Abstract
Classification criteria for antiphospholipid syndrome have not been updated since the revised Sapporo classification criteria were published in 2006. These criteria have limitations in that they omit nonclassical manifestations (hematologic and neurologic), include anticardiolipin and anti–β2-glycoprotein I immunoglobulin (Ig)M isotypes, and do not separately consider primary (no autoimmune disease) or secondary (usually systemic lupus erythematosus) disease. Recent findings in antiphospholipid antibody include fluctuation of antiphospholipid antibodies, recognition that IgA isotypes do confer risk, identification of the role of complementopathy in catastrophic antiphospholipid syndrome, and elucidation of the role of thrombosis risk equations.
Learning Objectives
Consider advances and refinements of antiphospholipid antibody assays
Review complementopathies and antiphospholipid syndrome
Consider the current thrombotic risk formulas for antiphospholipid syndrome
Clinical case
A 26-year-old woman with systemic lupus erythematosus (SLE) presented with photosensitive rash and arthritis, and the following laboratory values were measured:
Complement C3, 52 mg/dL (low) (normal, 81-157 mg/dL)
Anti–double-stranded DNA (anti-dsDNA) antibodies, 1:160 (high) (normal, 0)
Diluted Russell viper venom time confirmation, 1.6 (positive) (negative, <1.4)
Anticardiolipin immunoglobulin (Ig)M antibody, 16 MPL units (low positive)
Anti–β2-glycoprotein I IgA antibody, 41 std. IgA units (positive)
Key question: Is this 26-year-old patient with SLE at increased risk of future thrombosis?
Yes. Because she has low complement, she is at increased risk over having antiphospholipid antibodies alone.
Yes. Because she has high anti-dsDNA antibodies, she is at increased risk over having antiphospholipid antibodies alone.
a and b.
No, because antiphospholipid antibodies have been documented at only one point in time.
Answer: Number 1. In SLE, lupus anticoagulant, even if checked once, increases the risk of future thrombosis. Low C3 further increases the thrombosis risk.
Introduction
Antiphospholipid antibodies are found in the healthy general population, but they are frequently found in individuals with SLE. In the Hopkins Lupus Cohort, comprising 2,534 patients, 26% have had lupus anticoagulant antibodies, 47% have had anticardiolipin (aCL) antibodies (IgG, IgM, or IgA), and 28% have had anti–β2-glycoprotein I antibodies (IgG, IgM, or IgA). The Hopkins Lupus Cohort, based in Baltimore, is predominantly white or African American. There is an ethnically based difference, with 29% of white vs 22% of African American individuals having the lupus anticoagulant. There is also a gender difference: 40% of men vs 24.5% of women have the lupus anticoagulant. The prospective study of thrombotic risk in SLE showed that patients with SLE with lupus anticoagulant at baseline (as in the present clinical case) had a 50% risk of thrombosis by 20 years after diagnosis.1
Diagnosis of antiphospholipid syndrome
There are no diagnostic criteria for antiphospholipid syndrome (APS). The most recent classification criteria, the revised Sapporo classification criteria, were published in 2006.2 They subdivide APS into thrombotic (arterial, venous, or small vessel) or obstetric subtypes (multiple early pregnancy losses, one or more late intrauterine fetal demises, or severe preeclampsia). The laboratory criteria require that lupus anticoagulant, IgM or IgG aCL, or IgM or IgG anti–β2-glycoprotein I be positive twice over a 3-month period.
The classification criteria omit nonthrombotic and nonobstetric manifestations of antiphospholipid antibodies. The widely accepted nonclassical manifestations include hematologic (thrombocytopenia) and neurologic (in particular, chorea and longitudinal myelitis).
Limitations to current classification criteria for APS
The current APS classification criteria2 require that lupus anticoagulant, IgG or IgM aCL, or anti–β2-glycoprotein I IgG or IgM be present twice over a 3-month period. In SLE, however, the lupus anticoagulant explains the majority of the thrombotic or obstetric risk; aCL does not add to the risk.3 This message was also the conclusion of the multicenter PROMISSE (Predictors of Pregnancy Outcome in Systemic Lupus Erythematosus and Antiphospholipid Syndrome) study of antiphospholipid antibodies in pregnancy, in which only the lupus anticoagulant was associated with adverse pregnancy outcomes.4 The PROMISSE study included both SLE and non-SLE pregnancies with antiphospholipid antibodies.
