Abstract
Lower-risk myelodysplastic syndromes are defined using prognostic scoring systems that incorporate data on bone marrow blast percentage, degree and numbers of cytopenias, and cytogenetic abnormalities. Increasingly, these are incorporating molecular abnormalities to further refine risk. Therapy is geared toward predominating cytopenias, with erythropoiesis-stimulating agents luspatercept and lenalidomide used to ameliorate anemia, romiplostim and eltrombopag tackling thrombocytopenia, and hypomethylating agents and antithymocyte globulin palliating pancytopenia. Newer agents on the horizon are abrogating the downstream sequelae of specific molecular mutations. One challenge for the future is in further modifying response criteria to align with improvements that are clinically meaningful to patients.
Learning Objectives
Describe the current treatment paradigm and outcomes with use of agents for lower-risk myelodysplastic syndromes
Provide insight into novels agents (ie, targeted therapy) in clinical development for lower-risk myelodysplastic syndromes
Clinical case
A 72-year-old woman presented with isolated macrocytic anemia (hemoglobin, 7.8 g/dL) associated with worsening fatigue, requiring a packed red blood cell (pRBC) transfusion every 4 weeks. She described walking as “feeling like my legs were encased in cement.” Laboratory workup, including iron studies, evaluation for nutritional deficiencies, paroxysmal nocturnal hemoglobinuria flow cytometry, protein electrophoreses, and hemolysis were unrevealing. Her erythropoietin level was 80 mIU/mL. A bone marrow biopsy revealed erythroid dysplasia in 25% of ringed erythroid cells, consistent with myelodysplastic syndromes (MDS) with single lineage dysplasia-ring sideroblasts (< 5% marrow blasts). The patient had a normal, 46 (XX) karyotype. Molecular studies on the bone marrow aspirate revealed the SF3B1 mutation, with a variant allelic frequency of 26%. She had an International Prognostic Scoring System (IPSS) score of 0 (low risk) and an IPSS revised (IPSS-R) score of 2.0 (low risk). She was started on an erythropoiesis-stimulating agent (ESA; darbepoetin alfa at 500 μg every 3 weeks) with a robust erythroid response according to modified International Working Group (IWG) response criteria, remaining transfusion independent with hemoglobin >10.5 g/dL for 13 months. Then, her hemoglobin started to worsen.
Introduction, and the notion of risk
MDS are a heterogeneous collection of hematopoietic stem cell disorders in which a clonal mutation is identified in more than 90% of patients.1,2 The yearly incidence rate for MDS in the United States is at least 4.6 per 100 000 people, translating to over 20 000 new diagnoses.3 They are typically divided into lower- and higher-risk subtypes in an approximate two-thirds to one-third ratio of new diagnoses, with risk indicating likelihood of evolution to acute myeloid leukemia (AML) and truncated survival, defined according to prognostic scoring systems.4 The most commonly used for this purpose are the IPSS and its 15-year-younger revised successor (IPSS-R).5,6 These apply progressively higher “scores” to patients with greater numbers of or worse cytopenias, higher blast percentages, and worse cytogenetics. Thus, patients with a paucity of cytopenias (ie, hemoglobin of 10.1 g/dL and normal neutrophil and platelet counts), blasts <5%, and relatively favorable cytogenetics [ie, normal, −Y, or del(20q)], giving them a score of <1.5 in the IPSS and ≤3.5 in the IPSS-R, would be classified as having lower-risk MDS, whereas patients with scores higher than these have higher-risk disease.
The world of MDS would be complicated enough if we stopped right there. But these systems have their limitations, in being developed and validated in untreated patients, with an accuracy that deteriorates in patients receiving therapy; excluding therapy-related MDS; and being agnostic to molecular abnormalities, which can sway risk dramatically. Newer approaches to risk assessment incorporating molecular lesions into the IPSS-R using standard, multivariate analysis approaches that give a weight to IPSS-R score, age, and specific mutations (EZH2, SF3B1, and TP53) raise the C-index of the scoring systems (a measure of accuracy) in predicting overall survival (OS) from 66% to 68% to 70%, which appears numerically slight, but results in substantive separation of survival curves.7 Taking the level of statistical sophistication a step further, when machine-learning techniques focused on random survival forest algorithms are used to assess clinical, pathologic, and molecular variables, the C-index jumps to 74%.8 Given the complexity of interactions among germline and somatic mutations and their intersection with clinical data, we speculate that future risk assessment for prognosis and optimal therapy will require entering data to a secure website that can use machine-learning analytics to assess prognosis in patients regardless of therapy, and, dynamically, over the course of a patient’s MDS lifespan.
