Abstract
Myelodysplastic syndromes are clonal myeloid neoplasms that primarily present in older adults. Although leukemia develops in approximately 25% to 30% of individuals, the significantly shortened survival in this population is attributed more commonly to nonleukemic causes. The current prognostic scoring systems for leukemia and overall survival based on disease characteristics are becoming increasingly sophisticated and accurate with the incorporation of molecular data. The addition of patient-related factors such as comorbidity, disability, frailty, and fatigue to these new models may improve their predictive power for overall survival, treatment toxicity, and health care costs. To improve the generalizability of clinical trial results to the real world, geriatric assessment testing should become a standard of care in MDS clinical trials.
Learning Objectives
Review the evidence that patient-related factors add independent value to current prognostic models
Discuss the concept of frailty assessment and present several clinical tool options
Envision a future of personalized prognostic scoring systems in MDS that are derived from big data sets using computer algorithms and the most important disease- and patient-related factors
Clinical case
A 70-year-old male retired English professor is referred with moderate pancytopenia. His laboratory values are hemoglobin 8.2 g/dL, platelets 98 × 109/L, absolute neutrophil count 2.0 × 109/L, mean corpuscular volume 107 fL, red cell distribution width 16%, lactate dehydrogenase 400 IU/L, and creatinine clearance 50 mL/min. He received his second transfusion 3 weeks ago. His medical history includes coronary artery disease with 2-vessel stenting 1 year ago for angina, type 2 diabetes on oral hypoglycemics, and a history of low-risk localized prostate cancer treated with prostatectomy >5 years ago without any biochemical/radiologic recurrence. He denies neuropathy or nephropathy. He does not exercise in any dedicated fashion other than routine walking. He is independent of all activities of daily living (ADLs) and instrumental activities of daily living (IADLs) but reports feeling fatigued or slowed, limiting his activities. You grade his Eastern Cooperative Oncology Group (ECOG) performance status as 1.
He is moderately overweight, with a body mass index of 30 kg/m2. Current medications include metformin, aspirin, metoprolol, and rosuvastatin. A bone marrow aspirate and biopsy reveals dyserythropoesis and dysmegakarypoesis, blasts of 7%, and abnormal localization of immature precursors. His cytogenetics reveal trisomy 8, and his next-generation sequencing results from a 40-gene panel reveal TET2 mutation (variant allele frequency 40%) and RUNX1 mutation (variant allele frequency 25%). He is diagnosed with myelodysplastic syndrome (MDS) with excess blasts type 1.
What is his prognosis?
Clinical prognostic scoring systems in MDS
There is no shortage of clinical prognostic scoring systems that incorporate disease-related characteristics in MDS primarily focusing on cytopenias, cytogenetics, bone marrow blast percentage, World Health Organization (WHO) classification, and molecular mutations.1-4 They are used to predict leukemia-free survival (LFS) and overall survival (OS). Age is an additional significant prognostic factor, with MDS patients >75 years having a 40% greater risk of dying than younger individuals over a 27-month period,5 and it remains an independent risk factor incorporated into the international prognostic scoring system revised (IPSS-R) and the MD Anderson prognostic scoring system.3,4 According to the most commonly used scoring systems in clinical trials, our patient’s disease has an IPSS score of 1.5 (high-intermediate, median OS 1.2 years) and an IPSS-R score of 6 (high risk, median OS 1.6 years). Factoring in his RUNX1 mutation, his prognosis is predicted to be slightly worse,6,7 although the impact of comutations, variant allele frequency, and other clinical characteristics is still being resolved in an international effort6 without a “ready for use” deployable tool.
One critical limitation of these scoring systems for predicting OS is the primary focus on the disease (the seed) with neglect of the host factors (the soil) in which the disease has taken hold. If we are going to personalize our prognostic scoring systems and therapies based on dynamic disease biology, we must also personalize our predictive and prognostic scoring systems based on dynamic host biology.
