Abstract
Iron deficiency (ID) affects billions of people worldwide and remains the leading cause of anemia with significant negative impacts on health. Our approach to ID and iron deficiency anemia (IDA) involves three steps (I3): (1) identification of ID/IDA, (2) investigation of and management of the underlying etiology of ID, and (3) iron repletion. Iron repletion options include oral and intravenous (IV) iron formulations. Oral iron remains a therapeutic option for the treatment of ID in stable patients, but there are many populations for whom IV iron is more effective. Therefore, IV iron should be considered when there are no contraindications, when poor response to oral iron is anticipated, when rapid hematologic responses are desired, and/or when there is availability of and accessibility to the product. Judicious use of red cell blood transfusion is recommended and should be considered only for severe, symptomatic IDA with hemodynamic instability. Identification and management of ID and IDA is a central pillar in patient blood management.
Learning Objectives
Recognize absolute and functional iron deficiency
Evaluate underlying etiology of iron deficiency
Select appropriate iron-repletion option for a given patient population
Clinical case
A 43-year-old woman was seen by her general practitioner for fatigue. Screening blood work revealed a hemoglobin (Hb) of 6.5 g/dL. She was sent to a local emergency room for urgent transfusion of red blood cells.
Epidemiology of iron deficiency
The World Health Organization estimated worldwide prevalence of anemia to be 42% in children, 29% in non-pregnant women, and 38% in pregnant women in 2011.1 In 2013, iron deficiency (ID) was identified as the predominant cause of anemia among the 1.93 billion anemic people (27% of the world’s population) globally, making iron deficiency anemia (IDA) a major global health issue.2,3 The people most at risk are women and children, regardless of socioeconomic status or geography. In 2017, the Global Burden of Diseases Study reported that dietary ID remains the fourth and twelfth leading cause of years lived with disability in women and men, respectively.4 Yet, the true prevalence and clinical impact of ID remains difficult to capture. Population-based studies are limited, functional iron deficiency (FID) is common, and the prevalence of ID without anemia remains uncertain.
Absolute ID vs FID
Iron is an essential element for cellular life, and yet free iron is a source of cellular damage and toxicity. To meet but not exceed daily iron requirements for erythrocyte production and cellular metabolism (25 mg/day), iron is absorbed via the diet (1-2 mg/day) and salvaged from erythrocyte breakdown by macrophages (20-25 mg/day); any remaining iron requirements are met through the body’s residual iron stores (total of 3-5 g in adults).5 Daily iron loss (∼1-2 mg/day) cannot be regulated, and thus, tight hemostatic controls exist to regulate iron absorption, recycling, and storage (recently reviewed in detail by Wang and Babitt6 ).
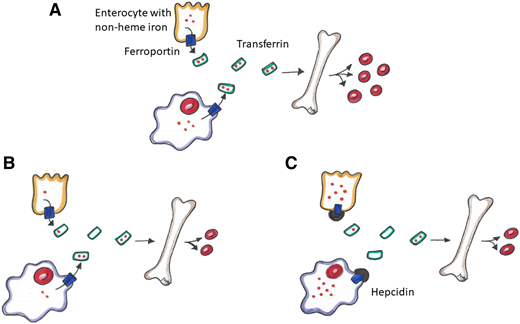
Total body iron is distributed among Hb in erythrocytes, myoglobin in muscles, iron-dependent proteins for cellular metabolism, and storage iron (predominantly in the liver, spleen, and bone marrow). A minority of the body’s total iron is found in the circulation, where it is bound to transferrin. Iron absorption and tissue iron availability are closely regulated by hepcidin.5 Complex regulatory pathways exist, but they are not within the scope of this review. Simply, hepcidin is a protein produced predominantly by hepatocytes, and it exerts control over systemic iron homeostasis by degrading ferroportin. Ferroportin is the key iron exporter expressed on macrophages and duodenal enterocytes that allows the recycling of iron from broken down/senescent erythrocytes into plasma and the absorption of iron from the gut into circulation, respectively (Figure 1A).5 Hepcidin expression is increased by high body iron levels and inflammation and decreased by erythropoiesis, hypoxia, and ID.5
Iron homeostasis. (A) Normal metabolic pathway of non-heme iron through dietary absorption across the enterocyte and red blood cell (RBC) recycling by macrophages. Non-heme iron is transported across ferroportin, bound to transferrin and transported to the bone marrow to promote erythropoiesis. (B) Iron metabolism in an absolute iron-deficient state. There is less iron to transport to the bone marrow resulting in decreased erythropoiesis. (C) Iron metabolism in a functional iron-deficiency state. Hepcidin disables ferroportin, thus sequestering iron in the enterocyte and macrophage resulting in decreased erythropoiesis.