A second problematic issue with the classification criteria is that antiphospholipid antibodies can fluctuate in patients with or without SLE. They may increase with SLE disease activity and decrease with effective SLE treatment. One-time baseline laboratory measurements are available in the present clinical case. However, even baseline lupus anticoagulant confers risk of thrombosis in SLE.1 Thus, the “3-month rule” can be difficult to translate into practice. Is a patient with positive results at 0 months and 1 month but negative results at 3 months to be counted in the classification criteria?
A third problem is that IgM aCL is not associated with lifetime thrombosis risk in SLE.3 In thrombotic APS, not limited to SLE, IgM antibodies had added clinical value only in patients with positive results for IgG antibodies.5 There is an association with IgA isotypes of aCL and of anti–β2-glycoprotein I, not limited to SLE, although IgA is not included in the classification criteria.2 The association of IgA isotypes with thrombosis is now generally accepted6-9 in primary or secondary APS. In fact, IgA isotypes are included in the Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) classification criteria for SLE.10
Progress in new antiphospholipid antibody assays
Lupus anticoagulant
The lupus anticoagulant is the single most important antiphospholipid antibody in SLE or non-SLE in that it confers the greatest thrombotic and obstetric risk. It requires an initial sensitive screen (such as diluted Russell viper venom time [dRVVT] or sensitive partial thromboplastin time), mixing study, and confirmatory step. Once a patient is anticoagulated, however, the assay becomes more problematic. A frequent issue today is the patient who is already receiving a new direct oral anticoagulant (DOAC). DOACs may lead to a risk of overestimation in lupus anticoagulant testing.11 The presence of either of a direct factor Xa or IIa inhibitor may falsely prolong the dRVVT screen and/or confirm test. However, the extent of this prolongation is different for both the dRVVT screen and confirmation, depending on the type of DOAC present. DOAC-Stop (Haematex Research, Sydney, Australia) will allow clinical laboratories to perform a valid lupus anticoagulant assessment in this situation.
Anti–phosphatidylserine/prothrombin
Anti–phosphatidylserine/prothrombin (anti-PS/PT) correlates highly with the presence of the lupus anticoagulant. In one Chinese study, 92% of lupus anticoagulant samples had positive results for IgG and/or IgM anti-PS/PT.12 Anti-PS/PT therefore might serve as a surrogate for lupus anticoagulant if it is not possible to perform a valid lupus anticoagulant test.
Anti–β2-glycoprotein I domain-specific autoantibodies
β2-glycoprotein I is one of several potential targets of antiphospholipid antibodies. The main conformational epitope is in domain I. Antibodies directed against domain I were more frequent in patients with APS than in asymptomatic carriers.13 However, antibodies to domain I were not associated with thrombosis in one series.7 Patients with APS can make antibodies to other epitopes, such as domain 4/5, as well. Domain-specific autoantibodies are not routinely available or used in clinical practice.
Functional assays
Thrombin generation.
Antiphospholipid antibodies can affect the activity of activated protein C (APC), leading to one potential pathway of hypercoagulability. In one study, APC resistance (using a thrombin generation–based test) was found in the majority of patients with the lupus anticoagulant. The resistance was more marked in those with a history of thrombosis.14 The endogenous thrombin potential (ETP) was associated with antiphospholipid antibodies and with thrombosis history in SLE in another study.15 However, ETP is not used in clinical practice or followed longitudinally.
Annexin A5 resistance.
Antiphospholipid antibodies can disrupt the anticoagulant shield formed by annexin A5. This then exposes thrombogenic anionic phospholipids, facilitating thrombosis. Rand et al16 pioneered the coagulation assay for resistance to annexin A5 and proved that this mechanism occurs in some but not all patients with APS.
Complementopathies.
A fresh approach to the understanding of APS has come from the field of complementopathies. Paroxysmal nocturnal hemoglobinuria is an acquired genetic mutation leading to loss of the complement regulatory proteins CD59 and CD55. CD59 is a glycosylphosphatidylinositol membrane protein that inhibits the terminal complement pathway. CD55 (decay-accelerating factor) is another anchor membrane protein that inhibits complement activation. Mutations leave the red blood cells vulnerable to lysis (but also predispose to thrombosis). Atypical hemolytic uremic syndrome, which causes a thrombotic microangiopathy, can be caused by mutations that activate the alternative pathway of complement.17 Both diseases can now be treated by blocking complement with eculizumab.
