCB unit with attached segment for confirmatory identity testing is mandatory |
| If several UCB units are available, choice of the best one should be also guided by the following: |
| a. ABO compatibility |
| b. NIMA and KIR status† |
| c. Avoid RBC-replete units (or accept only if no RBC-depleted unit is available) |
| d. Cryovolume (to be considered in case of further dilution needed after thaw) |
| e. Select more recent units because they may be linked to optimal banking practices |
| Cryopreserved cell dose |
| Number of cells in the UCB unit for malignant disease |
| a. Single UCBT TNC/kg: ≥2.5-3 × 107 and/or CD34+/kg ≥1.5 × 105* |
| b. Double UCBT: TNC/kg: ≥1.5 × 107 for each unit and/or CD34+/kg ≥1 × 105 for each unit |
| HLA typing |
| a. High-resolution typing for HLA-A, -B, -C, and -DRB1 of patients and UCB units |
| b. Avoid UCB units with >2 HLA mismatches and avoid HLA-C mismatches |
| c. In double UCBT, unit-to-unit HLA match is not required |
| Adapt cell dose and HLA matching to graft indication |
| a. Nonmalignant diseases: increase cell dose (>5.0 × 107 TNC/kg) and find the best HLA match. If the criterion for the minimum number of cells in a single UCB unit is not achieved, a double UCBT should be considered also in nonmalignant diseases |
| b. Cell dose should be considered first over HLA match for high–body weight patients |
| Perform anti-HLA antibody screening in patients and avoid CBU against which patient has DSA, when possible, especially in those with nonmalignant diseases (due to the risk of rejection) |
| Cord blood bank accreditation status (favor units from accredited cord blood banks) |
| Selection of UCB unit with attached segment for confirmatory identity testing is mandatory |
| If several UCB units are available, choice of the best one should be also guided by the following: |
| a. ABO compatibility |
| b. NIMA and KIR status† |
| c. Avoid RBC-replete units (or accept only if no RBC-depleted unit is available) |
| d. Cryovolume (to be considered in case of further dilution needed after thaw) |
| e. Select more recent units because they may be linked to optimal banking practices |
DSA, donor-specific antibodies; KIR, killer cell immunoglobulin-like receptor; KIR-L, killer cell immunoglobulin-like receptor ligand; NIMA, noninherited maternal antigen; RBC, red blood cells; TNC, total nucleated cell dose; UCB, umbilical cord blood; UCBT, umbilical cord blood transplantation.
If the minimum number of cells for a single UCBT is not achieved, a double UCBT should be considered, as well as a clinical trial investigating the use of ex vivo expanded CBU or the addition of another cellular product.
No sufficient data to support unit selection on the basis of NIMA or KIR-L status.
Low-resolution HLA matching for UCB units is generally based on 3 loci (HLA-A and -B at antigenic level and HLA-DRB1 at allelic level), with a maximum of 2 of 6 HLA mismatches being considered acceptable because a higher incidence of NRM is associated with greater mismatches. More recently, in a study analyzing the effect of HLA-C on UCBT, Eurocord and the National Marrow Donor Program/Center for International Blood and Marrow Transplant Research8 reported higher NRM in patients receiving a UCB unit with a mismatch at HLA locus C. In addition, concomitant mismatching at HLA-C and -DRB1 was associated with the highest risk of mortality. Later, a collaborative study by the same group9 analyzed the effect of full allelic typing for HLA-A, -B, -C, and -DRB1 on UCBT outcomes, with significant reduction in mortality for 8/8 and 7/8 reported. The advantage of allele-level matching was also recently confirmed in children with nonmalignant disease.10 These important findings helped in reassessing the strategy for UCB unit selection and supported the need for public CBB to expand the UCB unit inventory, including the typing at locus C and the allele-level matching.
As indicated in Table 1, the current criteria for donor selection recommend considering allele-level HLA matching at HLA-A, HLA-B, HLA-C, and HLA-DRB1 for both malignant and nonmalignant diseases and selecting a UCB unit with no more than 2 HLA mismatches.
Ethnicity
The access to HLA-matched unrelated donors is a major barrier to transplantation, especially for ethnic minorities. Recent data reported by Barker et al11 highlighted that more than half of the UCBT recipients referred to Memorial Sloan Kettering Cancer Center are of non-European descent, with nearly half (46%) of the patients of African descent in need of an URD transplant ultimately undergoing UCBT (Figures 2A–2C). This confirms the need for alternative donors for patients from minority backgrounds, some of whom may also lack a haploidentical donor, as was the case for the patient in clinical case 1. Furthermore, in recent years, despite an increasing number of donors available in international registries, the inequality in finding an 8/8 HLA-matched URD among the different ethnic groups persists,4 thus emphasizing the need for alternative graft sources.
