Abstract
Hodgkin lymphoma (HL) in older patients, commonly defined as ≥60 years of age, is a disease for which survival rates have historically been significantly lower compared with younger patients. Older HL patients appear to have different disease biology compared with younger patients, including increased incidence of mixed cellularity histology, Epstein-Barr virus–related, and advanced-stage disease. For prognostication, several studies have documented the significance of comorbidities and functional status in older HL patients, as well as the importance of achieving initial complete remission. Collectively, selection of therapy for older HL patients should be based in part on functional status, including pretreatment assessment of activities of daily living (ADL), comorbidities, and other geriatric measures (eg, cognition, social support). Treatment of fit older HL patients should be given with curative intent, regardless of disease stage. However, attention should be paid to serious treatment-related toxicities, including risk of treatment-related mortality. Although inclusion of anthracycline therapy is important, bleomycin-containing regimens (eg, doxorubicin, bleomycin, vinblastine, dacarbazine) may lead to prohibitive pulmonary toxicity, and intensive therapies (eg, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) are too toxic. Brentuximab vedotin given sequentially before and after doxorubicin, vinblastine, and dacarbazine to fit, untreated advanced-stage older HL patients was recently shown to be tolerable and highly effective. Therapy for patients who are unfit or frail because of comorbidities and/or ADL loss is less clear and should be individualized with consideration of lower-intensity therapy, such as brentuximab vedotin with or without dacarbazine. Altogether, therapy for older HL patients should be tailored based upon a geriatric assessment, and novel targeted agents should continue to be integrated into treatment paradigms.
Learning Objectives
Describe the epidemiologic, pathologic, and clinical differences between young and older Hodgkin lymphoma patients
Discuss the importance of geriatric measures, such as comorbidities and activities of daily living, in the prognosis and treatment of older patients
Review the varied contemporary treatment options available for older patients, including the integration of novel targeted therapeutic agents
Case presentation
A 69-year old Hispanic man presents with increasing generalized fatigue over a 4-month period. For the preceding 3 weeks, the patient developed drenching night sweats (ie, changing bed clothes 3-4 times per night). Physical examination reveals diffuse palpable nontender adenopathy in the left supraclavicular and axillary and bilateral cervical and inguinal areas. The patient’s hemoglobin is 9.5 g/dL with mean corpuscular volume 85 fL, percentage transferrin saturation 9%, ferritin 532 ng/mL, normal B12 and folate, absolute reticulocyte count of 48 300 cells/μL, and erythrocyte sedimentation rate is 119 mm/h.
Introduction
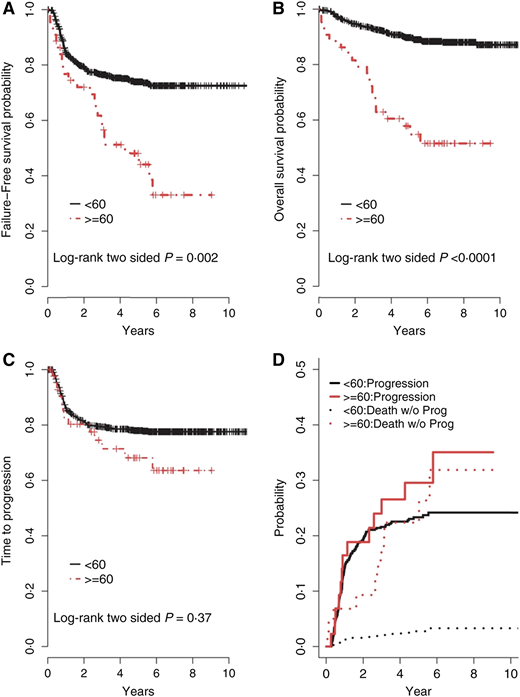
Survival rates for Hodgkin lymphoma (HL) have improved substantially over the past several decades. Using stage-adapted polychemotherapy regimens and innovative radiation techniques, 5-year progression-free survival (PFS) has reached almost 90% in younger patients; however, this progress has not translated into similar benefits for older patients. Historically, survival rates for HL patients aged ≥60 years have been disproportionately inferior compared with younger patient populations1-5 and markedly worse compared with age- and sex-matched controls.6,7 Findings from a subgroup analysis of the North American Intergroup E2496 trial that randomized advanced-stage HL patients to ABVD vs Stanford V included increased toxicity and significantly inferior outcomes for older HL patients compared with younger HL patients (Figure 1).
Outcomes comparing older HL patients with younger patients. (A) The 3- and 5-year failure-free survival for patients aged ≥60 years was 56% and 48%, respectively, compared with 76% and 74%, respectively, for patients aged <60 years (P = .002). (B) The 3- and 5-year OS for patients aged ≥60 years was 70% and 58%, respectively, compared with 93% and 90%, respectively, for patients aged <60 years (P < .0001). (C) The 2- and 5-year time-to-progression (TTP) for patients aged ≥60 years was 80% and 68%, respectively, compared with 81% and 78%, respectively, for patients aged <60 years (P = 0.37). (D) The rates of progression were determined with competing risk analysis, because death without progression is a competing risk for disease progression. The incidence rates of progression, including competing risks for patients aged ≥60 years, at 2 and 5 years were 19% and 30%, respectively, compared with 19% and 23%, respectively, for patients aged <60 years (P = .30); however, the incidence rates of death without progression for patients aged ≥60 years at 2 and 5 years were 13% and 22%, respectively, compared with 2% and 9%, respectively, for patients aged <60 years (P ≤ .0001). Reprinted with permission from Evens et al.10
Outcomes comparing older HL patients with younger patients. (A) The 3- and 5-year failure-free survival for patients aged ≥60 years was 56% and 48%, respectively, compared with 76% and 74%, respectively, for patients aged <60 years (P = .002). (B) The 3- and 5-year OS for patients aged ≥60 years was 70% and 58%, respectively, compared with 93% and 90%, respectively, for patients aged <60 years (P < .0001). (C) The 2- and 5-year time-to-progression (TTP) for patients aged ≥60 years was 80% and 68%, respectively, compared with 81% and 78%, respectively, for patients aged <60 years (P = 0.37). (D) The rates of progression were determined with competing risk analysis, because death without progression is a competing risk for disease progression. The incidence rates of progression, including competing risks for patients aged ≥60 years, at 2 and 5 years were 19% and 30%, respectively, compared with 19% and 23%, respectively, for patients aged <60 years (P = .30); however, the incidence rates of death without progression for patients aged ≥60 years at 2 and 5 years were 13% and 22%, respectively, compared with 2% and 9%, respectively, for patients aged <60 years (P ≤ .0001). Reprinted with permission from Evens et al.10
Inferior outcome for older HL patients has been attributed to a variety of factors, including presence of comorbidities, poor performance status, histologic and biologic differences (eg, increased mixed cellularity, Epstein–Barr virus (EBV)-related, and advanced-stage disease), inability to tolerate chemotherapy at full dose and schedule, and increased treatment-related toxicity and mortality. Compounding these factors has been the historic underrepresentation of older patients in HL clinical trials. More recently, there has been a renewed effort resulting in a multitude of new data generated analyzing prognostication of older HL patients in the modern era and a number of prospective clinical studies integrating targeted therapeutic agents into treatment paradigms.