Why is this important? MDS is predominantly a disease of the older adult, with a median age of presentation of 71 to 76 years,8,9 with only 6% cases diagnosed in those aged ≤50 years.9 An MDS diagnosis carries a significant increase in mortality that worsens with male sex, increasing age, and IPSS-R score. For our 70-year-old patient case, his survival is expected to be 43% that of age-matched controls in the United States.10 This excess in mortality appears predominantly driven by factors unrelated to leukemic transformation. A large Spanish retrospective registry-based study documented a continued increase in excess mortality in recent years, despite the advent of hypomethylating agents and increased accessibility of allogeneic stem cell transplants. This might be ascribed to a parallel increase in age at diagnosis (from 70 in the years of 1980-1999 to 76 after 2006) and associated comorbidities.11 In addition, predicting clinical outcomes for individual patients based on published trials in MDS may be erroneous, since older adults are underrepresented in clinical trials evaluating patients with hematologic cancers,12,13 and patients in oncology trials tend to have better performance status and fewer comorbidities and remain on cytotoxic chemotherapy for longer durations.14
“Real-life” data demonstrate that the clinical outcomes of new therapies in MDS usually fall short of those from clinical trials, with median survivals in high-risk MDS patients treated with azacitidine (AZA) ranging from 12 to 16.4 months15-19 instead of the expected 24 months in the practice-changing AZA-001 clinical trial.20
This is best exemplified by the Ontario, Canada experience with AZA. In an audit of 1101 patients with higher risk MDS/low-blast acute myelogenous leukemia (AML), the median survival was 11.6 months, and the median number of cycles received was 6 (instead of 9 cycles received on the AZA-001 trial). Ultimately, 30% of patients received <4 cycles of AZA with significantly inferior OS.21
Does the incorporation of patient-specific factors other than age into existing MDS risk assessment scores improve their performance?
Importance of comorbidity
Comorbidities increase with age, and a high prevalence of MDS patients have ≥1 comorbidities that either precede or follow their diagnosis. Commonly reported comorbidities and their frequencies are listed in Table 1 and are dominated by cardiac, endocrine, and other cancers. Comorbidities are significant, because they may affect therapeutic plans, tolerability, and outcomes in patients with cancer.22 They may also impact red blood cell transfusion thresholds, which are relevant to clinical trials in which transfusion independence is the primary end point. The independent impact of several comorbidity index scores have been evaluated in numerous, largely retrospective MDS studies summarized in Table 2. These include the Charlson Comorbidity Index (CCI),23 the Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI),24 the Adult Comorbidity Evaluation-27 (ACE-27),25 and the MDS-Comorbidity Index (MDS-CI).26 The independent contributions of CCI and HCT-CI to prognosis (for nonleukemic death and OS) have been demonstrated in different prognostic groups, making it difficult to determine which is the optimal comorbidity index to use. Some of the inconsistent findings are likely attributable to different disease and patient demographics, the use of disease-modifying treatments, and the imprecision of retrospective ascertainment. The most validated index is the MDS-CI. The MDS-CI derives from a large Italian cohort of MDS patients. Five comorbidities (cardiac, moderate to severe hepatic, severe pulmonary, renal, solid tumor) were found to be independently associated with the risk of nonleukemic death in multivariable analysis. A dynamic MDS-CI was developed composed of 3 risk groups (65% low, 29% intermediate, and 6% high) and predictive of OS and nonleukemic death independent of age, sex, WHO classification, cytogenetics, and transfusion dependency. Importantly, 32% had an increase of their MDS-CI over time, observed particularly in transfusion-dependent patients.26 Our patient would score as high risk on the MDS-CI with his 3 comorbidities, but this may be less relevant to him, because the additional refinement of prognosis by the MDS-CI was primarily seen in patients with very low-, low-, and intermediate-risk WHO prognostic scoring system (WPSS) and IPSS-R5 scores. This suggests that the clinical relevance of mild or moderate comorbidity is trumped by the severity of MDS. In contrast, the comorbidities’ refinement of prognosis in higher risk MDS in some studies might be attributed to their impact as competing causes of death, the restriction of therapeutic options or enrollment on clinical trials, and/or poor treatment tolerance.