Other donor characteristics
Some other factors, such as the noninherited maternal antigen and the effect of donor killer cell immunoglobulin-like receptor ligand incompatibility, may play a role in UCBT outcomes; however, to date, there is no clear evidence supporting inclusion of these factors in the algorithm of donor selection.7 Similarly to donor selection using other cell sources, ABO compatibility should be considered when multiple UCB units are available, and preference should be given to a unit that is ABO compatible or has a minor incompatibility with the recipient, especially in the setting of double UCBT (Table 1).7
The bank of origin is another important factor to consider when selecting the UCB unit,12 because a wide variability in laboratory techniques still exists, with special reference to UCB unit characterization, processing, and storage. High standards for quality control programs have been implemented, such as the Foundation for Accreditation of Cellular Therapy–Netcord and American Association of Blood Banks programs. The accreditation systems aim to standardize all the banking steps with the final goal of achieving better quality and homogeneity in the banks’ UCB unit inventory. Nevertheless, the selection of a UCB unit from an accredited bank is not currently mandatory.
Donor-specific anti-HLA antibodies in UCBT recipients
The presence of DSA in the recipient is a known risk factor for engraftment failure after HSCT,13 and its role in UCBT has been demonstrated by several independent groups.14,15 The use of a UCB unit for which patients have DSA should be avoided to reduce the risk of rejection and NRM, namely in patients and diseases at risk of graft rejection.15 In the absence of other possible donors, as was the case for the patient in clinical case 1, strategies to remove DSA before proceeding to transplantation are indicated. Among the most commonly used methods for DSA desensitization are antibody removal by plasma exchange, depletion of B cells with anti-CD20 antibodies, and intravenous immunoglobulin infusion for antibody neutralization.16 Patient screening to detect antibodies directed against HLA of the available UCB unit should be included in the algorithm of donor choice and must be considered when selecting the UCB unit.
Clinical case 1 continued
The patient was prepared with a conditioning regimen that included thiotepa 5 mg/kg on days −6 and −5; fludarabine 50 mg/m2 on days −4, −3, and −2; and intravenous busulfan 3.2 mg/kg on days −4, −3, and −2. GVHD prophylaxis was based on cyclosporine (CsA) and mycophenolate mofetil. Neutrophil engraftment occurred at day +23 with full donor chimerism tested in bone marrow analysis, as well as platelet recovery at day +48. The patient did not develop any acute GVHD during the follow-up.
Given the patient’s high-risk disease with FLT3-ITD, sorafenib was introduced as a single maintenance agent at 70 days after UCBT and at 14 days after withdrawal of immunosuppressive therapy, respectively, with no hematological or organ toxicities. At day +403, the patient presented to the outpatient clinics with mild chronic GVHD with oral symptoms not affecting oral intake as well as eye dryness without sclerotic features or joint contractures. The results of lung function tests and computed tomography were negative for bronchiolitis obliterans. Topical immunosuppressive treatment and eye drops allowed control of the chronic GVHD manifestations, with no further events observed during the clinical course. Five years after UCBT, the patient is in good clinical condition with normal blood counts and persisting complete remission, with no concomitant immunosuppressive treatment.
Conditioning regimen in UCBT
Standard myeloablative conditioning (MAC) regimens for UCBT generally include total body irradiation (TBI; >6 Gy) or busulfan (>8 mg/kg orally or 6.4 mg/kg IV) associated with cyclophosphamide. Later, fludarabine also started to be used in MAC regimens with the intent of lowering toxicity.17
In the single UCBT setting, the “TBF” conditioning regimen, based on the use of thiotepa 10 mg/kg, busulfan 9.6 mg/kg, fludarabine 150 mg/m2, is commonly used, especially in European transplantation centers, because of its capability of improving engraftment and survival outcomes. The use of thiotepa as an antineoplastic agent, with myelosuppressive and immunosuppressive activities and ability to penetrate the blood–brain barrier, showed a good safety profile in combination with other alkylating agents, such as busulfan. The TBF conditioning regimen was first reported by Sanz and colleagues18 in a single-center experience and then widely adopted, even in other donor settings.19
However, there is still considerable heterogeneity in the choice of conditioning regimen for single UCBT due to different transplantation centers policies. On the other hand, for double UCBT, the choice of MAC regimen is more homogeneous, because the majority of centers adopted the regimen with TBI 12 Gy, cyclophosphamide 120 mg/kg, and fludarabine 75 mg/m2 proposed by the University of Minnesota group, which has been shown to produce encouraging results regarding myeloid recovery and long-term disease control.20
The successful use of nonmyeloablative regimens based on low-dose TBI in UCBT, first reported by Brunstein et al, showed quick neutrophil recovery and low NRM in patients with malignancies.21 Other reports in recent years support the feasibility of UCB after nonmyeloablative conditioning, allowing older or heavily pretreated patients to receive transplants.22,23 This is of importance because older patients are less likely to have a healthy sibling donor available.