Epidemiology
Historically, <5% to 10% of patients in HL randomized controlled trials were older than 60 years.2,8-10 The most accurate assessments of incidence have come from population-based studies. Two Swedish studies from 1979 to 1988 and from 1973 to 1994 showed that HL patients older than 60 years of age made up 31% and 26%, respectively, of the populations.1,11,12 The Scotland and Newcastle Lymphoma Group reported that 624 (20%) of 3373 patients in the population registry were older than 60 years. An additional analysis of the British National Lymphoma Investigation Group found that ∼15% of all HL patients were older than 65 years,13 but only 5% had been included in British National Lymphoma Investigation Group studies,9 whereas another United Kingdom population-based study confirmed the proportion of ∼20% of older HL patients.7
In addition, there are apparent differences in HL based on race. In an analysis of US Surveillance, Epidemiology, and End Results data, there were distinct age-related incidence patterns based on race.14 Incidence rates for older HL patients (ie, ages > 64 years) were highest among Hispanics, followed by whites and blacks. Furthermore, incidence of HL in Hispanics was distinctly not bimodal, with a small increase at ages 20 to 29 years, followed by an exponential-like rise, with peak incidence at ages 70 to 79 years.
Pathology
The German Hodgkin Study Group (GHSG) reported a comprehensive retrospective review of older HL patients.2 Mixed cellularity was more common in older patients (35%) vs younger HL patients (19%) (P < .001). Similar results have been seen in other studies,3,4,8,10,13,15 and EBV positivity is more common in older HL patients.7,16,17 Furthermore, EBV-associated disease has been associated with advanced-stage disease and is a negative prognostic factor in older HL patients.7,16 Older age was also shown to be associated with decreased FOXP3 regulatory T cells and increased granzyme-B+ cells in HL tumor samples, which correlated with poor outcomes.18
Case revisited
Excisional lymph node biopsy of the patient’s left supraclavicular node showed effacement by a mixed population of lymphocytes, plasma cells, eosinophils, neutrophils, and histiocytes. Scattered large cells stained positive for CD15, CD30, PAX5, and EBER. Diagnosis was consistent with classic HL, mixed cellularity type with EBV positivity by EBER in situ hybridization.
Clinical presentation
A comprehensive analysis of 372 patients aged ≥ 60 years, treated within clinical trials of the GHSG, found more frequent B symptoms, elevated erythrocyte sedimentation rate, and poorer performance status, and large mediastinal mass or other bulky disease observed less frequently vs younger patients.2 Several analyses have shown a male predominance and that older HL patients more commonly present with advanced-stage disease.4,7,12,15,19 In a recent Swedish registry analysis, the proportion of patients with advanced-stage disease increased in recent decades, although these changes could be due, in part, to the increasing use of positron emission tomography (PET)/computed tomography.11
Case revisited
Staging PET scan for the patient shows diffuse hypermetabolic disease in all aforementioned lymph node areas and bilateral hilar and retroperitoneal adenopathy, with the largest node being 5.8 cm with maximum standardized uptake value (SUVmax) of 17.9; there was no evidence of bone marrow involvement by PET. Bone marrow biopsy shows many aggregates of CD30+ lymphoid cells (accounting for 15%-20% of cellularity) suspicious for involvement by HL. Baseline echocardiogram is grossly normal, with ejection fraction of 60%, and pulmonary function tests show a slight restrictive deficit, with corrected diffusing limiting capacity of the lungs for carbon monoxide (DLCO) of 65%.
Functional status and comorbidity
The impact of a geriatric assessment (GA) in older patients with cancer has been well recognized, and it can be used to guide prognosis and delineate treatment options.20 GA utilizes validated patient-reported and objective tools to evaluate several domains that include functional status, comorbidities, psychological health, polypharmacy, nutrition, cognition, and social support. Each of these domains is predictive of comorbidity and mortality among older adults. In addition, GA was shown to be more effective than clinical judgment in identifying older B-cell lymphoma patients likely to benefit from aggressive curative therapy.21,22 Table 1 includes a synthesis of GA domains in the literature, as well as the American Society for Clinical Oncology recommendations in geriatric oncology by Mohile et al.20 When applicable, data relevant to older patients with HL were highlighted.