Comorbidities and their frequency in MDS
| Study (N) . | Cardiac . | Vascular . | Diabetes endocrine . | Tumor . | Pulmonary . | Hepatic . | GI . | Psych . | Renal . | Any comorbidity, grades low, intermediate, high . |
|---|---|---|---|---|---|---|---|---|---|---|
| Bammer et al74 (616) | 25 | 7 | 12 | 10 | 5 | 3 | 6 | 2 | 2 | 48, 52, 25, 24 |
| Naqvi et al25 (600) | CVS 54 | — | 16 | 28 | 9 | — | 7 | 8 | 2 | 77, 42, 21, 14 |
| Della Porta et al26 (840 + 540) | 25 | 5 | 16 | 10 | 3 | 17 (mild 14) | 6 | 2 | 4 | 54, 65, 29, 6 |
| Balleari et al5 (318) | 24 | 2 | 21 | 15 | 4 | 5 | 1 | 6 | 4 | 56,62,31,7 |
| Zipperer et al30 (1161) | 37 | — | — | 10 | 9 | 4 | — | — | 7 | —, 50, 36, 14 |
| Breccia et al40 (418) | 38 | 6 | 18 | 6 | 6 | 5 | 6 | 2 | 5 | 93, 50, 25, 25 |
| Falantes et al75 (232) | 29 | 11 | 30 | 15 | 17 | 10 | — | — | 12 | 62, 44, 42, 14 |
| Study (N) . | Cardiac . | Vascular . | Diabetes endocrine . | Tumor . | Pulmonary . | Hepatic . | GI . | Psych . | Renal . | Any comorbidity, grades low, intermediate, high . |
|---|---|---|---|---|---|---|---|---|---|---|
| Bammer et al74 (616) | 25 | 7 | 12 | 10 | 5 | 3 | 6 | 2 | 2 | 48, 52, 25, 24 |
| Naqvi et al25 (600) | CVS 54 | — | 16 | 28 | 9 | — | 7 | 8 | 2 | 77, 42, 21, 14 |
| Della Porta et al26 (840 + 540) | 25 | 5 | 16 | 10 | 3 | 17 (mild 14) | 6 | 2 | 4 | 54, 65, 29, 6 |
| Balleari et al5 (318) | 24 | 2 | 21 | 15 | 4 | 5 | 1 | 6 | 4 | 56,62,31,7 |
| Zipperer et al30 (1161) | 37 | — | — | 10 | 9 | 4 | — | — | 7 | —, 50, 36, 14 |
| Breccia et al40 (418) | 38 | 6 | 18 | 6 | 6 | 5 | 6 | 2 | 5 | 93, 50, 25, 25 |
| Falantes et al75 (232) | 29 | 11 | 30 | 15 | 17 | 10 | — | — | 12 | 62, 44, 42, 14 |
CVS, cardiovascular system; GI, gastrointestinal; Psych, psychiatric.
Impact of comorbidities on OS
| Study, year (N) . | Prospective? Comorbidity scale(s) . | MDS score . | Predictive factors for OS/EFS by MVT analysis . | Novel composite prognostic score derived? . | Applicability/comments . |
|---|---|---|---|---|---|
| Bammer et al,74 2014 (616) | No, HCT-Cl, CCl | IPSS | HCT-CI, IPSS, age | No | HCT-CI scores higher in men than women; more CVD in men |
| Sperr et al,76 2013 (400) | No, HCT-CI | IPSS | HCT-CI, ferritin >900, age | Yes | |
| Zipperer et al,30 2008 (171) | No, HCT-CI, CCI | IPSS | HCT-CI, IPSS | No | Only in int-2 and high risk |
| Naqvi et al,25 2011 (600) | No, ACE-27 | IPSS | ACE-27, age, IPSS | Yes | Only in int-1, int-2, and high-risk disease; patients ≤age 65 |
| Daver et al,28 2014 (600) | No, ACE-27 | IPSS-R | ACE-27, age, IPSS-R | Yes | Only in int-, high-, and very high-risk patients of all ages; same cohort from Naqvi et al26 |
| Della Porta et al,26 2010 (840 derivation and 540 validation) | No, MDS-CI, CCI, HCT-CI | WPSS | MDS-CI, WPSS | No | Only in WPSS very low-, low-, and intermediate-risk disease |
| Balleari et al,5 2015 (318) | No, MDS-CI, HCT-CI | IPSS, IPSS-R | MDS-CI, IPSS or IPSS-R, age >75, male | No | Only in IPSS/IPSS-R int-2/int and high-, very high- risk patients |
| van Spronsen et al,29 2014 (222) | No, MDS-CI | IPSS, MDAS, WPSS, WPSSR, IPSSR | MDS-CI, IPSS-R, age, PS, fibrosis transfusions | No | Other MDS scoring systems were valid, but IPSS-R was best |
| Zipperer et al,30 2014 (1161) | No, MDS-CI | IPSS-R | MDS-CI, IPSS-R | No | Only when combined very low/low and intermediate-/high-/very high-risk |
| Breccia et al,77 2012 (418) | No, MDS-CI, CCI, HCT-CI | WPSS | CCI or MDS-CI, age >60, Hgb <8, Plt <50, bleeding | No | Higher MDS-CI and CCI scores associated with RBC transfusion dependency |
| Falantes et al,75 2016 (232) | No, MDS-CI | IPSS-R, IPSS | MDS-CI, age >75 y, diabetes on Tx, Hgb <10 | Yes | Focus on NLD; patient population restricted to IPSS-low and int |
| Buckstein et al,69 2016 (445) | Yes, MDS-CI, CCI, HCT-CI | IPSS-R, IPSS | CCI frailty, IPSS | Yes | In separate models, MDS-CI was also prognostic, but the model with CCI had the largest R2; frailty refined prognosis in all but IPSS-R very high risk |