Antithymocyte globulin and GVHD prophylaxis
The use of antithymocyte globulin (ATG) as part of the conditioning regimen before UCBT is still debatable, especially in patients with malignancies. There is evidence indicating that delayed T-cell reconstitution reported after UCBT compared with HSCT using bone marrow or peripheral stem cells might be explained by the fact that ATG severely impacts T-cell recovery after UCBT, with a detrimental effect on relapse incidence and survival.24 The use of ATG in patients receiving UCBT should be handled cautiously outside clinical trials. Individualized dosing and therapeutic drug monitoring could help in investigating the optimal dose schedule of ATG to improve outcomes.
The landscape of GVHD prophylaxis in HSCT dramatically changed over the last few years with evidence of the efficacy of posttransplantation cyclophosphamide (PT-Cy) in unmanipulated haploidentical transplant recipients.25 PT-Cy provides selective depletion of the alloreactive T cell, with no toxicity to the hematopoietic stem cells and early immune reconstitution. The application of PT-Cy also in the HLA-identical and URD setting has been reported.26 The feasibility of PT-Cy at a reduced dose of 30 mg/kg in UCBT recipients has been reported by Bacigalupo et al.27 The applicability of this GVHD platform in single UCBT after a myeloablative regimen is currently under investigation in a prospective clinical trial (NCT03802773).
Also important is that Brunstein et al28 demonstrated the feasibility of the use of the adoptive transfer of ex vivo expanded, UCB-derived T-regulatory cells (Tregs) to prevent GVHD in a clinical trial that included patients with hematological malignancies receiving a sirolimus and mycophenolate mofetil GVHD prophylaxis. The adoptive transfer of Tregs was safe and resulted in a low risk of acute GVHD in comparison with contemporary control subjects.
Maintenance therapy after UCBT
The patient in clinical case 1 had FLT3-ITD acute myeloid leukemia, was at high risk of recurrence, and had a dismal prognosis. UCBT allowed avoiding delay in transplantation for this patient with high-risk disease and from an ethnic minority background.29 After successful engraftment of the UCBT, the patient underwent a maintenance therapy with sorafenib, which has been shown to be effective for similar cases. Sorafenib is a potent multikinase inhibitor approved for late-stage hepatocellular carcinoma and renal cell carcinoma. A possible synergistic effect of sorafenib and alloreactive donor T cells in facilitating long-term disease control has been suggested.30 It has also been proposed in murine models in which sorafenib apparently exacerbated GVHD. In clinical case 1, signs of chronic GVHD were observed after treatment with sorafenib, suggesting an immunomodulatory effect. Interestingly, the patient did not experience acute GVHD, and her mild chronic GVHD was manageable with topical immunosuppressive treatment and dose adjustment. Currently, several other similar agents, such as lestaurtinib, midostaurin, crenolanib, gilteritinib, and quizartinib, are under evaluation in HSCT as well as UCBT recipients.
Clinical case 2
A 17-year-old white man with a diagnosis of severe aplastic anemia was referred to our department after his first cycle of immunosuppressive therapy with horse ATG and CsA failed. The patient’s weight was 72 kg. At that time, no suitable HLA-matched URD was available in the international registries. The patient was enrolled in the APCORD clinical trial (NCT01343953), and according to the cell dose requirement for the protocol (proceeding to a double UCBT in the absence of a single UCB with at least 2 HLA mismatches with more than 4 × 107/kg at cryopreservation), 2 UCB units were selected. HLA matching of the first UCB unit was 6/8 with full mismatch at locus B, ABO matched, and sex mismatched with the patient. The TNC at cryopreservation was 2.8 × 107/kg, and the CD34+ count was 2.2 × 105/kg. HLA matching of the second UCB unit was 6/8 with full mismatch at locus B, major ABO incompatibility, and sex matched with the patient. The TNC at cryopreservation was 3.1 × 107/kg, and the CD34+ count was 2.0 × 105/kg. The result of recipient screening for anti-HLA DSAs was negative.