Domains assessed and examples of validated tools as part of a full GA
| . | Components . | Tools/measurements . | Definition of impairment (when applicable, studies of HL are referenced) . |
|---|---|---|---|
| Functional status* | Katz ADL | Bathing, dressing, maintaining continence, transferring, going to the toilet, and feeding | Dependence on ≥1 ADL |
| Associated with worse overall survival (OS) in HL15 | |||
| Lawton instrumental ADL | Ability to use telephone, shopping, managing medications, housekeeping, preparing meals, laundry, transportation, and managing finances | Assistance required with or dependence on ≥1 instrumental ADL | |
| Associated with worse progression-free survival (PFS) and OS in HL26 | |||
| Falls | — | ≥1 self-reported fall in the previous 6 mo | |
| Objective physical performance (eg, Timed “Up and Go”) | Ability to get out of a chair and walk 3 m | A score ≥ 12 indicates that the patient is at risk for falling | |
| Comorbidities* | CIRS-G55 | Scores diseases in 14 organ systems and grades each according to severity with rules for classification | A score > 10 |
| Associated with worse event-free survival and PFS in HL (univariate)26 | |||
| Charlson Comorbidity Index | Consists of 19 comorbidities weighted according to their influence on mortality | ≥2 comorbidities (adapted Charlson) | |
| Associated with inferior outcomes in HL17,24 | |||
| Psychological health | Depression (eg, Geriatric Depression Scale-15) | 15-item self-reported assessment | A score > 5 suggests presence of depression |
| Cognition | Mini-Cog | Assesses word recall and clock draw | A score < 3 suggests dementia |
| Blessed Orientation Memory Concentration | Assesses orientation, memory, and concentration | A score ≥ 6 suggests moderate impairment; a score ≥ 11 suggests severe impairment. | |
| Nutritional status | Weight loss | — | Unintentional weight loss > 10% in the past 6 mo |
| Body mass index | — | <21 kg/m2 | |
| Polypharmacy | Number of medications | Scheduled and/or as needed medications | ≥5 medications |
| Potentially inappropriate medications | Beers criteria56 | — | |
| Social support | Presence of caregivers | — | — |
| Medical Outcomes Study Social Support and Social Activity Survey57 | Assesses perceived social support and social activity limitations due to health | — | |
| Geriatric syndromes | Dementia, delirium, osteoporosis, falls, failure to thrive, sarcopenia, pressure ulcer, incontinence, neglect/abuse | — | Presence of any syndrome |
| . | Components . | Tools/measurements . | Definition of impairment (when applicable, studies of HL are referenced) . |
|---|---|---|---|
| Functional status* | Katz ADL | Bathing, dressing, maintaining continence, transferring, going to the toilet, and feeding | Dependence on ≥1 ADL |
| Associated with worse overall survival (OS) in HL15 | |||
| Lawton instrumental ADL | Ability to use telephone, shopping, managing medications, housekeeping, preparing meals, laundry, transportation, and managing finances | Assistance required with or dependence on ≥1 instrumental ADL | |
| Associated with worse progression-free survival (PFS) and OS in HL26 | |||
| Falls | — | ≥1 self-reported fall in the previous 6 mo | |
| Objective physical performance (eg, Timed “Up and Go”) | Ability to get out of a chair and walk 3 m | A score ≥ 12 indicates that the patient is at risk for falling | |
| Comorbidities* | CIRS-G55 | Scores diseases in 14 organ systems and grades each according to severity with rules for classification | A score > 10 |
| Associated with worse event-free survival and PFS in HL (univariate)26 | |||
| Charlson Comorbidity Index | Consists of 19 comorbidities weighted according to their influence on mortality | ≥2 comorbidities (adapted Charlson) | |
| Associated with inferior outcomes in HL17,24 | |||
| Psychological health | Depression (eg, Geriatric Depression Scale-15) | 15-item self-reported assessment | A score > 5 suggests presence of depression |
| Cognition | Mini-Cog | Assesses word recall and clock draw | A score < 3 suggests dementia |
| Blessed Orientation Memory Concentration | Assesses orientation, memory, and concentration | A score ≥ 6 suggests moderate impairment; a score ≥ 11 suggests severe impairment. | |
| Nutritional status | Weight loss | — | Unintentional weight loss > 10% in the past 6 mo |
| Body mass index | — | <21 kg/m2 | |
| Polypharmacy | Number of medications | Scheduled and/or as needed medications | ≥5 medications |
| Potentially inappropriate medications | Beers criteria56 | — | |
| Social support | Presence of caregivers | — | — |
| Medical Outcomes Study Social Support and Social Activity Survey57 | Assesses perceived social support and social activity limitations due to health | — | |
| Geriatric syndromes | Dementia, delirium, osteoporosis, falls, failure to thrive, sarcopenia, pressure ulcer, incontinence, neglect/abuse | — | Presence of any syndrome |
Other measures to consider20 include estimates on risk for chemotherapy toxicity (eg, Cancer and Age Research Group Score58 and the Chemotherapy Risk Assessment Scale for High-Age Patients Score59 ) and estimates on noncancer life expectancy (eg, Schonberg Index or Lee Index60 ).
ADL, activities of daily living; CIRS-G, Cumulative Illness Rating Scale-Geriatric; OS, overall survival; —, no data.
Studies suggest that the GA domain is associated with outcomes in older HL patients.
Several analyses have documented the frequent occurrence and prognostic importance of comorbidities in older patients.15,17,23-25 In a study of 194 HL patients registered between 1993 and 1996, the most frequent comorbidity was cardiovascular disease (18%), followed by chronic obstructive lung disease (13%), diabetes mellitus (10%), and hypertension (3%).24 Furthermore, patients with a severe comorbidity received systemic chemotherapy less frequently and had significantly inferior overall survival (OS). A contemporary retrospective analysis of “real-world” older HL patients found that 61% had ≥1 severe comorbidity, 26% were classified as “unfit,” 17% had the presence of a geriatric syndrome, and 13% had loss of activities of daily living (ADL) at diagnosis.15 Moreover, loss of ADL at diagnosis was a strong prognostic factor for survival in this retrospective data set. On multivariate regression, age ≥ 70 years and loss of ADL were the strongest prognostic factors for survival. Moreover, patients with both factors present at diagnosis had a 3-year OS of 0%. Advanced age within the older HL population has been shown to predict survival, with patients aged >70 years having inferior outcomes/survival vs those aged 60 to 69 years.5,12,15,25 It is also important to highlight that the International Prognostic Score is not prognostic for outcome of older HL patients.5,15,26
In a recent multicenter phase 2 study, older HL patients were treated with 2 initial doses of single-agent brentuximab vedotin (BV), followed by doxorubicin, vinblastine, dacarbazine (AVD) for 6 cycles with subsequent post-chemotherapy consolidative BV.26 In this prospective study, geriatric-based measures (eg, comorbidity score and loss of instrumental ADL) were strongly associated with patient outcomes. For HL patients treated in this study with a lower Cumulative Illness Rating Scale-Geriatric (CIRS-G) comorbidity score (ie, <10 vs ≥10), 2-year PFS rates were 100% vs 45%, respectively (P < .0001). Furthermore, patients with no loss of instrumental ADL vs loss of any instrumental ADL at baseline had 2-year PFS rates of 94% vs 25% (P < .0001), which persisted in multivariable analyses. GA has also been incorporated in other prospective studies of brentuximab vedotin in older HL patients. Together, these data support the feasibility of incorporating GA in trials of older patients with HL and suggest the need to further study the associations between geriatric-based measures and patient outcomes.
Case revisited
The patient has a history of coronary artery disease status postangioplasty 5 years prior, well-controlled hypertension, type 2 diabetes (noninsulin requiring), and a remote history of basal cell cancer (CIRS-G score = 6). The patient is a retired electrician, lives alone, and performs all self-care and instrumental ADLs without restriction (Eastern Cooperative Oncology Group performance status = 1).