| Study, year (N) . | Prospective? Comorbidity scale(s) . | MDS score . | Predictive factors for OS/EFS by MVT analysis . | Novel composite prognostic score derived? . | Applicability/comments . |
|---|---|---|---|---|---|
| Bammer et al,74 2014 (616) | No, HCT-Cl, CCl | IPSS | HCT-CI, IPSS, age | No | HCT-CI scores higher in men than women; more CVD in men |
| Sperr et al,76 2013 (400) | No, HCT-CI | IPSS | HCT-CI, ferritin >900, age | Yes | |
| Zipperer et al,30 2008 (171) | No, HCT-CI, CCI | IPSS | HCT-CI, IPSS | No | Only in int-2 and high risk |
| Naqvi et al,25 2011 (600) | No, ACE-27 | IPSS | ACE-27, age, IPSS | Yes | Only in int-1, int-2, and high-risk disease; patients ≤age 65 |
| Daver et al,28 2014 (600) | No, ACE-27 | IPSS-R | ACE-27, age, IPSS-R | Yes | Only in int-, high-, and very high-risk patients of all ages; same cohort from Naqvi et al26 |
| Della Porta et al,26 2010 (840 derivation and 540 validation) | No, MDS-CI, CCI, HCT-CI | WPSS | MDS-CI, WPSS | No | Only in WPSS very low-, low-, and intermediate-risk disease |
| Balleari et al,5 2015 (318) | No, MDS-CI, HCT-CI | IPSS, IPSS-R | MDS-CI, IPSS or IPSS-R, age >75, male | No | Only in IPSS/IPSS-R int-2/int and high-, very high- risk patients |
| van Spronsen et al,29 2014 (222) | No, MDS-CI | IPSS, MDAS, WPSS, WPSSR, IPSSR | MDS-CI, IPSS-R, age, PS, fibrosis transfusions | No | Other MDS scoring systems were valid, but IPSS-R was best |
| Zipperer et al,30 2014 (1161) | No, MDS-CI | IPSS-R | MDS-CI, IPSS-R | No | Only when combined very low/low and intermediate-/high-/very high-risk |
| Breccia et al,77 2012 (418) | No, MDS-CI, CCI, HCT-CI | WPSS | CCI or MDS-CI, age >60, Hgb <8, Plt <50, bleeding | No | Higher MDS-CI and CCI scores associated with RBC transfusion dependency |
| Falantes et al,75 2016 (232) | No, MDS-CI | IPSS-R, IPSS | MDS-CI, age >75 y, diabetes on Tx, Hgb <10 | Yes | Focus on NLD; patient population restricted to IPSS-low and int |
| Buckstein et al,69 2016 (445) | Yes, MDS-CI, CCI, HCT-CI | IPSS-R, IPSS | CCI frailty, IPSS | Yes | In separate models, MDS-CI was also prognostic, but the model with CCI had the largest R2; frailty refined prognosis in all but IPSS-R very high risk |
CVD, cardiovascular disease; EFS, event-free survival; Hgb, hemoglobin; MVT, multivariate testing; NLD, nonleukemic death; Plt, platelet; PS, performance status; RBC, red blood cell; Tx, treatment.
Perhaps the severity of the comorbidity is more important than the actual tally. One comorbidity scale that evaluates severity is the ACE-27,27 a validated index for patients with cancer that categorizes diseases into 1 of 3 levels of comorbidity with the overall comorbidity score based on the highest ranked and graded single ailment. In a retrospective cohort study of 600 patients at MD Anderson, 4 categories of comorbidity further refined the survival of patients with IPSS-R intermediate-, high-, and very high-risk patients. A final combined multivariable survival model and weighted risk score was derived, including age, ACE-27 score, and IPSS-R resulting in 4 risk groups with survivals of 53, 27, 13, and 7 months, respectively. No correlation was found between leukemic evolution and severity of comorbidity.28 The patient discussed above has 4 comorbidities (coronary artery disease, diabetes, past cancer, and obesity), but all are graded mild; therefore, his ACE-27 score would be 1, and his composite score would be 7 (intermediate-2 [int-2]) with a predicted median survival of 13 months. Of note, this might still represent an underestimate of survival, since the combined survival model above was calculated from time of referral to MD Anderson and not diagnosis.28 The MDS-CI has also been validated by other groups to add independent prognostic refinement to the IPSS-R.5,28-30 It is interesting to note that MDS-related causes of death declined in one study as the MDS-CI scores increased,30 suggesting that optimizing life-limiting comorbidities such as cardiovascular disease might improve survival.