Double UCBT
When the TNC dose available in a single UCB unit is not enough for transplantation, infusing 2 UCB units sequentially in one transplantation procedure (double UCBT), is feasible.7 The requirements of cell dose and number of HLA disparities for double units are the same as for single units, with no need of interunit HLA match. The first findings of higher risk of acute GVHD and lower risk of relapse reported in adults after double UCBT were not confirmed in prospective trials in children and young adults.5,31
In the setting of nonmalignant disorders, the patient in clinical case 2, who had an acquired bone marrow failure syndrome, was enrolled in a prospective protocol evaluating the benefit of single vs double UCBT.32 In this case, the use of a second UCB unit to reach an acceptable cell dose for transplantation did not increase the risk of acute or chronic GVHD. This is extremely important in HSCT for patients with nonmalignant disease to avoid long-term disabilities related to chronic GVHD.
Other considerations
Delayed immune reconstitution and increased risk of infection after UCBT, and consequently NRM, are major concerns.7 New strategies to enhance immune reconstitution are warranted to improve outcomes after UCBT. A recent report by Bejanyan et al33 comparing outcomes of 96 UCB recipients with those of other patients who received peripheral blood graft transplants from matched sibling donors during the first year after transplantation showed slower T-cell subset recovery after UCBT. Infections were more frequent in UCB recipients, but donor type had no effect on NRM or survival. In the first month after transplant, low CD4+ T-cell counts (total and naïve) were associated with increased infection risk, TRM, and chronic GVHD. This indicates that in UCBT recipients, careful infection monitoring and prophylaxis are of utmost importance.
Although few cost-effectiveness HSCT studies are available, the cost of UCBT is estimated to be higher than the cost of MUD HSCT.34 The increased cost can be attributed in part to the cost of the UCB unit acquisition, which is estimated to be approximately $30 000 to $60 000,34 but also to the length of hospitalization for this type of procedure often associated with delayed engraftment.7 Reducing costs is of utmost importance for the future of UCBT. Strategies for achieving that goal include improving UCB collection and donor selection, developing new techniques to improve engraftment, and diversifying the use of UCBT with other therapeutic approaches.35
Clinical case 2 continued
The conditioning regimen used was fludarabine 30 mg/m2 (day −6 to day −3), cyclophosphamide 30 mg/kg (day −6 to day −3), ATG 2.5 mg/kg at day −3 and day −2 (5 mg/kg total dose), and TBI (2 Gy, day −2), with GVHD prophylaxis consisting of CsA and mycophenolate mofetil. Neutrophil engraftment occurred at day +17 and platelet recovery at day +32. Day +30 chimerism revealed 100% cells of donor origin from the first UCB unit. Tapering of immunosuppression started 6 months after double UCBT. Neither acute GVHD nor chronic GVHD occurred, and the patient is currently at 1 year after double UCBT with full donor chimerism. Tapering of CsA is ongoing, with no infectious complications having occurred.
UCBT in children
Cord blood transplantation was first adopted in pediatric patients, for whom the issue of cell dose is less critical and the possibility of reducing the risk of GVHD is feasible. The unique features of cord blood–derived hematopoietic stem cells make this source attractive for some specific diseases, such as inherited disorders of metabolism, immunodeficiencies and bone marrow failure.36,37 For patients with inherited bone marrow failure syndromes, such as Fanconi anemia, UCBT using adapted less toxic conditioning regimens has the advantage of reducing the risk of chronic GVHD and the associated long-term complications. This is of great importance for patients with Fanconi anemia who have a higher risk of developing solid tumors, such as of the head and neck.38
Furthermore, the ready availability of UCB makes it a very attractive graft source in other nonmalignant diseases, such as immunodeficiency and inborn errors of metabolism, in which proceeding quickly to transplantation is of utmost importance.39,40 Notably, because the concerned patients are mostly young children, cell dose is usually not a barrier; therefore, physicians could focus on the best HLA match for their patient to optimize the graft selection.39,40 Importantly, results of UCBT for children with inborn errors and metabolic disorders39,40 in comparison with other stem cell sources were reported as showing some advantages for UCBT, with reduced risk of GVHD and long-term disabilities.