Iron homeostasis. (A) Normal metabolic pathway of non-heme iron through dietary absorption across the enterocyte and red blood cell (RBC) recycling by macrophages. Non-heme iron is transported across ferroportin, bound to transferrin and transported to the bone marrow to promote erythropoiesis. (B) Iron metabolism in an absolute iron-deficient state. There is less iron to transport to the bone marrow resulting in decreased erythropoiesis. (C) Iron metabolism in a functional iron-deficiency state. Hepcidin disables ferroportin, thus sequestering iron in the enterocyte and macrophage resulting in decreased erythropoiesis.
In an absolute ID state, total body iron stores are reduced (Figure 1B). Suppressed hepcidin levels lead to reduced ferroportin degradation, which in turn facilitates absorption of iron from the gut (with help from divalent meta-transporter 1 [DMT1]) and allows iron export from macrophages and hepatocytes into the circulation. DMT1 and ferroportin are also upregulated by hypoxia-inducible factor 2α, which further facilitates gastrointestinal (GI) iron absorption.7 Transferrin production increases in the liver and decreases the levels of iron-bound transferrin in the plasma in ID, further reducing hepcidin levels.
Unlike absolute ID, FID (sometimes used interchangeably with the term iron-restricted erythropoiesis) is a state of imbalance between iron demand and serum iron availability, and it may occur despite adequate body iron stores.8 FID is most frequently observed in the setting of systemic inflammation and/or infection, in which inflammatory cytokines stimulate increased hepcidin production and thus impair iron absorption from the gut and facilitate iron trapping in macrophages by degrading ferroportin (Figure 1C).5 By reducing iron bioavailability, iron-deficient erythropoiesis occurs. Cytokines may also have an impact on ferroportin production and cellular iron transport through hepcidin-independent pathways, may dampen endogenous erythropoietin activity, and may shorten erythrocyte lifespan.9 The pathophysiology, diagnosis, and management of FID is reviewed in detail by Weiss et al.9
Approach to ID and IDA
We recommend a 3-step approach to ID and IDA (I3): (1) identification, (2) investigation, and (3) iron repletion.
Step 1. Identification
ID can occur in the presence or absence of anemia (World Health Organization definitions for anemia: adult males, Hb <13 g/dL; adult females, Hb <12 g/dL; pregnant females, Hb <11 g/dL).2 Clinical symptoms may serve as the first clue to the identification of ID/IDA and may include those secondary to ID (eg, alopecia, glossitis, pica and decreased cognitive abilities, attention and concentration10-12 ), as well as those associated with all anemias (eg, fatigue, dyspnea, syncope). IDA in the prenatal period can negatively impact maternal, fetal, and neonatal health.13,14 Signs and symptoms of ID and IDA are discussed in detail in Lopez et al.10
Serum ferritin is the most reliable initial test for diagnosing absolute ID.15 Ferritin is an intracellular iron storage protein that correlates with the body’s iron stores in the absence of inflammation. A ferritin of ≤15 μg/L was traditionally used for the diagnosis of ID in adults.10 However, a threshold of ferritin ≤30 μg/L achieves a higher sensitivity (92%) while maintaining a high 98% specificity for the diagnosis and is thus commonly used.16 A ferritin level of 35 to 45 μg/L was associated with a positive likelihood ratio of 1.83 for ID.17
The diagnosis of ID becomes more challenging with concomitant inflammatory conditions and in the elderly because ferritin is an acute-phase reactant that increases with age. In these circumstances, low transferrin saturation (TSAT) (<16% or <20% with inflammation) can be used with higher ferritin thresholds (<100 μg/L) for diagnosis.18 However, optimal thresholds remain unclear. In heart failure, ferritin <100 μg/L or ferritin <300 μg/L with TSAT <20% has been recommended for the diagnosis of ID.19 In the preoperative setting, cutoffs of ferritin at <100 μg/L and/or TSAT at <20% have been used.20 In patients with chronic kidney disease, the Kidney Disease Improving Global Outcomes guideline recommends considering iron therapy if ferritin is ≤500 μg/L and TSAT is ≤30%,21 whereas recent clinical trials in dialysis patients used ferritin <200 μg/L or TSAT <20% as indications for iron therapy.22 Other biochemical features of ID include low mean cell hemoglobin (hypochromia), mean corpuscular volume (microcytosis), and high red cell distribution width; these changes are slow to occur because of the long lifespan of red blood cells and are not specific for ID. An earlier marker of ID is the reticulated hemoglobin content (CHr), which is decreased (<29 pg) in ID (reticulated-Hb equivalent is provided instead of reticulated-Hb content on certain analyzers and the normal range may vary).23 In certain cases, the diagnosis of ID is made only after a successful trial of iron supplementation. Bone marrow biopsy remains the gold standard for the diagnosis of ID but is infrequently pursued. Table 1 summarizes common indices for diagnosis of ID. Additional and novel markers are discussed in detail elsewhere (see Ginzburg, in this book24 ).