What is the best treatment for our patient?
Based on his higher risk IPSS/IPSS-R scores, a decision analyses31 and international guidelines that include the National Comprehensive Cancer Network32 and the European Leukemia network,33 our patient should be treated with a hypomethylating agent with consideration of a reduced-intensity allogeneic stem cell transplant. You recommend AZA to start and discuss the role of allogeneic stem cell transplant. Based on his hematopoietic stem cell transplant index (HCT-CI) score of 5, age, and high-risk disease, his projected 2-year non-relapse mortality is 39% following an allogeneic stem cell transplant.34,35 Based on this, many centers would discourage this approach in the absence of a clinical trial.36 With these statistics in hand, the patient is not interested in an allogeneic stem cell transplant but would consider a hypomethylating agent if it was well tolerated and could extend his life.
Will our case patient tolerate AZA, and what will be his expected OS?
Several tools that use data from a geriatric assessment, such as the Chemotherapy Risk Assessment Scale for High-Age Patients and the Cancer and Aging Research Group calculators, have been developed to assist in the prediction of chemotherapy toxicity, but their derivation was primarily in solid tumor patients, so their applicability to MDS patients is unproven.37,38
Performance status and comorbidities also offer prognostic value for patients receiving AZA therapy. ECOG performance status ≥2 was found to be an independent component of the French prognostic score for OS developed in AZA treated patients with higher risk disease in addition to circulating blasts, transfusion dependency ≥4 units/8 weeks, and karyotype.17 However, only a minority (20%) of patients in the French AZA registry fell into this poor performance status category. Because our patient’s ECOG performance status is 1, he would fall into the French intermediate risk category (score 1-3) with a predicted median OS of 15 months. Performance status was also important at predicting outcome with AZA in the German PIAZA study. ECOG performance status (PS) of even 1 was associated with a progression-free survival that was 50% that of ECOG PS 0 (9.8 months vs 18.4 months, P = .002) in 150 patients with higher risk MDS, low-blast AML, or chronic myelomonocytic leukemia.39
Comorbidity may also impact treatment outcome with AZA. The MDS-CI has been shown to effectively risk stratify the OS from diagnosis of 60 patients treated with AZA (20 months for MDS-CI low risk, 12 months for MDS-CI intermediate risk, and 8 months for MDS-CI high risk (our patient).40
With AZA treatment, do comorbidities increase the probability of unplanned emergency room visits or hospitalizations?
What additional health costs are borne by a public payer unrelated to AZA drug cost in patients with comorbidities (1 Canadian dollar = 0.74 US dollars in 2019)?
Comorbidity assessed using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) may also be useful in predicting the tolerability and health care resource utilization associated with AZA. The ADG is a person-focused, diagnosis-based method of categorizing illnesses, often for large administrative database studies for predicting 1-year mortality in general ambulatory populations and health resource utilization.41 Individual diseases or conditions are placed into a single ADG based on 5 clinical dimensions: duration of the condition, severity, diagnostic certainty, etiology, and specialty care involvement. The 32 possible ADGs are derived from both inpatient health administrative data as well as ambulatory health care data derived from physician billing claims.42 In an Ontario provincial registry using public administrative databases, we found that only transfusion dependence (hazard ratio [HR], 1.3; P = .0016) and a higher ADG score group of 10 to 12 (HR, 1.3; P = .0025) and 13+ (HR, 1.6; P < .0001) compared with 0 to 7 were associated with a higher number of emergency room visits, any hospitalization within the first 6 months of treatment, and a shorter time to hospitalization in 877 higher risk MDS/low-blast AML patients treated with AZA. A higher ADG comorbidity score (13+) was further predictive of increased health care spending ($2029 above the base estimate of $13 599 per cycle of AZA, excluding drug cost).43
What are the limitations of using comorbidity and PS exclusively for “staging the aging”?