For the purpose of this paper, we define lower-risk MDS as those patients classically defined as such using the IPSS or IPSS-R, also recognizing that mutations such as SF3B1 shift risk in a beneficial direction, whereas lesions such as TP53, EZH2, ASXL1, RUNX1, STAG2, etc move risk in a deleterious direction.9
Ameliorating anemia
The majority of patients with lower-risk MDS have some degree of anemia. Anemia in these patients can be divided into those with no transfusion needs but a compromised hemoglobin level; those with a low transfusion burden, defined as transfusion of 3 to 7 units of pRBCs in a 16-week period; and those with a high transfusion burden requiring ≥8 units of pRBCs in the same period.10 No prospective trial of a drug to treat lower-risk MDS has ever demonstrated a survival advantage for that drug compared with continued transfusion support, nor has early initiation of a drug. Thus, for those with no transfusion burden and good quality of life, as defined by the patient, with disease that might instead be referred to as a “mild displeasure syndrome,” regular observation and delay in treatment is appropriate.
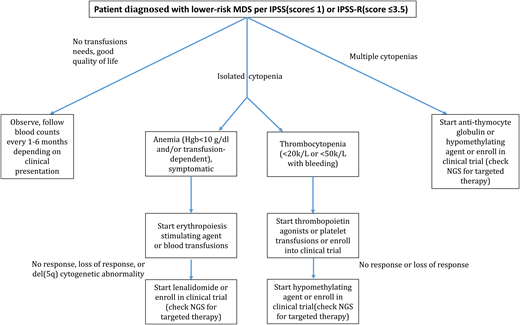
For those with low-transfusion-burden MDS, treatment should only be initiated if it is less burdensome to the patient than the transfusions themselves. Thus, a patient receiving 1 unit pRBC every 5 weeks may prefer this to an injection of a drug every 3 weeks. Alternatively, patients with frequent transfusion needs and/or a compromised quality of life will likely appreciate a therapeutic intervention, the most common being an ESA (Figure 1).
Treatment algorithm for lower-risk MDS. *Use of growth factors for patients with isolated neutropenia is not supported.
Treatment algorithm for lower-risk MDS. *Use of growth factors for patients with isolated neutropenia is not supported.
ESAs are approved by most regulatory agencies for chronic anemia, though not specifically for MDS. For all comers, response rates to ESAs range from 20% to 40%, and tend to be higher in those with low baseline serum erythropoietin levels (<500 U/L, ideally <100 U/L) and minimal or no transfusion needs.11,12 One recent study randomized 147 low (42%) or no (58%) transfusion burden, lower-risk MDS patients in a 2:1 ratio to receive darbepoetin alfa (dosed at 500 μg every 3 weeks or escalated to every 2 weeks) or placebo.13 During the first 24 weeks (the blinded portion of the study), the transfusion incidence for those receiving darbepoetin was 36%, compared with 59% for the placebo group (P = .008). Formal hematologic improvement occurred in only 14.7% of darbepoetin-treated patients, compared with 0% in the placebo group (P = .016). Quality of life did not differ between the arms. During the 48-week open-label period of the study, hematologic improvement rates rose to 35% for those treated with darbepoetin. The mean response duration was 235 days (SE, 21 days).
Thus, though easy to use, and appealing to patients and practitioners as they are not a type of chemotherapy and have been available for use for decades, ESAs are not a panacea in treating MDS. Once these agents fail patients, a limited number of options remain.
Iron chelation, while heavily promoted, has never been shown prospectively in a randomized trial to prolong OS. Single-institution studies, or those with historical controls of nonchelated patients, suffer from selection biases for which statistical adjustment of potential contributing variables is insufficient. Thus, although chelation can be considered in lower-risk MDS patients with heavy transfusion burdens who have end-organ evidence of iron deposition, its use should be judicious, and rare.