Biochemical and hematologic values in keeping with ID
| Test . | Value for ID . |
|---|---|
| Ferritin | <15-30 μg/L |
| Mean corpuscular volume | <75 fL with previous normal value |
| Transferrin saturation | <20% |
| Reticulate hemoglobin content | Decreased (<29 pg) |
| Bone marrow (not recommended for routine screening) | Absent iron stores |
| Test . | Value for ID . |
|---|---|
| Ferritin | <15-30 μg/L |
| Mean corpuscular volume | <75 fL with previous normal value |
| Transferrin saturation | <20% |
| Reticulate hemoglobin content | Decreased (<29 pg) |
| Bone marrow (not recommended for routine screening) | Absent iron stores |
Values may vary depending on patient comorbidities.
Step 2. Investigation
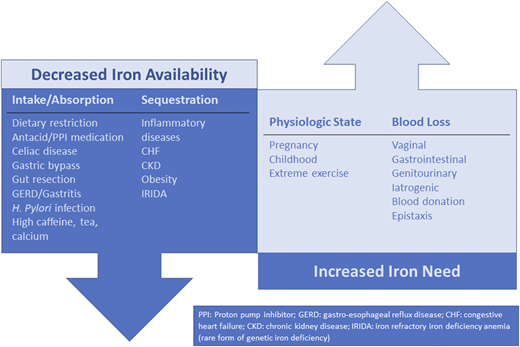
ID is not a final diagnosis; rather, it is indicative of an underlying etiology that is decreasing iron availability and/or increasing iron needs.10,25 To effectively manage ID/IDA, the underlying etiology must be identified and, if possible, treated. Figure 2 outlines a number of key causes of ID/IDA. In a woman of reproductive age, vaginal blood loss must be considered and explored with a detailed bleeding history.13 GI blood loss or malabsorptive states must be considered in all patients. Although it is especially important in men and in all patients older than age 50 years, it is important to remember that celiac disease26 and GI malignancy27 can occur in both sexes at any age. Blood donors are at risk of ID, and thus current or previous blood donation is an important element to obtain from the patient history28 Additional considerations include detailed medication history (eg, oral anticoagulants, antacids, proton pump inhibitors) and dietary history.
Underlying etiologies of ID. There are other causes of ID that are not represented in this figure.
Underlying etiologies of ID. There are other causes of ID that are not represented in this figure.
Back to the case
A detailed history was obtained in the emergency room. The patient had increased dyspnea after her regular 4-mile run over the last few months and daily headaches. There were no GI symptoms and no history of blood donations. The patient followed a balanced diet and took no medications or supplements. Further review of systems revealed heavy menses that began after the third uncomplicated pregnancy with 2 days of changing tampons every hour, frequently leaking through and waking her up 2 to 3 times per night to change pads. There was no other significant bleeding history beyond menorrhagia. On physical examination, there was no acute distress, and vital signs were within normal limits. Apart from pale conjunctiva, the remainder of the examination was unremarkable. Laboratory studies showed Hb, 6.2 g/dL; mean corpuscular volume, 65 fL; leukocyte count, 7200/mm3; platelet count, 480 000/mm3; reticulocytes, 0.6%; red blood cell distribution width, 15.7%; ferritin, 4 ng/mL, and transferrin saturation, 3%.