Poorer performance scores reliably identify patients at risk for more adverse outcomes; however, a minority (20% to 30%) of patients in MDS clinical trials and registries have Karnofsky performance scores of <8044 or an ECOG PS that exceeds 1. Furthermore, 38% of patients with a (physician-judged) PS of 0 to 1 have been found to have some disability on more careful geriatric testing.45 Comorbidity is a component but not a perfect surrogate of PS and “fitness.” Not all patients with high comorbidity scores are “unfit,” and not all unfit patients have high comorbidity scores.
What is the impact of patient-reported outcomes on survival?
The quality of life in MDS patients is inferior compared with age-matched controls. The EL-NET investigators discovered the pronounced symptom burden experienced by many patients with MDS, predominantly in the dimensions of pain/discomfort, mobility, anxiety/depression, and usual activities.46 Fatigue is one of the most commonly reported troublesome symptoms.47,48 In addition to being a symptom that contributes negatively to quality of life, this patient reported outcome may, in and of itself have prognostic deterioration value for OS. In one study of higher risk MDS patients, every 10 points of fatigue (evaluated by the EORTC QLQ-C30), was associated with inferior survival (HR, 1.11-1.13) independent of the IPSS, IPSS-R, and WPSS risk scoring systems.49 In a follow-up study that included validation in an independent cohort of higher risk MDS patients at Dana Farber, a fatigue score >45/100 provided the best result in distinguishing OS between patients with IPSS int-2 and high-risk disease. In fact, patients with IPSS int-2 and high fatigue and patients with IPSS high risk and low fatigue were found to have similar median OS of 15 to 16 months, allowing for the generation of a new fatigue-IPSS risk score in high-risk patients that reclassified them into 3 risk groups of 23 months, 16 months, and 10 months survival, respectively.50
Are geriatric assessment tests feasible and do they add value in MDS?
The gold standard for detecting the older patient’s vulnerability and frailty to poor outcome and treatment complications is the comprehensive geriatric assessment (CGA), which is strongly recommended by the International Society of Geriatric Oncology for cancer patients aged >70 years.45 The CGA is multidimensional and encompasses the following domains: cognition, mood, ADLs, IADLs, mobility, polypharmacy, nutritional status, and social support. In a prospective study from Freiburg, Germany, Deschler et al found it feasible to administer 8 geriatric assessment instruments in 195 patients aged ≥60 years with MDS (n = 63) or AML (n = 132). Patients with any impairment in ADL, a Karnofsky index <80%, and a fatigue score (European Organization for Research and Treatment of Cancer quality of life questionnaire [QLQ-C30]) of ≥50 had significantly worse OS than those treated with hypomethylating agents or best supportive care independent of cytogenetics, blasts, and comorbidity.51 An Australian study demonstrated the feasibility of nurse-led “tailored” CGA in 98 patients with MDS and oligoblastic AML. Only 28% had ECOG scores of ≥2, yet 78% had ≥1 impairments in CGA domains indicating functional, cognitive, or psychological impairments. This culminated in 64% being referred to geriatricians for impactful “fine-tuning.” Inability to complete IADLs and ADLs were independently poor prognostic factors of OS. IADL impairment in the 25 evaluable patients who received AZA was associated with significantly shorter survival (6 vs 19 months, P < .001), a higher failure rate to complete 6 cycles of AZA (71% vs 17%, P = .01), and a lower number of cycles of AZA (3.7 vs 12; P < .001). IADL impairment predicted a higher complication rate as well, with 50% ceasing treatment due to recurrent infections, prolonged hospital admissions, or poor tolerance compared with 17% ceasing treatment if they were IADL independent.52 In Canada, IADL dependence was also 1 of the 3 significant variables independently predictive of OS in 239 lower risk, transfusion-dependent MDS patients in addition to IPSS-R and the receipt of iron chelation therapy. Its importance in the survival model is evidenced by an HR that matched that of IPSS-R higher risk (HR, 5; P = .0001).53 Fortunately, our patient has no ADL or IADL impairment to negatively impact his ability to complete an adequate trial of AZA.
Simple considerations such as nutrition/inflammation and walking ability may also be valuable. In a retrospective study from Dana Farber of 114 patients with largely higher risk MDS, ease at taking a long walk (no trouble vs some trouble; OS 53 vs 21 months) and low serum albumin (<3.5 g/dL; OS 4 vs 26 months) were found to be powerful independent prognostic factors of “equal impact” to the disease-related factors in a survival model.54 The prognostic importance of a low serum albumin (<3.4 g/dL) for both OS and LFS was also demonstrated in a cohort of 200 MDS patients from Turkey.55
What is the impact of frailty?