The term “immunothrombosis” describes the multiple interactions of the immune system (monocytes, neutrophils, and complement proteins) and coagulation. C3a and C5b, for example, can lead to platelet activation, increase tissue factor, activate endothelial cells, increase von Willebrand factor, and lead to P-selectin exposure. C5a, through its receptor on neutrophils, induces tissue factor, which then catalyzes factor X. C5b-9 (the membrane attack complex) increases exposure of prothrombinase assembly sites on platelets, leading to thrombin propagation.18 Prompted by thrombin, plasmin cleaves C5 into C5a.
The understanding that complement has a role in primary and secondary APS came from both obstetric APS and thrombotic APS studies in animal models and in patient studies, both SLE and non-SLE. In obstetric APS, it was recognized that the benefit of prophylactic doses of heparin in reducing miscarriage was due to the fact that it blocked complement activation.19 Some cases of HELLP syndrome (hemolysis with elevated liver enzymes and low platelets) were found to arise from germline mutations in the alternative pathway of complement.20-22 In fact, HELLP could be considered as a “pregnancy-induced” atypical hemolytic uremic syndrome.23 The redefinition of HELLP as a complementopathy led to case reports of successful treatment with eculizumab.24,25 A subset of cases of preeclampsia, also an obstetric APS manifestation, were also linked to the alternative pathway of complement.26 In the PROMISSE multicenter study, which included patients with and without SLE, complement activation was associated (P = .02) with adverse pregnancy outcomes in pregnancies with antiphospholipid antibodies.27
In thrombotic APS animal models, thrombosis can be prevented by inhibitors of complement activation or selection of animals that are complement deficient.28-32 Actual complement deposition was proved in the vessel wall in a patient with primary APS undergoing coronary bypass surgery for an arterial thrombus.33
Modified Ham test to quickly identify complementopathies.
The Ham test was originally used to help diagnose paroxysmal nocturnal hemoglobinuria. Vaught et al23 modified the Ham test to allow for the rapid identification of complementopathies. For example, the modified Ham test can differentiate HELLP from healthy pregnant patients and most patients with preeclampsia.23 In these conditions, in which effective treatment is needed quickly, sending blood to a reference laboratory to identify germline mutations and/or autoantibodies against complement regulatory proteins is inadequate. The modified Ham test, however, is not generally available.
Catastrophic antiphospholipid syndrome and complementopathy
Catastrophic antiphospholipid syndrome (CAPS) is the most devastating form of primary or secondary APS, with 50% mortality still observed. Forty-two percent of patients with CAPS have defined SLE or a lupus-like syndrome.34 Thrombotic manifestations occur in multiple organs over a short period of time. Anti–β2-glycoprotein I, one of the antiphospholipid antibodies, is the key to understanding how CAPS (and APS in general) could be a complementopathy, because β2-glycoprotein I is a homolog for factor H. Factor H regulates the alternative pathway by binding to C3b and disrupting the alternative pathway C3 convertase, and it is a cofactor for factor I to inactivate C3b as well.
The modified Ham test has identified complementopathy in patients with CAPS (both SLE and non-SLE) at our institution as well as in a subset of patients with general APS (R. Brodsky, personal communication, 1 August 2019). Rapid identification of CAPS could change treatment. Current treatment is “triple therapy” of intravenous methylprednisolone pulse, heparin, and plasmapheresis.35 In patients without a response, rituximab can be added.36 However, therapy specifically directed against the causative factor—complementopathy—would be a complement inhibitor such as eculizumab. Several case reports have proved its benefit.24,25
Thrombotic risk scores
Triple positivity
In an effort to better predict thrombotic risk from antiphospholipid antibodies in patients with either primary (no autoimmune) or secondary (usually SLE) disease, several risk formulas have been constructed. Perhaps the best known is “triple positivity,” requiring that the patient have lupus anticoagulant, aCL, and anti–β2-glycoprotein I. There is little opportunity to study thrombosis risk scores in primary antiphospholipid “carriers,” leading to most studies including a large number of patients with SLE. In one Finnish study, 61% of the 119 patients were “secondary” with an autoimmune disease, predominantly SLE.37 All 9 (7.6%) who developed a thrombotic event had an autoimmune disease. The triple-positivity risk score has been disproved in both patients with primary and secondary antiphospholipid antibodies in that IgM aCL is not associated with lifetime thrombosis risk in SLE3 and only the lupus anticoagulant was associated with adverse pregnancy outcomes in the PROMISSE study,4 which included patients with primary and secondary antiphospholipid antibodies. In the present clinical case, the patient’s aCL was both low titer and IgM. However, triple positivity remains the most widely used risk score.