Therapy-associated toxicities
Treatment-related mortality
Therapy-associated toxicities have a major impact on the outcomes of older HL patients. The reduced tolerability of conventional chemotherapy results in more toxicities overall and more severe toxicities (including fatal outcomes), the inability to maintain the scheduled dose density, and a shorter survival for relapsing or progressing patients.1,3,4,12,27-29 Studies in older HL patients have identified the most common toxicities as leukopenia, infections, and cardiopulmonary events.2,5,30,31 Severe hematologic toxicities were significantly more frequent in older vs younger HL patients treated in the randomized E2496 study.10 Not surprisingly, early termination of scheduled therapy in older patients has a negative impact on survival.2,12 Furthermore, acute toxic deaths in older HL patients reported in the literature include 19% with cyclophosphamide, vincristine, procarbazine, and prednisone (COPP)/doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and 18% with mechlorethamine, vincristine, procarbazine, and prednisone (MOPP)/ABVD.31 In the randomized study comparing a baseline bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) regimen with COPP-ABVD (HD9elderly), the treatment-related mortality (TRM) among 75 advanced-stage HL patients aged 66 to 75 years was 21% and 8%, respectively (Table 2).30 Due to early mortality risk, when analyzing disease-specific survival rates in older and more frail cancer populations, the presence of competing risks should be incorporated into analyses to account for non-HL–related events (Figure 1D).6
Selected historical chemotherapy studies for newly diagnosed older HL patients in advanced stages
| Reference . | N . | Therapy . | Outcomes . | Selected toxicities ≥ grade 3 . | TRM (%) . |
|---|---|---|---|---|---|
| Weekes et al4 | 31 | ChlVPP | 5-y EFS, 24% | NR | 13% |
| 5-y OS, 30% | |||||
| 25 | ChlVPP/ABV | 5-y EFS, 52% | 16% | ||
| 5-y OS, 67% | |||||
| Levis et al23 ,* | 57 | VEPEMB | 5-y RFS, 66% | Anemia (18%), infection (14%) | 3% |
| 5-y OS, 32% | |||||
| Ballova et al30,* | 26 | COPP/ABVD | 5-y HD-FFTF, 55% | Anemia (24%), thrombocytopenia (16%), infection (12%) | 8% |
| 5-y OS, 50% | |||||
| 42 | BEACOPP baseline | 5-y OS, 50% | Thrombocytopenia (49%), anemia (41%), infection (23%), cardiac (15%) | 21% | |
| Halbsguth et al45,* | 60 | BACOPP | 2-y PFS, 71% | Infection (23%), thrombocytopenia (22%) | 12% |
| 2-y OS, 76% | |||||
| Böll et al44,* | 59 | PVAG | 3-y PFS, 58% | Infection (22.8%), anemia (17.5%), thrombocytopenia (15.8%) | 2% |
| 3-y OS, 66% | |||||
| Proctor et al13,* | 72 | VEPEMB | CR, 61% | Neutropenic sepsis (15%), neutropenia (12%), myocardial infarction (2%) | 4% |
| 3-y PFS, 52% | |||||
| 3-y OS, 62% | |||||
| Evens et al10,* | 45 | ABVD and Stanford V | 5-y PFS, 48% | BLT (24%, primarily ABVD), gastrointestinal hemorrhage (5%, Stanford V only) | 9% |
| 5-y OS, 58% |
| Reference . | N . | Therapy . | Outcomes . | Selected toxicities ≥ grade 3 . | TRM (%) . |
|---|---|---|---|---|---|
| Weekes et al4 | 31 | ChlVPP | 5-y EFS, 24% | NR | 13% |
| 5-y OS, 30% | |||||
| 25 | ChlVPP/ABV | 5-y EFS, 52% | 16% | ||
| 5-y OS, 67% | |||||
| Levis et al23 ,* | 57 | VEPEMB | 5-y RFS, 66% | Anemia (18%), infection (14%) | 3% |
| 5-y OS, 32% | |||||
| Ballova et al30,* | 26 | COPP/ABVD | 5-y HD-FFTF, 55% | Anemia (24%), thrombocytopenia (16%), infection (12%) | 8% |
| 5-y OS, 50% | |||||
| 42 | BEACOPP baseline | 5-y OS, 50% | Thrombocytopenia (49%), anemia (41%), infection (23%), cardiac (15%) | 21% | |
| Halbsguth et al45,* | 60 | BACOPP | 2-y PFS, 71% | Infection (23%), thrombocytopenia (22%) | 12% |
| 2-y OS, 76% | |||||
| Böll et al44,* | 59 | PVAG | 3-y PFS, 58% | Infection (22.8%), anemia (17.5%), thrombocytopenia (15.8%) | 2% |
| 3-y OS, 66% | |||||
| Proctor et al13,* | 72 | VEPEMB | CR, 61% | Neutropenic sepsis (15%), neutropenia (12%), myocardial infarction (2%) | 4% |
| 3-y PFS, 52% | |||||
| 3-y OS, 62% | |||||
| Evens et al10,* | 45 | ABVD and Stanford V | 5-y PFS, 48% | BLT (24%, primarily ABVD), gastrointestinal hemorrhage (5%, Stanford V only) | 9% |
| 5-y OS, 58% |
The studies included a minimum of 25 patients.
ABV, adriamycin, bleomycin, vinblastine; BACOPP, bleomycin, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; BLT, bleomycin lung toxicity; ChlVPP, chlorambucil, vinblastine, procarbazine, and prednisone; COPP, cyclophosphamide, vincristine, procarbazine, and prednisone; CR, complete remission; EFS, event-free survival; FFTF, freedom from treatment failure; HD, Hodgkin disease; NR, not reported; OS, overall survival; PFS, progression-free survival; PVAG, prednisone, vinblastine, doxorubicin, and gemcitabine; RFS, relapse-free survival; TRM, treatment-related mortality; VEPEMB, vinblastine, cyclophosphamide, procarbazine, etoposide, mitoxantrone, and bleomycin.
Prospective clinical study.
Bleomycin lung toxicity
The incidence of bleomycin lung toxicity (BLT) in most studies of older patients with HL ranges from 5% to 31%, with increased risk seen with advancing age (ie, ages >70 years vs 60-69 years) and the number of cycles given.10,15,32-34 The primary risk factor for BLT is advancing age, in part owing to declining creatinine clearance because bleomycin is primarily metabolized renally, with 50% of the dose eliminated in 4 hours after its administration and 70% eliminated in the next 24 hours.35 The incidence of BLT among older HL patients treated in the E2496 study was 24%, with an associated death rate of 18%10 ; the vast majority of BLT cases occurred with ABVD. The incidence of pulmonary toxicity in a French retrospective series of 147 older HL patients was 31%, which occurred at a median of 5 months from the first cycle. Furthermore, the associated death rate due to BLT was 23%.34 Additional data supporting the number of doses/cycles of bleomycin as a risk factor come, in part, from GHSG HL data in older adults. BLT was rare in early-stage older patients who received 2 cycles of ABVD, but it occurred in 10% of patients who received 4 ABVD cycles, including several lethal events.33 The incidence of BLT in the “real-world” Chicago series was 32%, which was associated with a mortality of 25%.15 Moreover, the incidence of BLT was 38% vs 0% among patients who did or did not receive granulocyte colony-stimulating factor (G-CSF), respectively (P < .0001). Preclinical and retrospective studies have suggested that the risk of BLT is increased when G-CSF is given concurrently with bleomycin,36 which has been postulated as being due primarily to pulmonary recruitment of free-radical neutrophils.