Frailty is defined as a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function that increases an individual’s vulnerability for developing increased dependency and/or death.56 Cancer may contribute to or accelerate the frailty syndrome, and it may be an outcome of cancer treatment. Frailty has been associated with increased mortality in older patients with cancer, and its relevance in the management of hematologic malignancy, including MDS, has been excellently reviewed recently.57 The 2 leading theories of frailty’s pathophysiology include the frailty phenotype and the accumulated deficit theories.58 In the frailty phenotype theory, frailty arises from aging-related cellular and physiological changes that lead to a condition of vulnerability. The components include 5 criteria: weight loss, low physical activity, weak grip strength, slow gait speed, and exhaustion.59 The accumulated deficit theory postulates that vulnerability results from accumulated medical, physical, and social conditions that drive the increased vulnerability observed in frailty.60 The concept is that a global system loses robustness as it develops various illnesses or functional declines, termed “deficits,” and includes comorbidities and disability as deficits of age. An individual’s frailty index is expressed as a ratio calculated as the number of deficits an individual has over the number of deficits considered in the index (usually a minimum of 30). It is scored as 0 to 1, with the lower end of the scale reflecting the robust and resilient and the upper end of the scale reflecting the vulnerable and frail.61
The best way to detect vulnerability and frailty is with a CGA. However, outside a clinical trial, a CGA may be too time consuming and resource intensive to deploy routinely, and there are a limited number of geriatricians.
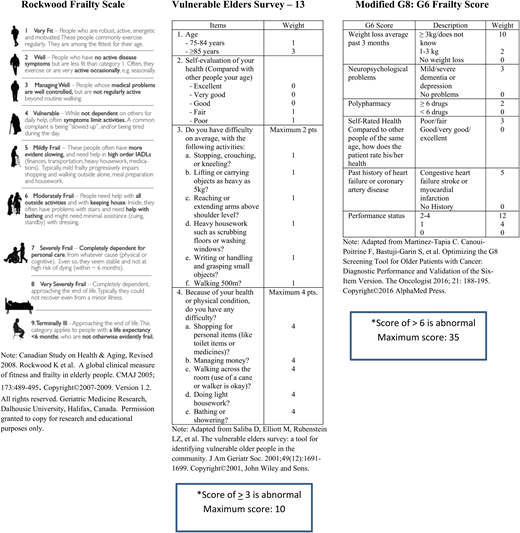
Fortunately, there are available a number of quickly completed frailty screening tools endorsed by the International Society of Geriatric Oncology62 with the caveat that there is no “one tool fits all” that provides the optimal combination of sensitivity and specificity to identify older persons with an abnormal CGA.63 Nevertheless, the consensus is that using a validated frailty tool such as the Vulnerable Elders Survey-13,64 the Clinical Frailty Scale,65 the modified Geriatric-866 (Figure 1) and others58 may be useful to identify the vulnerable and frail at risk for adverse outcomes67 and worthy of further assessment/intervention.
(A) Geriatric Medicine Research, Dalhousie University, Halifax, Canada. Canadian Study on Health & Aging, Revised 2008. Version 1.2. Adapted from Rockwood et al65 with permission. Permission granted to copy for research and educational purposes only. (B) Adapted from Saliba et al64 with permission. (C) Adapted from Martinez-Tapia et al66 with permission.
(A) Geriatric Medicine Research, Dalhousie University, Halifax, Canada. Canadian Study on Health & Aging, Revised 2008. Version 1.2. Adapted from Rockwood et al65 with permission. Permission granted to copy for research and educational purposes only. (B) Adapted from Saliba et al64 with permission. (C) Adapted from Martinez-Tapia et al66 with permission.