In a nationwide asymptomatic antiphospholipid carrier cohort in Finland, 119 people were followed for a mean of 9.1 years. Seven triple-positive carriers were identified, and only one had prospective thrombosis. A key point was that single aCL or anti–β2-glycoprotein I positivity was not associated with thrombosis. Double- or triple-positive carriers had higher first thrombotic event rates, but not significantly higher.37 A second longitudinal study included only triple-positive individuals and did not separately analyze risk of lupus anticoagulant alone.38
Antiphospholipid score
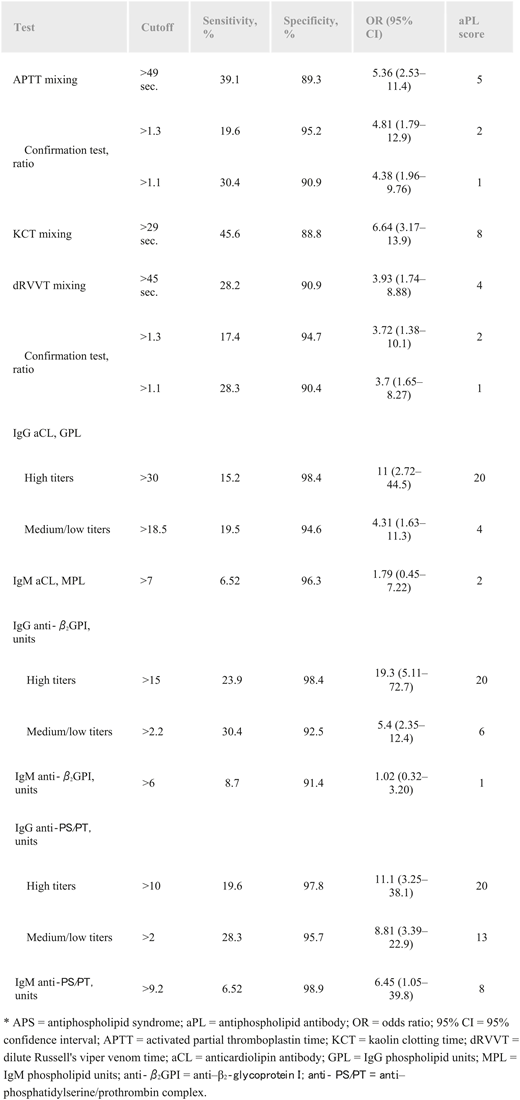
The antiphospholipid score (aPL-S) was devised by a Japanese group. Twenty-four patients with SLE with APS and 89 without were part of the total group of 296 patients. The study included 3 tests for lupus anticoagulant (activated partial thromboplastin time, kaolin clotting time, and dRVVT) and 6 solid-phase antiphospholipid assays (including IgG aCL, IgM aCL, IgG anti–β2-glycoprotein I, IgM anti–β2-glycoprotein I, IgG anti-PS/PT, and IgM anti-PS/PT). The laboratory tests were performed on the first set of patients (77 with SLE, 16 with APS with SLE, and 140 without SLE) to assess the diagnostic value of the aPL-S, and then the aPL-S was evaluated retrospectively to assess the risk of developing thrombosis. The IgG assays were given different scores depending on high vs medium/low measurements (Figure 1). The area under the receiver operating characteristic curve was 0.752. An aPL-S score ≥30 was an independent risk factor for thrombosis (P = .006).39 This score is not widely used, because most laboratories do not perform all the included tests.
Relative risk of clinical manifestations of APS for each aPL-S test. Reprinted from Otomo et al39 with permission.
Relative risk of clinical manifestations of APS for each aPL-S test. Reprinted from Otomo et al39 with permission.