Acute leukemia remains the most common indication for UCBT in pediatric patients, with a strong graft-versus-leukemia effect, including patients with positive minimal residual disease before UCBT.41 Two important prospective trials were conducted, one in the United States5 and one in France,31 randomizing participants to single or double UCBT. Very low toxicity and NRM were reported, with excellent overall survival approaching 70% in both trials. Both studies confirmed the benefit of a single UCBT in the pediatric setting, with overall survival similar to that of double UCBT, and showed no indication for the addition of a second UCB unit when a single unit with an adequate cell dose is available.
Ex vivo expansion of cord blood stem cells
Currently, multiple strategies are under investigation, with the main aim being to increase the progenitor cells of a cord blood graft.42 Delaney et al showed that a rapid myeloid reconstitution after UCBT was possible with a Notch-mediated ex vivo expansion of human cord blood progenitor cells and infusion of a nonmanipulated UCB together with another unit expanded ex vivo.43 The same group is currently assessing the use of an “off-the-shelf’” expanded UCB product in a phase 2 study that is currently ongoing (NCT01690520).
A different platform for progenitor cell expansion was reported by De Lima and colleagues, who used mesenchymal stromal cell coculture (mesoblast),44 allowing shorter time to engraftment than the historical control. The use of a single UCB unit expanded ex vivo with nicotinamide as a “stand-alone graft” in patients with hematological diseases was recently reported by Horwitz et al.45
Other strategies of UCB ex vivo expansion42 or the use of agents to enhance UCB homing to the marrow have also been described. In addition, some groups have also reported encouraging results with the use of the direct intra–bone marrow injection of the UCB unit or coinfusion of UCB with a haploidentical T-cell–depleted graft. Table 2 provides a summary of the main cord blood expansion platforms and other strategies to improve engraftment after UCBT. Promising results have been reported with the aforementioned strategies; however, they remain experimental, and definitive conclusions cannot yet be drawn regarding their reproducibility, cost efficiency, and long-term outcomes.
Cord blood expansion and other strategies to improve engraftment after umbilical cord blood transplantation
| Agent/strategy . | Mechanism . | Clinical trial identifier . | Patients, N . | Median d to PMN engraftment . | Institution, country/manufacturer . | Author . | Reference . |
|---|---|---|---|---|---|---|---|
| Nicotinamide | Inhibits HSC differentiation and facilitates homing | NCT01816230 | 36 | 11.5 | Duke University, United States/Gamida Cell | Horwitz et al | 45 |
| Notch ligand | Provides HSC proliferative signal | NCT01690520 | 10 | 16 | Fred Hutchinson Cancer Research Center, United States | Delaney et al | 43 |
| Mesenchymal stem cells (Mesoblasts) | Provide signal for HSC expansion; improve stroma | NCT01854567 | 31 | 15 | MD Anderson Cancer Center, United States/Mesoblast | De Lima et al | 44 |
| 16,16-dimethyl prostaglandin E2 | Facilitates homing, proliferation, and self-renewal | NCT00890500 | 12 | 17 | Dana-Farber Cancer Institute, United States | Cutler et al | 53 |
| Oral sitagliptin | DPP-IV inhibition | NCT00862719 | 24 | 21 | Indiana University School of Medicine, United States | Farag et al | 54 |
| Complement fragment 3a priming | Facilitates homing | NCT00963872 | 29 | 7 | University of Minnesota, United States | Brunstein et al | 55 |
| FT-VI | Fucosylation | NCT01471067 | 22 | 17 | MD Anderson Cancer Center, United States | Popat et al | 56 |
| Aryl hydrocarbon receptor antagonist (StemRegenin1) | Blocking of HSC differentiation by SR-1 inhibition of aryl hydrocarbon receptor | NCT01474681 | 17 | 15 | University of Minnesota, United States | Wagner et al | 57 |