Neurotoxicity
In more contemporary studies, especially ones incorporating BV, neurotoxicity has been examined (Table 3). A multicenter prospective study examined extended dosing of single-agent BV, which was followed by expanded patient cohorts that combined bendamustine or dacarbazine with BV, for older HL patients deemed ineligible in the investigator’s judgment for frontline conventional combination.37,38 In these 2 studies, the incidence rates of grade 3 neuropathy for single-agent BV and BV+dacarbazine in the frontline setting for older adults were 30% and 27%, respectively. In the aforementioned study utilizing sequential (before and after) and overall more limited dosing of BV with AVD, the risk of grade 3 neuropathy was much lower (4%).26 In most series, BV-related neuropathy improved over time in the majority of older patients and was resolved in 30% to 40%.
Selected contemporary clinical studies for newly diagnosed older HL patients in advanced stages
| Reference . | N . | Therapy . | Outcomes . | Febrile neutropenia (%) . | Peripheral neuropathy (≥grade 3; %) . | TRM (%) . |
|---|---|---|---|---|---|---|
| Forero-Torres et al37 | 27 | BV | 2-y PFS, ∼30% | 0 | 26 | NR |
| 2-y OS, NR | ||||||
| Friedberg et al38 | 42 | BV + bendamustine or dacarbazine | 2-y PFS, ∼50% | NR | 21 | NR |
| 2-y OS, NR | ||||||
| Evens et al26 | 48 | BV sequentially with AVD | 2-y PFS, 84% | 8 | 4 | 2 |
| 2-y OS, 93% | ||||||
| Böll et al49 | 25 | Lenalidomide + AVD | 3-y PFS, 70% | 4 | NR | NR |
| 3-y OS, 84% | ||||||
| Evens et al51 | 84 | A+AVD | 2-y mPFS, 70.3% | 37 | 18 | 4 |
| 102 | ABVD | 2-y OS, NR | 17 | 3 | 5 | |
| 2-y mPFS, 71.4% | ||||||
| 2-y OS, NR | ||||||
| Boell et al50 | 49 | BV + CAP | 1-y PFS, 73.9% | 27 | 0 | 2 |
| 1-y OS, 92.6% |
| Reference . | N . | Therapy . | Outcomes . | Febrile neutropenia (%) . | Peripheral neuropathy (≥grade 3; %) . | TRM (%) . |
|---|---|---|---|---|---|---|
| Forero-Torres et al37 | 27 | BV | 2-y PFS, ∼30% | 0 | 26 | NR |
| 2-y OS, NR | ||||||
| Friedberg et al38 | 42 | BV + bendamustine or dacarbazine | 2-y PFS, ∼50% | NR | 21 | NR |
| 2-y OS, NR | ||||||
| Evens et al26 | 48 | BV sequentially with AVD | 2-y PFS, 84% | 8 | 4 | 2 |
| 2-y OS, 93% | ||||||
| Böll et al49 | 25 | Lenalidomide + AVD | 3-y PFS, 70% | 4 | NR | NR |
| 3-y OS, 84% | ||||||
| Evens et al51 | 84 | A+AVD | 2-y mPFS, 70.3% | 37 | 18 | 4 |
| 102 | ABVD | 2-y OS, NR | 17 | 3 | 5 | |
| 2-y mPFS, 71.4% | ||||||
| 2-y OS, NR | ||||||
| Boell et al50 | 49 | BV + CAP | 1-y PFS, 73.9% | 27 | 0 | 2 |
| 1-y OS, 92.6% |
The studies included a minimum 25 patients.
A+AVD, BV + doxorubicin, vinblastine, and dacarbazine; CAP, cyclophosphamide, doxorubicin, and prednisone; mPFS, modified PFS; NR, not reported; OS, overall survival.
Case revisited
The patient was treated with ABVD chemotherapy. With cycle 1 of therapy, the patient experienced significant toxicity, including vomiting, ileus, abdominal pain, and febrile neutropenia. The patient was given repeated G-CSF thereafter. The majority of disease-related symptoms improved. During the second cycle of therapy, the patient developed prominent dry cough, shortness of breath, and slight hypoxia. A repeat PET scan following the second cycle of therapy showed near-complete remission of disease, as well as findings consistent with BLT. He was treated with pulse steroids, and bleomycin was discontinued; the patient continued AVD therapy. Following the third cycle of chemotherapy, the patient developed grade 2 sensory neuropathy (ie, difficulty using the phone) and, subsequently, grade 3 neuropathy after cycle 5 (ie, difficulty with bathing and dressing), at which time all therapy was stopped.
Therapy for newly diagnosed patients
Early stage
Most published early-stage HL studies have included few older patients. In the GHSG HD8 trial, patients in the early unfavorable stage were randomized to 4 courses of chemotherapy (COPP/ABVD) and involved field radiotherapy (IFRT) or extended field radiotherapy.39 Analysis of the older subgroup of patients in this study demonstrated lower 5-year freedom from treatment failure (FFTF) and OS in older patients (FFTF, 64% vs 87%; P < .001; OS, 70% vs 94%; P < .001). Importantly, older patients had poorer outcomes when treated with extended field radiotherapy compared with IFRT, with 5-year FFTF of 58% (vs 70%; P = .034) and 5-year OS of 59% (vs 81%; P = .008).40
An analysis focusing on older patients treated within the GHSG HD10 and HD11 trials included 117 early-stage HL patients treated with 2 to 4 cycles of ABVD followed by IFRT.33 Mean delay of treatment was twice as high in the older patients (2.2 vs 1.2 weeks) and World Health Organization grades 3 and 4 toxicity was also more frequent in this group (68% vs 50%) compared with younger patients. This resulted in a higher TRM in older patients. Boll et al analyzed outcomes of older HL patients treated in the GHSG HD10 and HD13 trials, which included randomization to 2 cycles of ABVD or AVD followed by IFRT or 4 cycles of ABVD and IFRT.32 In patients receiving 2 cycles of chemotherapy, respiratory AEs were rare (eg, BLT 1.5%); however, the incidence of BLT was 10% (including several related deaths) for early-stage patients who received 4 cycles of ABVD. Additional studies have analyzed other chemotherapy platforms for older early-stage patients (eg, VEPEMB [vinblastine, cyclophosphamide, procarbazine, etoposide, mitoxantrone, bleomycin] or CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone] followed by IFRT)23,41 or IFRT alone,28,42 the last of which may be considered for frail patients.
Advanced stage
Historical data.