Global Antiphospholipid Syndrome Score
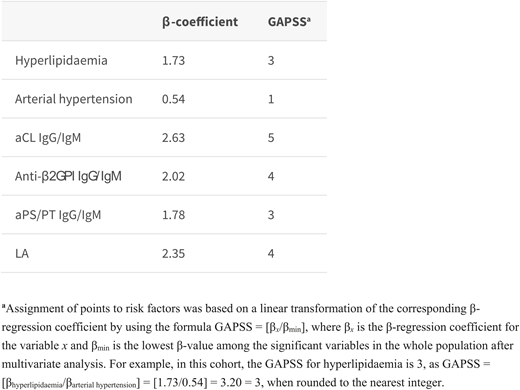
An Italian group combined antiphospholipid antibodies (aCL, anti–β2-glycoprotein I, anti-PS/PT, and lupus anticoagulant) and independent cardiovascular risk factors (hyperlipidemia and arterial hypertension) to create the Global Antiphospholipid Syndrome Score (GAPSS). GAPSS cutoffs of 10 or higher were statistically significant. An adjusted version of GAPSS omitted anti-PS/PT (Figure 2).40 Pooled studies were analyzed later by Sciascia et al.41 Many of these pooled studies included patients with and without SLE.40,42-44 The highest levels of GAPSS were in patients with arterial thrombosis.
Multivariate logistic regression analysis for the development cohort and scoring system. Reprinted from Sciascia et al40 with permission.
Multivariate logistic regression analysis for the development cohort and scoring system. Reprinted from Sciascia et al40 with permission.
Toward an SLE thrombosis risk equation
None of the existing APS thrombosis risk formulas—triple positivity, aPL-S, or GAPSS—incorporates the concept of complementopathy in thrombosis risk. SLE is the prototypic autoimmune disease representing the role of complement, in terms of both low levels of complement and of complement activation. Low complement is even part of the SLICC classification criteria for SLE.10
A 3-variable thrombosis risk equation was constructed for SLE. Thrombosis within 5 years of the variable testing was the outcome. The first variable was lupus anticoagulant because it is the most important antiphospholipid antibody for thrombosis in SLE.45,46 In addition, it is the most important for APS pregnancy morbidity.4 If the patient was unable to have lupus anticoagulant tested, then anti-PS/PT was shown also to be associated with thrombosis. The second variable was low C3. (Low C4 in SLE does not necessarily equate to complement consumption, because it can occur as a result of genetic deletion.)
The third variable was complement split products bound to platelets. It has long been recognized that platelets are part of APS, with thrombocytopenia being one of the potential clinical manifestations. Antiphospholipid antibodies can bind to and activate platelets.47-49 However, the complement split product C4d bound to platelets is a new assay developed by Navratil et al.50 Their group showed that platelet C4d was associated with both ischemic stroke (P = .002) and pulmonary emboli (P = .007) in SLE.51 This association has been confirmed for venous and arterial thrombosis in the Hopkins Lupus Cohort.52 This is likely not limited to SLE, because platelet C4d was associated with stroke severity (P = .003) and with stroke subtypes (cardioembolic and large artery atherosclerosis) in a general population study.53 In a recent study that included both primary and secondary APS as well as aPL-positive SLE, cell-bound C4d (on both red cells and platelets) was shown to be a marker of complement activation in APS.54
In the Hopkins Thrombosis Risk Score, each component was associated with thrombosis in the past 5 years: abnormal platelet C4d (odds ratio, 8.4), low C3 (odds ratio, 9.5), and lupus anticoagulant (odds ratio, 5.42) (all P < .005). The composite risk score was higher in the presence of thrombosis (1.93) than without thrombosis (P < .01). In particular, it was higher in venous than in arterial thrombosis. Adding arterial thrombosis risk factors did not improve the risk score. The Hopkins Thrombosis Risk Score is derived only from patients with SLE and needs to be evaluated by other centers and assessed prospectively as well. In the present clinical case, platelet C4d measurement was not performed, but the patient’s low complement would increase her risk beyond having lupus anticoagulant alone.
Conclusions
The role of complement is sufficiently well defined in APS, both primary and secondary, that it is time to include it both in assessing risk and in picking the best treatment on the basis of mechanism of action. The modified Ham test can help to define complementopathy in urgent clinical situations, such as CAPS and HELLP, but it is not widely available. The Hopkins Thrombosis Risk Score for patients with SLE requires prospective and external validation. It is the first risk score to include the role of complement.
Acknowledgments
The Hopkins Lupus Cohort is funded by National Institutes of Health grant R01AR069572. Hopkins antiphospholipid research is also funded by the Gene D. Rubin Trust and the Sally Nyborg Foundation for Lupus Research.
Correspondence
Michelle Petri, Division of Rheumatology, Johns Hopkins University School of Medicine, 1830 E. Monument St, Suite 7500, Baltimore, MD 21205; e-mail: mpetri@jhmi.edu.
References
Competing Interests
Conflict-of-interest disclosure: M.P. is a consultant to and receives grant support from Exagen Inc.
Author notes
Off-label drug use: None disclosed.