| Intrabone infusion | Facilitates homing | NCT00696046 | 32 | 23 | University of Genoa, Italy | Frassoni et al | 58 |
| Copper chelation (carlecortemcel-L) | Expansion by blocking HSC differentiation | NCT00469729 | 101 | 21 | Cardinal Bernardin Cancer Center, Loyola University, United States | Stiff et al | 59 |
| Agent/strategy . | Mechanism . | Clinical trial identifier . | Patients, N . | Median d to PMN engraftment . | Institution, country/manufacturer . | Author . | Reference . |
|---|---|---|---|---|---|---|---|
| Nicotinamide | Inhibits HSC differentiation and facilitates homing | NCT01816230 | 36 | 11.5 | Duke University, United States/Gamida Cell | Horwitz et al | 45 |
| Notch ligand | Provides HSC proliferative signal | NCT01690520 | 10 | 16 | Fred Hutchinson Cancer Research Center, United States | Delaney et al | 43 |
| Mesenchymal stem cells (Mesoblasts) | Provide signal for HSC expansion; improve stroma | NCT01854567 | 31 | 15 | MD Anderson Cancer Center, United States/Mesoblast | De Lima et al | 44 |
| 16,16-dimethyl prostaglandin E2 | Facilitates homing, proliferation, and self-renewal | NCT00890500 | 12 | 17 | Dana-Farber Cancer Institute, United States | Cutler et al | 53 |
| Oral sitagliptin | DPP-IV inhibition | NCT00862719 | 24 | 21 | Indiana University School of Medicine, United States | Farag et al | 54 |
| Complement fragment 3a priming | Facilitates homing | NCT00963872 | 29 | 7 | University of Minnesota, United States | Brunstein et al | 55 |
| FT-VI | Fucosylation | NCT01471067 | 22 | 17 | MD Anderson Cancer Center, United States | Popat et al | 56 |
| Aryl hydrocarbon receptor antagonist (StemRegenin1) | Blocking of HSC differentiation by SR-1 inhibition of aryl hydrocarbon receptor | NCT01474681 | 17 | 15 | University of Minnesota, United States | Wagner et al | 57 |
| Intrabone infusion | Facilitates homing | NCT00696046 | 32 | 23 | University of Genoa, Italy | Frassoni et al | 58 |
| Copper chelation (carlecortemcel-L) | Expansion by blocking HSC differentiation | NCT00469729 | 101 | 21 | Cardinal Bernardin Cancer Center, Loyola University, United States | Stiff et al | 59 |
FT-VI, enzyme fucosyltransferase; DPP-IV, dipeptidylpeptidase IV; HSC, hematopoietic stem cell; PMN, polymorphonuclear leukocytes.
Results of UCBT compared with other graft sources in patients with acute leukemia
Table 3 summarizes the different comparative studies of UCBT with other stem cell sources. The main results of comparative studies on UCBT with HSCT from URDs46-48 highlighted the delayed neutrophil and platelet recovery in UCBT recipients, some excess in NRM, and decreased incidence of acute or chronic GVHD, with ultimately comparable overall and leukemia-free survival. Similar findings were also demonstrated in the setting of double UCBT with either a MAC or reduced intensity conditioning regimen. Some investigators reported a benefit of double UCBT with expanded graft over URD transplantation in patients with positive minimal residual disease49 ; however, this result should be confirmed in different settings.
Results of comparative studies on BMT, PBSC and UCBT in adults with hematological malignancies
| Series . | Graft source, no. of patients . | Conditioning regimen intensity . | Conditioning regimen . | ATG (yes/no) . | Median follow-up, mo . | Comments (UCBT vs others) . | Reference . |
|---|---|---|---|---|---|---|---|
| UD vs UCBT for adults with acute leukemia | sUCB, n = 98 | MAC | sUCB: TBI-based, n = 64 | Yes | 27 | Delayed myeloid recovery | 47 |
| BM, n = 584 | Bu-based, n = 34 | Decreased aGVHD and cGVHD | |||||
| BM: TBI-based, n = 426 | Comparable OS and DFS | ||||||
| UCBT vs UD for adults with acute leukemia | sUCB, n = 287 | MAC | TBI-based, n = 720 | No | — | For AML: lower OS and DFS, higher NRM, no difference in RI | 60 |
| BM, n = 533 | Non TBI-based, n = 100 | For ALL: similar OS and DFS, no differences in RI and NRM | |||||