Step 3. Iron repletion
Red blood cell transfusion
Choosing Wisely campaigns across jurisdictions and specialties have highlighted the importance of restrictive red blood cell (RBC) transfusion and promotes the use of alternative therapeutic options to transfusion when available and appropriate.29-32 Recommendations from the American Society of Hematology and American Association of Blood Banks Choosing Wisely campaigns advise clinicians to avoid transfusing RBCs for ID without hemodynamic instability.29,33 Therefore, RBC transfusion for severe IDA should be restricted for cardiovascular compromise and/or debilitating symptoms. In the great majority of patients, there is time to address the cause of IDA and fuel self-driven erythropoiesis with iron supplementation.
Oral iron
Oral iron supplementation is an inexpensive and effective option for treating ID in stable outpatients. In North America, multiple formulations of iron are available, but iron salts such as ferrous gluconate, ferrous sulfate, and ferrous fumarate remain the standard first-line therapy for treating ID. Other common iron formulations include ferrous ascorbate, ferrous succinate, carbonyl iron, ferric citrate, liposomal iron, heme iron polypeptide, and polysaccharide iron complexes (PICs). Table 2 provides examples of common dosages and elemental iron contents of selected oral iron formulations.
Common doses and elemental iron content of select available iron formulations in the United States and Canada
| Drug class . | Example . | Dose per tablet (mg) . | Elemental iron content per tablet (mg) . | Dose . | Special instructions . |
|---|---|---|---|---|---|
| Iron salts | Ferrous gluconate | 240 | 27 | 1-3 tablets, once per day or once every other day | Take on empty stomach; consider vitamin C; take at a different time of day than antacid or proton pump inhibitor. Acidic environment required. |
| 325 | 38 | ||||
| Ferrous sulfate | 325 | 65 | 1-2 tablets, once per day or once every other day | ||
| Ferrous fumarate | 325 | 106 | 1 tablet, once per day or once every other day | ||
| Heme iron polypeptide | Proferrin | 398 | 11 | 1-3 tablets per day | Can be taken with a meal. Acidic environment not required for absorption. |
| Polysaccharide iron complex | Feramax | 150 | 150 | 1 tablet once per day | Can be taken with a meal. Acidic environment not required for absorption. |
| Ferric citrate | Auryxia | 210 | 210 | 3-5 tablets once per day | Can be taken with a meal. Acidic environment not required for absorption. |
| Drug class . | Example . | Dose per tablet (mg) . | Elemental iron content per tablet (mg) . | Dose . | Special instructions . |
|---|---|---|---|---|---|
| Iron salts | Ferrous gluconate | 240 | 27 | 1-3 tablets, once per day or once every other day | Take on empty stomach; consider vitamin C; take at a different time of day than antacid or proton pump inhibitor. Acidic environment required. |
| 325 | 38 | ||||
| Ferrous sulfate | 325 | 65 | 1-2 tablets, once per day or once every other day | ||
| Ferrous fumarate | 325 | 106 | 1 tablet, once per day or once every other day | ||
| Heme iron polypeptide | Proferrin | 398 | 11 | 1-3 tablets per day | Can be taken with a meal. Acidic environment not required for absorption. |
| Polysaccharide iron complex | Feramax | 150 | 150 | 1 tablet once per day | Can be taken with a meal. Acidic environment not required for absorption. |
| Ferric citrate | Auryxia | 210 | 210 | 3-5 tablets once per day | Can be taken with a meal. Acidic environment not required for absorption. |
The list of examples and doses in this table is not exhaustive. Liquid formulations are also available.71 Approximately 10% of elemental iron ingested is absorbed.