The investigational drug luspatercept is an ActRIIB/IgG1 Fc fusion protein that blocks transforming growth factor β ligands to inhibit aberrant Smad2/3 signaling, thus enhancing late-stage erythropoiesis. In a single-arm study of 58 anemic, lower-risk MDS patients (11 of whom had no transfusion burden), luspatercept was administered at doses of up to 1.75 mg/kg every 3 weeks.14 Of 42 evaluable patients, 16 (38%) achieved pRBC transfusion independence, whereas 32 of 51 patients receiving higher concentrations of luspatercept (61%) achieved hematologic improvement. This number rose to 77% in SF3B1-mutated patients, the molecular abnormality associated with ring sideroblasts.
Consequently, a follow-up trial randomized lower-risk, transfusion-dependent MDS patients with ring sideroblasts, most of whom had the SF3B1 mutation, 2:1 to receive luspatercept or placebo.15 Patients had either been exposed to an ESA that had failed them, or were unlikely to respond with a high serum erythropoietin level. Those treated with luspatercept achieved a pRBC transfusion independence response rate lasting 8 weeks or longer, the study’s primary end point, of 38%, compared with 13% for those receiving placebo (P < .0001), for an absolute difference of 25%. The median duration of response was 31 weeks (range, 21-41 weeks). Adverse events appeared broadly comparable between the luspatercept and placebo arms.
Clinical case (continued)
With worsening anemia now requiring pRBC transfusions and loss of response to ESA therapy, a repeat bone marrow evaluation was performed with concerns of progressive disease. It continued to show isolated oligoblastic erythroid dysplasia with ring sideroblasts, but now with a previously cryptic del(5q) cytogenetic abnormality.
Dealing with del(5q) MDS
The del(5q) mutation is one of the most common cytogenetic abnormalities found in MDS patients, with a prevalence ranging from 16% to 28%.16-18 The drug lenalidomide interferes with cell-cycle regulation through inhibition of phosphatase activity in the common deleted region, and through ubiquitination and degradation of CK1α in patients with the del (5q) mutation. It was approved by the US Food and Drug Administration (FDA) based on the phase 2 trial MDS-003,19 in which 148 transfusion-dependent, lower-risk MDS patients with del(5q) were treated with lenalidomide at 10 mg daily or for 21 of 28 days. Two-thirds of patients (n = 99) had transfusion-independence responses by week 24 of therapy. Of the 85 patients with informative cytogenetics, 73% had a cytogenetic response (45% with complete response; 28% with partial response). The median duration of response to lenalidomide was 2.2 years. The most common serious treatment associated side effects included grade ≥3 neutropenia and thrombocytopenia, necessitating dose adjustments.
Follow-up on the long-term outcomes of the MDS-003 trial found that achievement of transfusion independence and/or a cytogenetic response [ie, suppression of the del(5q) clone] was associated with improved OS and reduced risk of AML progression. Those with isolated del(5q) at baseline had longer median OS, at 3.9 years, vs 2.7 years in patients with additional cytogenetic abnormalities.20 The randomized MDS-004 trial confirmed the efficacy of 10 mg of lenalidomide daily, with a transfusion-independence response rate of 61% (compared with 6% for patients receiving placebo), as the preferred dose for treatment of MDS patients with del(5q).21 A subsequent pooled analysis of these trials showed that likelihood of achieving durable transfusion independence, cytogenetic response, and OS was significantly higher for patients treated during their first cycle with higher doses of lenalidomide (eg, 10 mg daily), and for those undergoing subsequent dose reduction, to remain on lenalidomide.22
For patients with lower-risk MDS without del(5q), who also experienced responses in earlier-phase studies, lenalidomide has been investigated off-label in advanced-phase trials.23,24 Patients not responding to ESAs or in whom ESAs were unlikely to work were randomly assigned in a 2:1 ratio to receive either lenalidomide (n = 160) or placebo (n = 79). Almost 27% of lenalidomide-treated patients achieved the primary end point of pRBC transfusion independence lasting 8 weeks or more, compared with 2.5% of those receiving placebo (P < .001). Response duration was a median of 31 weeks, with no measurable improvement in quality of life for the entire cohort. Approximately 62% of lenalidomide-treated patients experienced severe neutropenia, and 36% experienced thrombocytopenia. Lenalidomide had no impact on transformation to AML.
Clinical case (continued)
The patient develops progressive thrombocytopenia, with a platelet count that dips to 35 × 109/L and has 2 episodes of epistaxis. A repeat bone marrow assessment reveals MDS with multilineage dysplasia. Molecular studies now reveal a TP53 abnormality.