Collectively, outcomes have improved slightly for older HL patients over the last few decades43 ; however, a standard treatment paradigm has been absent. Three- to 5-year PFS rates for untreated advanced-stage older HL patients has ranged from 28% to 55% with ABVD therapy, with associated OS rates of 31% to 67%.5,8,10,13,30,31 Moreover, toxicity was prohibitive in a number of these studies, including TRM rates of 8% to 23% (Table 2). Decreased tolerance, increased toxicity, and factors such as frailty and comorbidities pose a significant challenge in implementing aggressive treatment regimens for advanced-stage older patients. In the GHSG analysis, reduced dose density was identified as a major determinant of inferior outcome for older HL patients.2 Landgren et al reported that older HL patients who received ABVD-based chemotherapy with a relative dose intensity > 65% had significantly improved OS vs a relative dose intensity ≤ 65% (P = .001).12
Efforts to improve outcomes for older HL patients over the past several decades have included development of chemotherapy platforms with decreased intensity and regimens with individualized dosing in an attempt to mitigate toxicity (eg, chlorambucil, vinblastine, procarbazine, prednisone/cyclophosphamide, etoposide, adn bleomycin [CVP/CEB]; ChlVPP [chlorambucil, vinblastine, procarbazine, prednisone]; VEPEMB; prednisone, vinblastine, doxorubicin, gemcitabine; bleomycin, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; and vincristine, doxorubicin, bleomycin, etoposide, and prednisone [ODBEP]).4,5,13,19,27,31,44,45 As highlighted in Table 2, therapy with several of these regimens was better tolerated; however, survival rates were modest. An older nonanthracycline regimen studied in advanced-stage HL is carmustine, cyclophosphamide, vinblastine, procarbazine, and prednisone (BCVPP), which was better tolerated and associated with improved outcomes compared with MOPP in a prior Eastern Cooperative Oncology Group study.46
Proctor et al reported results from the SHIELD project, which consisted of a prospective clinical trial and a registry “real-world” component.13 A total of 103 older HL patients received VEPEMB, 72 of whom had advanced-stage disease; only nonfrail patients were eligible for the prospective study. For advanced-stage patients, the complete remission (CR) rate was 61%, and 3-year PFS and OS rates were 58% and 66%, respectively. For prognostication, achievement of CR strongly predicted survival. Factors associated with CR were comorbidity score and ADL. In the same study, there was an additional observational group of older HL patients (frail and nonfrail) treated according to physician discretion. Among registry “real-world” patients studied, ABVD was the most common treatment selected by investigators. Among advanced-stage patients, overall response rate (ORR) was modest, and the TRM was 18%. Furthermore, among 13 frail HL patients in this substudy, all died (12 from HL), with a median OS of 7 months.
Prephase and inclusion of anthracyclines.
Use of a “prephase” therapy to improve patient performance status prior to the start of definitive therapy has not been well studied in HL. In diffuse large B-cell lymphoma, Pfreundschuh showed that use of steroids as a prephase ≥1 week prior to the start of therapy improved patient performance status and reduced the number of therapy-associated deaths.47 We follow a common prephase paradigm in most newly diagnosed older HL patients by utilizing a short course of pulse steroids (eg, prednisone, 60-100 mg daily for 5 days), as was recommended in the aforementioned sequential BV-AVD study.26 At a minimum, this ameliorates disease-related symptoms while pretherapy testing and approval processes are being completed. It should be highlighted that timing of pulse steroids should be given after PET staging is completed.
In addition, anthracycline is likely an important component of therapy for older HL patients. The Nebraska Group compared ChlVPP with the hybrid ChlVPP/doxorubicin, bleomycin, and vincristine (ABV) in a nonrandomized study of 262 previously untreated HL patients.4 In older patients treated with ChlVPP, the 5-year event-free survival and OS rates were 24% and 30%, respectively, compared with 52% and 67% for patients treated with ChlVPP/ABV. In a small study, Kolstad et al used CHOP for older HL patients.41 They treated 29 patients with CHOP-21 using 2 to 4 cycles with IFRT for early-stage disease and 6 to 8 cycles, with or without IFRT, for advanced-stage disease. The CR rate was 93% and the 3-year PFS and OS rates for advanced-stage patients were 67% and 72%, respectively. In older HL patients who are otherwise fit, but in whom anthracycline is contraindicated, BCVPP may be a treatment option. Additionally, Stanford V contains 50% of the usual doxorubicin dosing compared with most other regimens; thus, it may be a consideration in otherwise fit patients in whom decreased anthracycline dosing is warranted.
Contemporary clinical trial data.
BV has been integrated into the frontline treatment of untreated older HL patients (Table 3). An initial study examined single-agent BV for older untreated HL patients deemed ineligible in the investigator’s judgment for frontline conventional combination treatment.37 The ORR was 92% with a CR rate of 72%; however, the relapse rate was high, with 2-year PFS rates < 40%. This single-agent BV study was amended to combine concurrent bendamustine or dacarbazine.38 The bendamustine arm was closed prematurely because of toxicity. Response rates were good in the concurrent BV/dacarbazine arm; however, this approach did not appear curative for most patients (ie, 2-year PFS rates of ∼50%) and may be best considered when anthracycline or combination chemotherapy is not feasible in unfit or frail patients.
In the aforementioned study of BV given before and after standard AVD therapy for untreated older HL patients,26 the choice of sequential therapy (vs concurrent) was predicated on assumptions that (1) initial single-agent BV therapy may improve performance status, establish earlier disease control, and increase the likelihood of successful potentially curative therapy; (2) there would be minimization of overlapping neurotoxicity with concurrent AVD; and (3) consolidation would decrease the risk of relapse. Overall, 77% of patients completed the initial BV dosing and 6 AVD cycles, and 73% received ≥1 dose of BV consolidation. The ORR and CR rates after the initial 2 lead-in doses of BV were 82% and 36%, respectively, and they were 95% and 90%, respectively, after 6 AVD cycles. The most common grade 3/4 AEs were neutropenia (44%), febrile neutropenia and pneumonia (8%), and diarrhea (6%). By intent-to-treat, the 2-year PFS and OS rates were 84% and 93%, respectively. In addition, response to the initial 2 doses of BV (ie, CR or PR vs not) was associated with survival (ie, 2-year PFS 100% vs 50%, respectively, P = .007).
Response-adapted therapy has not been well studied in older HL patients, but if ABVD therapy is used, it would be rational to omit bleomycin following an interim negative PET-2 vis-à-vis the RATHL study design for advanced-stage patients.48 However, therapy should not be intensified to BEACOPP therapy with positive PET-2, because older patients do not tolerate this regimen, as elucidated before. Changing therapy to a noncrossresistant regimen should be tested further in clinical trials (eg, BV-based therapy) for early nonresponders.