| UD vs single UCBT for adults with acute leukemia | BM, n = 472 | MAC | BM, TBI-based, n = 321 | Yes, 28% | 26 | Delayed myeloid recovery (vs BM) | 48 |
| PBSC, n = 888 | PBSC, TBI-based, n = 583 | Yes, 18% | 24 | Increased NRM but comparable OS and LFS (vs BM) | |||
| UCB, n = 165 | UCB, TBI-based, n = 90 | Yes, 72% | 29 | ||||
| Sibling, UD vs single UCBT | MRD, n = 204 | MAC | TBI + Cy | 36 | Comparable OS and LFS | 20 | |
| MUD, n = 152 | TCF (dUCBT) | Delayed myeloid recovery | |||||
| MMUD, n = 52 | Increased NRM, reduced relapse | ||||||
| dUCB, n = 128 | |||||||
| UD vs double UCBT for adults with acute leukemia | 8/8 PBSC, n = 313 | RIC | 8/8 PBSC Bu-based, n = 119 | Yes | 36 | Overall results comparable to 8/8 PBSC | 61 |
| 7/8 PBSC, n = 111 | 7/8 PBSC Bu-based, n = 46 | 24 | |||||
| dUCB, n = 161 | dUCB, TBI-based, n = 121 | 24 | |||||
| Non–T-cell–depleted haploidentical transplantation vs UCBT for adults with acute leukemia | AML, n = 918 (haploidentical = 360, UCBT = 558) | MAC and RIC | For MAC, TBF, n = 198 | Yes, in 60% of UCBT and 49% of haploidentical | AML, haploidentical 20 | Delayed engraftment | 19 |
| ALL, n = 528 (haploidentical = 158 UCBT = 370). | For RIC, TCF, n = 289 | AML UCBT 31 | Lower incidence of cGVHD | ||||
| ALL haploidentical 19 | Similar relapse, LFS, and NRM | ||||||
| ALL UCBT 17 | |||||||
| UCB vs MUD vs MMUD in AL and MDS | UCBT, n = 140 | MAC | Bu-based, n = 181 | In pre transplantation positive minimal residual disease: | 49 | ||
| MUD, n = 344 | TBI-based, n = 401 | Comparable outcomes after UCBT and MUD, lower relapse for UCBT | |||||
| MMUD, n = 98 | |||||||
| Influence of stem cell source on transplantation outcomes for pediatric AML | MRD, n = 61 | MAC | TBI-based, n = 136 | Yes, 21% | 144 | Comparable RI, LFS, and NRM for all sources | 62 |
| MUD, n = 73 | Chemotherapy-based, n = 181 | MRD allowed better OS | |||||
| UCB, n = 183 |
| Series . | Graft source, no. of patients . | Conditioning regimen intensity . | Conditioning regimen . | ATG (yes/no) . | Median follow-up, mo . | Comments (UCBT vs others) . | Reference . |
|---|---|---|---|---|---|---|---|
| UD vs UCBT for adults with acute leukemia | sUCB, n = 98 | MAC | sUCB: TBI-based, n = 64 | Yes | 27 | Delayed myeloid recovery | 47 |
| BM, n = 584 | Bu-based, n = 34 | Decreased aGVHD and cGVHD | |||||
| BM: TBI-based, n = 426 | Comparable OS and DFS | ||||||
| UCBT vs UD for adults with acute leukemia | sUCB, n = 287 | MAC | TBI-based, n = 720 | No | — | For AML: lower OS and DFS, higher NRM, no difference in RI | 60 |
| BM, n = 533 | Non TBI-based, n = 100 | For ALL: similar OS and DFS, no differences in RI and NRM | |||||
| UD vs single UCBT for adults with acute leukemia | BM, n = 472 | MAC | BM, TBI-based, n = 321 | Yes, 28% | 26 | Delayed myeloid recovery (vs BM) | 48 |
| PBSC, n = 888 | PBSC, TBI-based, n = 583 | Yes, 18% | 24 | Increased NRM but comparable OS and LFS (vs BM) | |||
| UCB, n = 165 | UCB, TBI-based, n = 90 | Yes, 72% | 29 | ||||
| Sibling, UD vs single UCBT | MRD, n = 204 | MAC | TBI + Cy | 36 | Comparable OS and LFS | 20 | |
| MUD, n = 152 | TCF (dUCBT) | Delayed myeloid recovery | |||||
| MMUD, n = 52 | Increased NRM, reduced relapse | ||||||
| dUCB, n = 128 | |||||||
| UD vs double UCBT for adults with acute leukemia | 8/8 PBSC, n = 313 | RIC | 8/8 PBSC Bu-based, n = 119 | Yes | 36 | Overall results comparable to 8/8 PBSC | 61 |
| 7/8 PBSC, n = 111 | 7/8 PBSC Bu-based, n = 46 | 24 | |||||
| dUCB, n = 161 | dUCB, TBI-based, n = 121 | 24 | |||||
| Non–T-cell–depleted haploidentical transplantation vs UCBT for adults with acute leukemia | AML, n = 918 (haploidentical = 360, UCBT = 558) | MAC and RIC | For MAC, TBF, n = 198 | Yes, in 60% of UCBT and 49% of haploidentical | AML, haploidentical 20 | Delayed engraftment | 19 |
| ALL, n = 528 (haploidentical = 158 UCBT = 370). | For RIC, TCF, n = 289 | AML UCBT 31 | Lower incidence of cGVHD | ||||
| ALL haploidentical 19 | Similar relapse, LFS, and NRM | ||||||