Iron should be taken between meals, and inhibitors of iron absorption (calcium-containing foods such as dairy products, tea, and coffee) should be avoided when the iron supplement is taken. Medications that reduce gastric acidity such as antacids may also impair oral iron absorption and should be similarly avoided. Oral iron taken with vitamin C (orange juice or ascorbic acid) can enhance iron absorption.34 Adverse effects of oral iron are common, including nausea, constipation, diarrhea, vomiting, metallic taste, and dark stool (fecal occult blood testing will not be affected),35 and are dose-dependent. With consistent oral iron supplementation, reticulocytosis starts in 4 to 5 days, and Hb begins to improve by the second week. Oral iron therapy is often required for at least 3 to 6 months to replete iron stores and normalize ferritin levels, although more time may be required depending upon the severity of IDA and ongoing losses.
There are no biochemical markers to predict the likelihood of response to oral iron; however, malabsorption-related ID and FID decrease responsiveness. When selecting an oral iron formulation, cost, adverse effects profile, likelihood of efficacy, and dosing are all important considerations. Heme iron polypeptide and PIC are more expensive than iron salts, but they carry potentially improved tolerability and they taste better. There is no concrete evidence that one iron formulation is more efficacious than another, although a recent small randomized trial of iron supplementation in children found that ferrous sulfate resulted in a greater increase in Hb at 12 weeks compared with PIC.36 Patients have traditionally been instructed to take iron in divided daily doses to achieve 100 to 200 mg of elemental iron consumption per day. However, new evidence suggests that lower single daily doses and every-other-day dosing of iron can improve absorption and improve tolerability. A study of 54 women with ID without anemia demonstrated that higher and divided daily doses of iron increased hepcidin and resulted in less optimal iron absorption than reduced and once-per-day iron dosing.37 Two small open-label trials subsequently confirmed that divided doses of iron increased hepcidin compared with once-per-day dosing and failed to improve absorption.38 Every-other-day dosing also resulted in greater iron absorption than once-per-day dosing, with a statistically nonsignificant trend toward reduced nausea.39 Data from additional ongoing clinical trials will be of value.
Back to the case
The emergency room physician decided against RBC transfusion given the patient’s stability. Out of concern for the severity of anemia and likelihood of ongoing blood loss, she initiated treatment consisting of a single dose of a high-dose, short-infusion-time intravenous (IV) iron formulation. She was referred for gynecologic investigation regarding the cause for heavy menstrual bleeding (HMB), and a prescription for oral iron was provided along with instructions for administration. Close follow-up with her primary care physician was recommended to ensure adequate Hb recovery and HMB management while awaiting her consultation with a gynecology specialist.
IV iron
IV iron has traditionally been used for unresponsiveness to or intolerance of oral iron replacement therapy, or for patients for whom rapid iron replacement (for example, preoperative ID or symptomatic anemia) is desired. However, the paradigm of oral iron as first-line and IV iron as second-line therapy has taken a turn in past years because clinicians are recognizing the efficacy of IV iron over oral iron. The pharmacology, indications, and toxicities of IV iron14,39-41 as well as its use in gynecology and obstetric patients have been reviewed recently and will not be reviewed here.14 Instead, we present an update on available formulations of IV iron and dosages. We also highlight the use of IV iron when managing patients with chronic iron needs and the importance of IV iron in patient blood management (PBM).
Choosing a formulation
The choice of IV iron formulation may be limited by what is available, accessible, affordable, and/or approved in the center or patient population being treated. In general, a smaller dose per infusion product would be more suitable for a patient who is visiting the hospital frequently (for example, a patient with chronic kidney disease who receives iron during hemodialysis visits) whereas a larger-dose product is more convenient for a patient who requires urgent iron repletion. Some patients will tolerate certain formulations better than others.
Dosing
Table 3 shows the dosages and infusion times of IV iron formulations available in the United States and Canada. In general, the Ganzoni formula42 can be used to calculate the patient’s total body iron deficit to guide dosing. High-volume IV iron such as ferric carboxymaltose and iron isomaltoside are often given as a single dose of 1000 mg. Depending on the degree of ID, additional doses may be required to complete iron repletion, especially in a patient with ongoing losses.