Tackling thrombocytopenia
Two thrombopoietin receptor agonists have been explored in some depth off-label in lower-risk MDS patients. Romiplostim was studied in a phase 2 multicenter study in which 250 thrombocytopenic MDS patients were randomized in a 2:1 fashion to receive the drug or placebo. The number of clinically significant bleeding events (for those with platelet counts ≥20 × 109/L) and platelet transfusions (in those with platelet counts <20 × 109/L) were lower in patients receiving romiplostim compared with placebo (relative risk = 0.71 and 0.35, respectively; P < .0001), and IWG platelet response rates were higher (odds ratio, 15.6). Due to increased concerns of excess blasts and progression to AML, the trial was halted prematurely, though these rates equilibrated between arms with 5 years of follow-up.25
Eltrombopag was also studied in a phase 1/2 trial of adult patients with lower-risk MDS with thrombocytopenia (<30 × 109/L); 90 patients were randomly assigned (2:1) to receive eltrombopag (50 mg to 300 mg) or placebo. Platelet response was reported in 28 of 59 patients (47%) in the eltrombopag group vs 1 of 31 patients (3%) in the placebo group (odds ratio, 27.1; 95% confidence interval, 3.5-211.9; P < .0017). Although there was no difference in the rate of progression to AML or disease progression between the 2 groups (12% vs 16%; χ2 = 0.06; P = .81),26 use of eltrombopag or romiplostim should be avoided in patients with excess blasts. Long-term safety end points will need to be evaluated and the role of molecular profiles to monitor for clonal evolution will need to be established.
Modifying multilineage dysplasia
The hypomethylating agents azacitidine and decitabine have a well-established role in patients with higher-risk MDS. In lower-risk patients, their utility has been explored in 3 phase 2 studies. In 1 trial, 65 patients received decitabine at a dose of 20 mg/m2 for 3 days of a 28-day cycle; 23% achieved an IWG response.27 In the second trial, decitabine was administered to 25 patients at a dose of 0.1 to 0.2 mg/kg per day for 1 to 3 days per week in an effort to capitalize on its hypomethylating, but not cytotoxic, properties; 44% of patients achieved an IWG response, lasting a median of ∼2 years for erythroid and 3 years for platelet responders.28 Most recently, a phase 2 study conducted through the US MDS Clinical Research Consortium randomized 113 lower-risk MDS patients to decitabine 20 mg/m2 or azacitidine 75 mg/m2 for 3 days of a 28-day cycle. Overall response rates were 70% vs 49% (P = .03), cytogenetic response was 61% vs 25% (P = .02), and OS was 20 vs 13 months (P = .1), respectively.29 A recently completed trial (NCT02269280) has randomized lower-risk MDS patients to 1 of these 2 schedules or to 5 days of azacitidine, the most commonly used schedule in the United States, to determine whether, indeed, less drug may be at least similarly effective to FDA-labeled schedules, with less toxicity.
In a subset of patient with MDS, dysregulation of the immune system can lead to ineffective hematopoiesis, with a clinical and bone marrow picture that may be similar to acquired aplastic anemia. Two multicenter trials examined the off-label use of antithymocyte globulin (ATG) in lower-risk MDS. In a European study, 88 patients (of whom the majority had lower-risk MDS) were randomized to ATG plus cyclosporine vs best supportive care. The complete and partial response rates for ATG at 6 months was 29% with a median duration of response of 16.4 months, compared with 9% in the best supportive care arm (P = .02); response rates were higher for those with hypocellular marrows, but survival did not differ between arms.30 In the second study, conducted in the United States, ATG was administered to 27 patients of whom the majority had lower-risk MDS. The IWG response rate was 33%, with a median duration of response of 245 days. In a multivariate analysis, shorter disease duration, having a higher percentage of CD8+ terminal memory cells, and a higher CD4+ T-cell proliferative index (Ki-67+) independently discriminated for hematological response.31
The future of therapy for lower-risk MDS is not bleak
Patients with lower-risk MDS, particularly those for whom available drugs have failed, should be considered for clinical trials of new agents, if available. As MDS worsens and patients develop progressive cytopenias and transfusion needs, and/or undergo genetic evolution, hematopoietic cell transplantation can also be considered. Drugs under investigation include the following.