| ALL UCBT 17 | |||||||
| UCB vs MUD vs MMUD in AL and MDS | UCBT, n = 140 | MAC | Bu-based, n = 181 | In pre transplantation positive minimal residual disease: | 49 | ||
| MUD, n = 344 | TBI-based, n = 401 | Comparable outcomes after UCBT and MUD, lower relapse for UCBT | |||||
| MMUD, n = 98 | |||||||
| Influence of stem cell source on transplantation outcomes for pediatric AML | MRD, n = 61 | MAC | TBI-based, n = 136 | Yes, 21% | 144 | Comparable RI, LFS, and NRM for all sources | 62 |
| MUD, n = 73 | Chemotherapy-based, n = 181 | MRD allowed better OS | |||||
| UCB, n = 183 |
aGVHD, acute graft-versus-host disease; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; BM, bone marrow; BMT, bone marrow transplantation; Bu, busulfan; cGVHD, chronic graft-versus-host disease; DFS, disease-free survival; dUCB, double umbilical cord blood; dUCBT, double umbilical cord blood transplantation; LFS, leukemia-free survival; MAC, myeloablative conditioning regimen; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor; NRM, nonrelapse mortality; OS, overall survival; PBSC, peripheral blood stem cells; RI, relapse incidence; RIC, reduced intensity conditioning regimen; sUCB, single umbilical cord blood; TBI, total body irradiation; TCF, total body irradiation, fludarabine, cyclophosphamide; UBM, unrelated bone marrow; UCBT, umbilical cord blood transplantation; UD, unrelated donor.
Recently, the use of family mismatched donors has dramatically increased.1 Brunstein and colleagues50 reported the results of 2 parallel phase 2 trials on double UCBT and unmanipulated haploidentical transplantation with PT-Cy. This parallel trial was the basis for a phase 3 randomized trial (NCT01597778), the results of which are expected later in 2019. Single-center and registry studies have not yet reported any clear differences in overall outcomes between the 2 approaches.19
Importantly, studies evaluating long-term outcomes are needed to define the most appropriate stem cell source and conditioning regimen, as well as the best GVHD prophylaxis. The progress made most recently helps considerably in extending the donor pool for patients who lack an HLA-matched donor, offering the possibility of shortening the delay of donor procurement. The age of the donor could be a matter of concern in the setting of adult donors, owing to the occurrence of the clonal hematopoiesis of indeterminate potential.51 Cord blood stem cells are from the youngest donor, as they are collected at birth and used over the years when needed. Some evidence indicates that persons carrying a clonal hematopoiesis of indeterminate potential may have a 13 times greater risk of hematological malignancies than the general population, and in the haploidentical setting, some algorithms for donor selection recommend a younger donor even over a relative of a more advanced age.52
Although the use of UCBT has decreased in the last few years, mainly with the increased use of haploidentical transplantation, UCB remains an important source of stem cells, such as in the pediatric setting. New applications using this stem cell source are still being investigated, not only for the treatment of hematological diseases but also for autoimmune or inflammatory disorders, such as for developing chimeric antigen receptors transduced using cord blood–derived natural killer cells (NCT03056339).
Acknowledgments
The author thanks Eliane Gluckman, Mary Eapen, Vanderson Rocha, Fernanda Volt, Juliet Barker, and Regis Peffault de Latour for helpful discussion and critical reading of the manuscript, as well as colleagues from Eurocord and from the European Society for Blood and Marrow Transplantation.
Correspondence
Annalisa Ruggeri, Eurocord, 1 Ave Claude Vellefaux, 75010 Paris, France; e-mail: annalisaruggeri80@hotmail.com.
References
Competing Interests
Conflict-of-interest disclosure: A.R. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.