A recently published phase 1 study examined lenalidomide given concurrently (daily from days 1 to 21) with AVD chemotherapy for older HL patients.49 Twenty-five HL patients with a median age of 67 years (range, 61-76) were treated with escalating doses of lenalidomide, with dose limiting toxicity (DLT) evaluation of 20 patients elucidating a recommended phase 2 dose of 25 mg. Dose-limiting toxicities were mainly hematologic but also included 3 thromboembolic events, despite documented aspirin prophylaxis. The ORR was 79% for evaluable patients and 86% in patients treated with ≥20 mg lenalidomide. The GHSG and the Nordic Lymphoma Group recently presented data using BV concurrently with cyclophosphamide, doxorubicin, and prednisone for fit older HL patients with CIRS-G ≤ 6.50 Among 48 eligible advanced-stage patients, median age was 67 years (range, 60-84), and 50% had an International Prognostic Score of 4 to 7. The ORR was 98% with a 65% CR rate. Notably, there was no grade 3 neuropathy seen, and TRM was low.
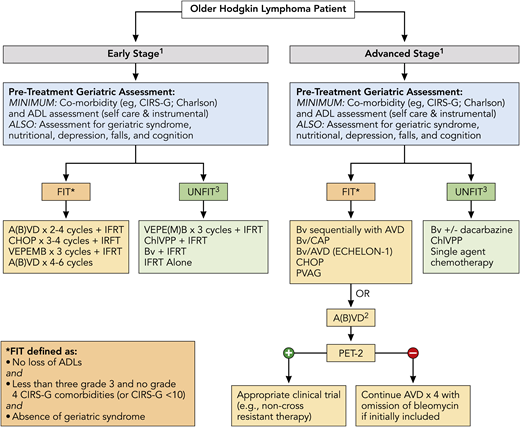
Finally, outcomes were recently analyzed across ages for the pivotal phase 3 ECHELON-1 study that examined BV + doxorubicin, vinblastine, and dacarbazine (A+AVD) vs ABVD in patients with untreated advanced-stage classic HL.51 Overall, 186 patients in the intent-to-treat population were aged ≥60 years. With a median follow-up of 2 years, modified PFS per independent review facility was similar for ABVD and A+AVD (70.3% vs 71.4%). For older patients with stage IV disease, there was an increase in median PFS per investigator, with A+AVD vs ABVD of 74.0 months vs 59.9 months (hazard ratio, 0.66; P = .20). Grades 3 and 4 AEs were seen in 87% and 80% of patients, respectively. Any grade of febrile neutropenia was seen in 37% and 17% of older patients, respectively, and pulmonary AEs were seen in 2% and 17%, respectively. Additionally, treatment-emergent peripheral neuropathy was reported in 65% and 43% of patients, respectively (≥grade 3 in 18% vs 3%, respectively); 65% and 60% of patients had complete resolution or improvement of these events. Figure 2 highlights overarching treatment recommendations for newly diagnosed older HL patients, including the importance of assessment of a baseline GA with therapeutic options directed in part on fit vs unfit status.
Treatment algorithm for newly diagnosed older HL patients. All patients should undergo a GA to determine fitness prior to initiation of treatment, which should include at least evaluation of ADL and comorbidities (see Table 1) and calculation of noncancer expected survival (https://eprognosis.ucsf.edu/leeschonberg.php). Treatment may be divided by disease stage (early stage vs advanced stage). Treatment options are based on published data and investigator experience (listed by order of preference); a clinical trial should always be considered. 1There should be consideration of a prephase of steroid therapy, prior to initiation of definitive therapy, especially in frail and/or symptomatic patients; this should be given after completion of a PET scan. 2ABVD may be used vis-à-vis the RATHL study design, with treatment decisions based on the PET scan following cycle 2; however, escalation to BEACOPP for positive PET-2 should not be done. 3Therapy for patients who are classified as unfit based primarily on cardiac status, but who are otherwise stable without loss of ADL and minimal other comorbidities, may include Stanford V or BCVPP therapy. A(B)VD, doxorubicin, bleomycin, vinblastine, dacarbazine, with inclusion of bleomycin at physician discretion or a priori exclusion of bleomycin (ie, AVD); BCVPP, carmustine, cyclophosphamide, vinblastine, procarbazine, and prednisone; Bv/CAP, brentuximab, cyclophosphamide, doxorubicin, and prednisone; PVAG, prednisone, vinblastine, doxorubicin, and gemcitabine; RATHL, Response Adapted Therapy in Advanced Hodgkin Lymphoma; VEPE(M)B, vinblastine, cyclophosphamide, procarbazine, etoposide, mitoxantrone, and bleomycin, with inclusion of mitoxantrone at physician’s discretion or a priori exclusion of mitoxantrone. * There are additional published definitions that have defined patient fitness, which include ages ≥80 years (regardless of other factors) and other variations in CIRS-G scoring (eg, <5 comorbidities score of 2, etc).
Treatment algorithm for newly diagnosed older HL patients. All patients should undergo a GA to determine fitness prior to initiation of treatment, which should include at least evaluation of ADL and comorbidities (see Table 1) and calculation of noncancer expected survival (https://eprognosis.ucsf.edu/leeschonberg.php). Treatment may be divided by disease stage (early stage vs advanced stage). Treatment options are based on published data and investigator experience (listed by order of preference); a clinical trial should always be considered. 1There should be consideration of a prephase of steroid therapy, prior to initiation of definitive therapy, especially in frail and/or symptomatic patients; this should be given after completion of a PET scan. 2ABVD may be used vis-à-vis the RATHL study design, with treatment decisions based on the PET scan following cycle 2; however, escalation to BEACOPP for positive PET-2 should not be done. 3Therapy for patients who are classified as unfit based primarily on cardiac status, but who are otherwise stable without loss of ADL and minimal other comorbidities, may include Stanford V or BCVPP therapy. A(B)VD, doxorubicin, bleomycin, vinblastine, dacarbazine, with inclusion of bleomycin at physician discretion or a priori exclusion of bleomycin (ie, AVD); BCVPP, carmustine, cyclophosphamide, vinblastine, procarbazine, and prednisone; Bv/CAP, brentuximab, cyclophosphamide, doxorubicin, and prednisone; PVAG, prednisone, vinblastine, doxorubicin, and gemcitabine; RATHL, Response Adapted Therapy in Advanced Hodgkin Lymphoma; VEPE(M)B, vinblastine, cyclophosphamide, procarbazine, etoposide, mitoxantrone, and bleomycin, with inclusion of mitoxantrone at physician’s discretion or a priori exclusion of mitoxantrone. * There are additional published definitions that have defined patient fitness, which include ages ≥80 years (regardless of other factors) and other variations in CIRS-G scoring (eg, <5 comorbidities score of 2, etc).