Intravenous iron formulations
| Compound . | Brand name . | Recommended amount per dose . | Infusion time . | Availability . | Reference . |
|---|---|---|---|---|---|
| Low-molecular-weight iron dextran | INFeD | 100 mg after uneventful 25-mg test dose | 2-6 h (+ test dose) | United States, Europe | https://www.pdr.net/drug-summary/INFeD-iron-dextran-2087; https://www.allergan.com/assets/pdf/infed_pi |
| Ferrous gluconate | Ferrlecit | 125 mg | 12.5 mg/min | United States, Europe, Canada | http://products.sanofi.us/ferrlecit/ferrlecit.html |
| Iron sucrose | Venofer | 200-300 mg | 100 mg/30 min | United States, Europe, Canada | http://www.venofer.com/Indications_Dosage |
| Ferumoxytol | Feraheme | 510 mg | 15 min | United States, Europe | https://www.feraheme.com/dosing-and-administration/ |
| Ferric carboxymaltose | Injectafer | 750 mg | 15 min | United States, Europe | https://injectaferhcp.com/iron-deficiency-anemia-dosing |
| Ferinject | 1000 mg | 15 min | United States, Europe | https://www.ferinject.co.uk/simplified-dosing-for-all-patients/ | |
| Iron isomaltoside | Monofer | ≤1000 mg | >15 min | United States, Europe | https://www.medicines.org.uk/emc/files/pil.5676.pdfinu |
| Monoferric | >1000 mg (maximum 20 mg/kg) | ≥30 min | Canada |
| Compound . | Brand name . | Recommended amount per dose . | Infusion time . | Availability . | Reference . |
|---|---|---|---|---|---|
| Low-molecular-weight iron dextran | INFeD | 100 mg after uneventful 25-mg test dose | 2-6 h (+ test dose) | United States, Europe | https://www.pdr.net/drug-summary/INFeD-iron-dextran-2087; https://www.allergan.com/assets/pdf/infed_pi |
| Ferrous gluconate | Ferrlecit | 125 mg | 12.5 mg/min | United States, Europe, Canada | http://products.sanofi.us/ferrlecit/ferrlecit.html |
| Iron sucrose | Venofer | 200-300 mg | 100 mg/30 min | United States, Europe, Canada | http://www.venofer.com/Indications_Dosage |
| Ferumoxytol | Feraheme | 510 mg | 15 min | United States, Europe | https://www.feraheme.com/dosing-and-administration/ |
| Ferric carboxymaltose | Injectafer | 750 mg | 15 min | United States, Europe | https://injectaferhcp.com/iron-deficiency-anemia-dosing |
| Ferinject | 1000 mg | 15 min | United States, Europe | https://www.ferinject.co.uk/simplified-dosing-for-all-patients/ | |
| Iron isomaltoside | Monofer | ≤1000 mg | >15 min | United States, Europe | https://www.medicines.org.uk/emc/files/pil.5676.pdfinu |
| Monoferric | >1000 mg (maximum 20 mg/kg) | ≥30 min | Canada |
A study by Koch et al43 examined clinical trials of iron deficient anemic patients from mixed etiologies who received treatment with IV iron to provide clinicians with evidence-based guidance for iron therapy dosing. A modified Ganzoni formula (subject weight in kilograms × [15 – current Hb g/dL] × 2.4 + 500) was used to determine the amount of iron repletion required. The authors found that the mean iron deficit for 4641 patients in 7 studies was approximately 1500 mg. Treatment with 1500 mg compared with 1000 mg resulted in a faster and higher Hb recovery with more time until additional iron repletion was required.43 A recent phase 3 randomized controlled double-blind safety trial compared 2 doses of 510 mg ferumoxytol to 2 doses of 750 mg of ferric carboxymaltose in patients with IDA.44 They demonstrated noninferiority of ferumoxytol to ferric carboxymaltose for clinical efficacy as measured by using the mean change in Hb from baseline to week 5. The dose of ferric carboxymaltose was 50% higher than the dose of ferrumoxytol; however, this translated to an 18% increase in Hb at 5 weeks.44 This latter trial supports the approach of treating with 1000 mg up front, and reassessing at 4 weeks to determine whether additional IV iron is required.
Doses of 3000 mg (1000 mg once per week for 3 weeks) of ferric carboxymaltose45 and doses as high as 8000 mg (200 mg once every 1 to 2 weeks) of iron sucrose46 have been used in clinical trial patients with inflammatory bowel disease to good effect. Iron isolmatoside has been administered safely in 2000-mg doses (1000 mg once per week) in iron deficient anemic patients.47 When IV iron is ordered, the clinician should refer to the product monographs because some recommend weight-based dosing.