FG-4592
Roxadustat (FG-4592), is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor, which works by inhibiting the hypoxia-inducible factor prolyl hydroxylase enzymes, allowing for transcription of genes for normal erythropoiesis by stimulating endogenous erythropoietin, increased iron transport/absorption, and decreased hepcidin levels. Roxadustat was first evaluated for the treatment of anemia in chronic kidney patients who were dialysis independent (vs placebo) and in dialysis-dependent chronic kidney patients (vs ESA therapy). Both studies met their primary end points: in the first, the mean hemoglobin change from baseline was +1.9 g/dL, compared with −0.4 g/dL for placebo-treated patients. In the second study, mean change in hemoglobin was +0.8 g/dL, similar to that for the ESA of +0.5 g/dL. (NCT02174627 and NCT02174731). FG-4592 is under investigation in a phase 3 trial to study the safety and effectiveness in the treatment of anemia in lower-risk MDS (NCT03263091).
Spliceosome inhibitors
With the advent of next-generation sequencing, we have been able to identify recurrent molecular mutations in MDS, such as those involving RNA-splicing factors, which has allowed for discovery and development of targeted therapy. H3B-8800 is a targeted oral drug, which modulates the SF3b complex. In several preclinical xenografts models of spliceosome mutations, H3B-8800 enhances antitumor activity, due to dependency of these cells on normal expression of spliceosome proteins in comparison with wild-type cells.32 H3B-8800 is being explored in a phase 1 trial to determine its therapeutic benefits, maximum tolerated dose, and safety in MDS, AML, and chronic myelomonocytic leukemia (NCT02841540).
APR-246 for TP53
TP53-mutant MDS and AML patients are challenging, as most have low response rates to standard therapies and a dismal OS, even with allogeneic transplant. APR-246 is a novel IV agent that releases the active drug methylene quinuclidinone, a p53 reactivator, which binds to the mutant protein and restore normal function of TP53.33 In results from a phase 1b/2 multicenter study of APR-246 plus azacitidine in patients (n = 12) with TP53-mutant MDS/AML with poor-risk karyotype, the overall response rate by IWG criteria was 95% in 20 patients for whom responses were evaluable (14 patients had a complete response [70%]). Median time to first response was 70 days (and response correlated with normalization of immunohistochemistry and decrease in the variable allele frequency of the TP53 mutant clone).34 Although ongoing trials are enrolling patients with higher-risk disease, we anticipate that the drug would be used in IPSS or IPSS-R lower-risk patients with the TP53 abnormality.
Lowering the boom while raising the bar of response
The most commonly used system assessing a patient’s response to an established or new drug is the modified IWG criteria, now reaching their teenage years in age.35 Although these have systematized assessments of whether a patient is benefitting from a given therapy, their broad definition of benefit may not clearly translate to a clinically meaningful improvement in a patient’s condition.
Most patients with lower-risk MDS have some degree of anemia. An improvement of that anemia is arbitrarily defined, according to the IWG criteria, as an increase of 1.5 g/dL or achievement of transfusion independence lasting 8 weeks or longer. These outcomes are measured against a baseline pRBC-transfusion frequency widely used to define transfusion dependence in clinical trials of ≥1 to 2 units every 4 weeks, averaged over an 8-week pretreatment period. These criteria ignore the impact of synchronous illness or bleeding on baseline hemoglobin levels or transfusions needs, however.36 Such events may resolve at precisely the same time as a new drug is initiated, causing the false conclusion that the drug was more effective than it actually was. Evidence of this has been seen in randomized trials in myeloid disorders, in which placebo control groups achieved hematologic improvement in up to 15% to 20%.15,37,38
Neither response criterion incorporates an improvement in quality of life. An improvement in anemia or significantly fewer red blood cell transfusions may thus be Pyrrhic victories at best to a patient who continues to suffer from the withering symptoms of anemia despite his or her numbers improving. Similarly, a response duration of 8 weeks is a pittance when compared with the amount of time a patient may have to spend receiving a drug to achieve 4 fortnights of transfusion relief.
Recognizing these limitations, a recent international proposal recommends a minimum of 16 weeks of improvement in anemia, with a 16-week assessment of transfusion needs, prior to an intervention.10 Additionally, we recommend comparing the anticipated benefit of an intervention against the time commitment required of a patient to achieve that benefit.
Correspondence
Mikkael A. Sekeres, Leukemia Program, Cleveland Clinic Taussig Cancer Institute, Desk CA-60, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: sekerem@ccf.org.
References
Competing Interests
Conflict-of-interest disclosure: M.A.S. has served on advisory boards for Celgene and Millennium/Takeda. B.J.P. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.