Therapy for relapsed disease
Prospective randomized studies have not specifically evaluated the treatment of relapsed older HL patients. Therefore, treatment recommendations in this setting are largely based on retrospective and subset study analyses. Treatment options for relapsed or refractory HL in older patients include intensified treatment, polychemotherapy, radiotherapy in selected patients, single-agent (palliative) chemotherapy, and best supportive care. With the development of novel drugs with impressive single-agent activity, potentially less toxic alternative treatments are available for older patients in whom conventional treatment is not an option because of comorbidity.
The selection of treatment strategies is guided in part by patient preference, comorbidity/functional status, and the duration of response to first-line therapy. In patients with long-lasting remission after first-line treatment, polychemotherapy regimens, such as prednisone, vinblastine, doxorubicin, and gemcitabine (PVAG); CHOP; or the oral prednisone, etoposide, chlorambucil, and lomustine (PECC) regimen,52 are valid options, the latter being a consideration for unfit patients. Further, drugs with known single-agent activity in HL include alkylating agents (eg, ifosfamide and procarbazine), gemcitabine, vinca alkaloids, and platinum derivatives.
Smaller retrospective single-center studies have suggested that high-dose chemotherapy, followed by autologous stem cell support, may be effective for selected older patients with relapsed HL.53 A recent large GHSG analysis examined 105 older relapsed/refractory HL patients.54 Different second-line treatment strategies were used, including intensified salvage regimens in 22%, conventional polychemotherapy and/or salvage radiotherapy with curative intent in 42%, and palliative approaches, such as single-agent chemotherapy and best supportive care, in 31%. A prognostic score applied using the risk factors early relapse, clinical stage III/IV, and anemia identified patients with favorable and unfavorable prognoses. Median OS for the entire cohort of relapsing older HL patients was 12 months and OS at 3 years was 31%. Survival was significantly different within different risk groups (ie, ≤1 RF: 3-year OS, 59%; ≥2 RFs: 3-year OS, 9%). In low-risk patients, the impact of therapy on survival was significant in favor of the conventional polychemotherapy/salvage radiotherapy approach. In high-risk patients, OS was low overall and did not differ significantly between treatment strategies.54
These findings may be useful in guiding treatment decisions, whereas there remains a critical need to evaluate the safety and efficacy of novel compounds in older patients with relapsed/refractory HL. Although only a handful of older HL patients who have received anti–PD-1 checkpoint inhibitors have been reported in the literature, these agents provide a viable targeted treatment option for relapsed or refractory older HL patients.
Case revisited
The patient remained in remission for ∼18 months following completion of the prior AVD. Now at age 71 years old, he presents with mild anemia, fatigue, and increasing retroperitoneal and inguinal adenopathy. Biopsy confirms mixed cellularity classic HL. Since the initial diagnosis, the patient had repeat angioplasty 12 months ago and now has insulin-requiring diabetes and mild renal insufficiency (creatinine clearance 65 mL/min). His prior sensory neuropathy returned to grade 1. Current CIRS-G score is 9, and he performs all ADL without restriction. Salvage multiagent chemotherapy and a clinical trial were discussed with the patient; he chose the latter and was treated with BV and checkpoint inhibitor combination therapy. The patient experienced transient grade 2 rash after cycle 2 and grade 2 sensory neuropathy following cycle 5. He achieved CR and deferred stem cell transplant. He came off study following 8 months of therapy and remains in remission 1.5 years off study.
Conclusions
Patients older than 60 years of age represent a distinct clinical subset of HL that will likely be magnified over time, because the most rapidly growing segment in the population is persons aged >65 years, especially those who are ≥80 years of age. Older HL patients often have different disease biology compared with younger patients, with an increased incidence of mixed cellularity histology and EBV-related disease, and they present more frequently with advanced-stage disease. For prognostication of older patients and delineation of appropriate therapeutic options, incorporation of GAs, including comorbidities and functional status, is important, and these measures should continue to be integrated and examined in prospective studies for older HL patients.
Although outcomes have seemingly improved, especially via the integration of targeted agents, the treatment of older HL patients remains challenging. GAs should be done (with at least assessment of ADL and comorbidities) to help guide the choice of optimum patient-specific therapeutic options. Generally, treatment of fit older HL patients without severe comorbidities should be given with curative intent using anthracycline-based chemotherapy paradigms that are similar overall to those used in younger patients. This may also include an abbreviated steroid prephase prior to definitive therapy: 2 to 4 cycles of chemotherapy and IFRT for early-stage disease and 6 cycles of chemotherapy for advanced stages. Intensive regimens, such as BEACOPP, are too toxic, whereas less-intensive nonanthracycline regimens, such as CVP/CEB, are insufficient. Outside of a clinical trial, ABVD may be considered for older HL patients; however, one should be mindful of the potential for severe treatment-related toxicities, especially BLT. Balancing the risk/benefit ratio, minimization or a priori omission of bleomycin may also be considered (ie, AVD), especially for patients older than 70 years of age. In the United States, integration of BV with AVD chemotherapy for untreated advanced-stage older HL patients is an additional option. Moreover, data incorporating BV sequentially before and after AVD chemotherapy represent the best-reported outcomes to date. The objectives of future investigations in fit patients should be to attempt to maintain these robust outcomes with less treatment (especially chemotherapy).
Therapy for unfit and frail patients or ones with severe comorbidities is less clear and should be highly individualized. Lower-intensity chemotherapy programs, including regimens that incorporate BV (alone or combined with dacarbazine) may be considered. Finally, integration of other novel targeted therapeutic agents, such as checkpoint inhibitors, is being actively evaluated for unfit and fit older HL patients (eg, NCT02758717, NCT03226249, NCT03033914), and concerted efforts should be made to continue to prospectively tailor therapy based upon a GA prior to therapy and to concurrently incorporate response-adapted concepts into treatment paradigms.
Correspondence
Andrew M. Evens, Rutgers Cancer Institute of New Jersey, 195 Little Albany St, New Brunswick, NJ 08901; e-mail: andrew.evens@rutgers.edu.
References
Competing Interests
Conflict-of-interest disclosure: A.M.E. serves on the advisory board or educational forum (with honorarium) for Bayer, Seattle Genetics, Affimed, Verastem Oncology, Pharmacyclics, Research to Practice, and Physicians’ Education Resource and has received research support from Takeda, Seattle Genetics, Merck, National Institutes of Health/National Cancer Institute, Leukemia and Lymphoma Society, and Oncology Research Information Exchange Network (ORIEN). J.C., K.P.L., and K.A.D. declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.