In one prospective study of patients with hematologic cancers that included MDS (23%) and AML (29%), an abnormal G8 (score of ≤14/17) was an independent risk factor of mortality in addition to a diagnosis of AML, impaired mobility, and risk of malnutrition. Of particular interest was the finding that the survival of patients with an abnormal G8 was short at 7.6 months irrespective of whether the patient received attenuated or no therapy versus standard therapy based on physician judgement.68
The Rockwood Clinical Frailty Scale (CFS), which uses clinical descriptors and pictographs, was developed to provide clinicians with an easily deployable tool that stratifies older adults according to level of vulnerability and divides patients into 9 levels of vulnerability ranging from 1 (very fit) to 9 (terminally ill) (Figure 1). The CFS was validated in a sample of 2305 older participants from the Canadian Study of Health and Aging with each category increment of the scale increasing the medium-term risks of death and entry into an institution.65 The MDS registry of Canada evaluated the impact of prospectively assessed frailty, comorbidity, disability and physical performance on OS and found that the incorporation of frailty (using the Rockwood scale) and comorbidity (CCI) improved the risk stratification of the IPSS-R by 30% and 5%, respectively, in a population of 445 MDS patients. A limitation of the derived composite prognostic score was the dichotomous definition of frailty (1-3 vs ≥4) necessitated by the smaller sample sizes of patients falling into scores of ≥4 (25%).69 While still impactful and informative, this prevented the more nuanced comparison of not frail (0-3) to vulnerable (4) to moderately frail (5-6) and severely frail (7-9) patients. Our patient would have been assigned a frailty score of 4, since he endorsed that symptoms limited his activities. In the Canadian composite risk score, he would have fallen into a high-risk group with a projected median survival of only 9.4 months. The Rockwood CFS has also been shown to add independent prognostic value to the IPSS-R and the CCI in a retrospective cohort of 118 patients diagnosed with MDS in a Japanese hospital. In this study, a CFS of ≥5 predicted a shorter 1-year OS (51% vs 90%, P < .001) and a higher incidence of infection-related mortality.70 An MDS frailty index based on cumulative deficits has been developed by the Canadian MDS registry.71
Based on his CFS of 4 and MDS-CI of 4, what is the probability that our patient will remain on AZA for 4 or more cycles, and what is his expected survival?
In the Canadian MDS registry, the survival of the 63 out of 313 (20%) patients completing less than 4 cycles AZA was considerably less at 4 months (95% confidence interval [CI], 4-8) compared with 25 months (95% CI, 21-27) for ≥4 cycles. A CFS of ≥4 was also associated with shorter OS in the IPSS-R higher risk patients (10 vs 19 months, P = .04). The factors predictive of completing ≥4 cycles included age, hemoglobin, platelet count, and MDS-CI, but by multivariable analysis, only MDS-CI was predictive. An MDS-CI score of ≥2 was particularly discriminating (HR, 8.4; 95% CI, 2.3-33; P = .001).72
In summary, our patient’s CFS of 4 and high-risk MDS-CI would predict for a lower probability of completing 4 cycles of AZA and a survival of only 10 months if treated with AZA (instead of the predicted 19 months survival attached to his high IPSS-R score). This knowledge might impact the patient’s decision and/or the physician’s recommendation to pursue AZA treatment.
Conclusions and future directions
The seed and the soil are inextricably intertwined. The MDS prognostic scoring systems are effective for estimating LFS and OS and will be improved further with the inclusion of molecular data. The OS estimates could be improved by the inclusion of patient factors. This is essential, since nonleukemia deaths predominate, the median age at diagnosis is increasing, and more therapies are emerging. Physicians are imperfect evaluators of “fitness.” There is compelling evidence in MDS that supports some form of geriatric assessment at diagnosis and prior to the initiation of therapy. Screening geriatric tools may identify areas of vulnerability, predict toxicity and survival, facilitate health interventions, and help guide shared decision making. Many of these screening tools (like IADL, EQ-5D, fatigue, and frailty) are not time consuming, and some components can be completed by the patients at home or in the clinic waiting rooms. Geriatric screening tools should be incorporated into all MDS clinical trials so that we can better discern (in parallel with more nuanced assessment of their methylome and genome) the patients who benefit from and tolerate therapy.
We have entered the era of big data and artificial intelligence. Last year, Nazha et al presented a more robust and validated personalized prediction model developed with artificial intelligence and machine learning algorithms from a training cohort of 1471 patients and validated in a cohort of 831 patients composed of 23 clinical variables (1 was age) inclusive of 11 somatic mutations with significantly better predictability than existing prognostic scoring systems for OS and LFS.73 It is probable that its predictability for OS could be further improved with the inclusion of some geriatric tests and/or comorbidities. Software programs that can independently extract diagnoses and laboratory values from clinical electronic patient records to create databases already exist. We should aspire toward an automated calculation of patients’ frailty, comorbidity, and chemotherapy risk scores displayed in electronic patient records next to routine laboratory results and the derivation of personalized prognostic scores using the most impactful patient and disease elements.
References
Competing Interests
Conflict-of-interest disclosure: R.J.B. has received research funding from Celgene and Otsuka, and honoraria from Celgene.
Author notes
Off-label drug use: None disclosed.