High-dose IV iron has recently become available in Canada. Our practice has thus been to use iron sucrose in doses of 200 to 300 mg once every 1 or 2 weeks until iron is replete (please note that different jurisdictions may vary on dosing recommendations). The number of doses is dependent upon the degree of anemia and ID as well as the etiology of ID. Blood is routinely drawn for complete blood count, reticulocytes, reticulated-Hb content, and ferritin levels before each infusion. For an iron-deficient non-anemic patient, we will replete with 300 to 600 mg total (1-2 doses) and reassess iron status in ∼3 months after infusion. For patients with moderate IDA (Hb, 8.0-10.9 g/dL),48 1200 to 1500 mg total may be required with reassessment 3 months after the final infusion. For patients with severe IDA (Hb, <8.0 g/dL),48 2000 to 3000 mg total may be required with reassessment 3 months after final infusion. For patients with recurrent IDA from chronic loss or malabsorption, patients are placed on a maintenance schedule after initial iron repletion that depends on their need with the goal of maintaining a normal Hb level and iron status between infusions (discussed in the next section).
Back to the case
Nonsteroidal anti-inflammatory drugs and tranexamic acid were effective in decreasing menstrual losses, and the patient eventually had an intrauterine device inserted. It was successful in decreasing menstrual losses and resulted in the maintenance of a normal Hb and ferritin level for the next year; however, she chose to have the intrauterine device removed because of constant spotting. When it was removed, her HMB recurred.
Chronic need
There are many populations who will require ongoing iron supplementation beyond initial iron repletion. Such populations include those with inflammatory bowel disease, ongoing GI blood loss (eg, from arteriovenous malformations, hereditary hemorrhagic telangiectasia), abnormal uterine bleeding refractory to or awaiting gynecologic intervention, and those with malabsorption (eg, from bariatric surgery, bowel resection). In our experience, maintenance iron therapy is often required. We routinely recheck complete blood count, reticulocytes, reticulated-Hb content, and iron parameters 3 to 6 months after initial iron repletion to determine whether ongoing iron supplementation is required and to establish the optimal route, dose, and frequency. For some patients, we are successful in maintaining normal iron stores and Hb level using once-per-day or every-other-day oral iron after IV iron repletion. In other patients, a regimen of once per month, once every 3 months, or once every 6 months IV iron is required, with the goal of maintaining normal iron status (ferritin >50 µg/L; TSAT >20%). As access to high-dose IV iron increases, we anticipate that intervals between iron therapy treatments will lengthen.
Oral vs IV: which should you choose?
Oral iron is inexpensive, is easy to access, and is available without a prescription. Oral iron will thus continue to be the first-line therapy in resource-limited regions; it is an appropriate first-line option for maintaining iron stores in healthy patients and in asymptomatic outpatients with mild ID/IDA in which there is no inflammation and in whom oral iron is well tolerated. Patients with menstrual losses may benefit from oral iron once every other day as a means of maintaining an iron replete status.38 Healthy blood donors also benefit from oral iron repletion.49
However, adherence to oral iron can be poor. This is in part the result of the high rates of adverse effects (predominantly GI; range 3.7% to 43.3%50 ) that occurs with oral iron therapy. A study of 96 women treated with oral iron for IDA found 40% noncompliance, with most citing GI intolerance as the reason for discontinuation.51 A 2015 systematic review and meta-analysis reported that patients treated with ferrous sulfate had significantly more GI adverse effects than those treated with placebo or IV iron; these findings were echoed in studies of gravidas and in inflammatory bowel disease.35,37,38 Therefore, IV iron may be favored over oral iron in many populations because of its increased efficacy, improved compliance, decreased discontinuation rate, and an acceptable safety profile. Our recommendation is to consider use of IV iron for patients in whom there is no contraindication, and for whom the product is available and easy to access for rapid hematologic response. IV iron is recognized as appropriate first-line therapy in inflammatory bowel disease (adult and pediatric),52,53 chronic kidney disease,54-56 chemotherapy-induced anemia,57 and after bariatric surgery.58 Treatment of ID in the absence of anemia with either oral or IV iron is recommended and has been reviewed in detail by Muñoz et al.59
Severe hypersensitivity reactions and serious adverse events, including anaphylaxis, are rare, and their recognition and management have been well-described in the literature.39,60 Hypophosphatemia is a recognized risk of IV iron and is more frequently observed with ferric carboxymaltose than with other iron formulations.61-63
Back to the case
With the recurrence of her HMB and in discussion with the gynecologist, she opted for a hysterectomy. Her most recent blood work showed Hb of 9.0 g/dL and ferritin of 5 µg/L. A referral for preoperative optimization of her IDA was requested.
PBM
Iron repletion is an important component of PBM, a multidisciplinary strategy that aims to conserve blood and optimize the use of blood products by optimizing and preserving the patient’s own blood.64-66 Recently published recommendations from the 2018 Frankfurt Consensus Conference on PBM67 highlighted the importance of (1) early detection and management of preoperative anemia, given its recognized associations with poor outcomes, including mortality (strong, with low certainty in the evidence of effects),65,67 and (2) use of iron supplementation in adults with IDA who are awaiting elective surgery (conditional, based on moderate certainty of evidence of effects), although there was insufficient evidence to recommend target iron indices, type of iron, dose, or route.
The International Consensus Statement on the perioperative management of anemia and ID provides further direction on classification and management of anemia and IDA in the preoperative setting.20 The authors recommend iron repletion in preoperative patients with ferritin 30 µg/L, TSAT <20%, or ferritin 100 µg/L in the setting of C-reactive protein >5 mg/L, targeting an Hb of >13.0 g/dL.20 For surgeries with 6 weeks or more lead time, oral iron once per day or once every other day is recommended; for those with less lead time, IV iron is recommended as safe and efficacious. A pooled observational analysis of 2547 patients undergoing orthopedic surgery demonstrated that very-short-term perioperative administration of 200 to 600 mg of IV iron, with or without erythropoietin stimulating agents, was associated with reduced RBC transfusions and length of hospital stay, with no increase in postoperative nosocomial infection or mortality.68 The PROTECT randomized double-blind placebo-controlled clinical trial showed that a single dose of 1000 mg iron isomaltoside reduced postoperative anemia in preoperatively non-anemic patients undergoing elective or subacute coronary surgery.69 Further studies with a focus on both important patient outcomes and formal cost analyses are required to develop more specific and stronger evidence-based guidelines for the management of perioperative anemia. Anemia and RBC transfusions remain independent risk factors in predicting poor patient outcomes and therefore require prompt identification and effective management.65 (See Lin in this book70 for a more detailed approach to managing anemia in the perioperative setting.)
Back to the case
Two doses of 1000-mg high-dose IV iron 2 weeks apart were administered. Eight weeks after the second infusion, Hb was 13.0 g/dL. An uncomplicated hysterectomy was performed. No allogeneic RBC transfusions were needed. Hb recovered postoperatively, and at 3 months postoperatively, iron indices and Hb were normal with no further need for oral or IV iron.
Conclusion
ID and IDA are prevalent worldwide with significant sequelae on health outcomes and quality of life. Our I3 approach emphasizes identification, investigation, and iron repletion as the steps for managing ID and IDA. Inflammatory states characterized by functional ID can be challenging to identify given the restrictions of currently available iron indices; however, new indices may be available in the near future with improved sensitivity and specificity. Investigation of the cause of ID is key to management. Etiologies are broad and must be explored thoroughly. Selecting an iron repletion option requires consideration of the underlying etiology of ID, severity of symptoms, likelihood of and the desired rapidity of hematologic response, risks, toxicities of treatment, resource availability, and patient preference. Oral iron remains appropriate in some, and the list of populations who will benefit from first-line therapy with IV iron is growing.
Acknowledgment
The authors thank Michael Auerbach, MD, for providing valuable insights and feedback during preparation of this manuscript.
Correspondence
Michelle P. Zeller, McMaster University Medical Centre, 1200 Main St W, HSC 3H54, Hamilton, ON L8N 3Z5, Canada; e-mail: zeller@mcmaster.ca.
References
Competing Interests
Conflict-of-interest disclosure: S.N. and M.P.Z. declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.