Abstract
Bleeding is the main complication of oral anticoagulant (OAC) therapy, with major bleeds occurring in about 2% to 4% of OAC-treated patients per year. Although direct oral anticoagulants (DOACs) reduce the risk of major, fatal, and intracranial hemorrhage, major DOAC-related bleeding is associated with substantial morbidity and mortality, with case-fatality rates of 8% to 15% reported. Specific reversal agents for dabigatran (idarucizumab) and factor Xa inhibitors (andexanet) correct laboratory indices of anticoagulant effect. Clinical studies suggest that the majority of patients receiving these agents for DOAC-associated major bleeds experience clinical hemostasis. However, uncertainty remains regarding the incremental benefit of these agents and prothrombin complex concentrates over supportive measures alone, based on cohort studies that lacked control groups. Similar methodologic limitations preclude firm conclusions regarding the harms associated with use of these agents. Importantly, patients with DOAC-related major bleeding have substantial short-term risks of thrombosis and mortality, emphasizing the need for individualized patient assessment and protocolized bleed management strategies that include assessment of candidacy for safe resumption of OACs. With expanding indications and increasing prevalence of DOAC-eligible patients, bleeding complications and their management represent an ever-greater major health problem.
Learning Objectives
Understand the evidence supporting the use of specific DOAC reversal agents and prothrombin complex concentrates for DOAC-treated patients with bleeding
Identify areas of uncertainty regarding the benefit and harm of reversal and hemostatic strategies
Clinical case
An 85-year-old man presented to the emergency department with severe back pain and presyncope. He had a history of atrial fibrillation, hypertension, type 2 diabetes mellitus, and a transient ischemic attack, and he was receiving rivaroxaban 20 mg by mouth daily, with the last dose received about 4 hours before presentation. Over the last several weeks, he had been receiving meloxicam daily for worsening pain caused by hip osteoarthritis. He had no known trauma. Examination revealed that his blood pressure was 84/58 mm Hg and his heart rate was 121 beats per minute. He was transferred to a monitored setting, and intravenous access was established, followed by resuscitation with isotonic IV fluids. Laboratory investigations revealed hemoglobin of 5.2 g/dL (last known value, 11.8 g/dL) and serum creatinine of 1.8 mg/dL (last known value, 0.9 mg/dL). Routine coagulation tests showed an elevated international normalized ratio of 1.8 and an activated partial thromboplastin time of 36 seconds. An urgent computed tomographic scan of the abdomen demonstrated a large retroperitoneal hematoma with extravasation of contrast. The emergency department physician requested approval for an anticoagulant reversal agent as per the institution’s protocol for anticoagulant-related major bleeding.
Bleeding is the main complication of anticoagulant therapy
Oral anticoagulants (OACs) are widely used to prevent and treat thrombosis, with approximately 24 million Medicare claims annually in the United States.1 Bleeding is the main complication of OAC therapy, with an annual risk of major bleeding of about 2% to 4% in contemporary studies.2-6 Although direct oral anticoagulants (DOACs) reduce the risk of major, fatal, and intracranial bleeding compared with warfarin, case-fatality rates of 8% to 15% are reported in DOAC-treated patients who have major bleeds.3-10 Intracranial hemorrhage, although uncommon, is associated with a risk of 30-day mortality that approaches 50%.11 As a class, DOACs increase the risk of bleeding from the gastrointestinal tract, the most frequent site of hemorrhage.5,12 In addition to acute bleeding complications, OACs are permanently discontinued in up to 50% of patients after bleeding, thereby exposing these patients to risks of thrombosis and death.13 Concerns about bleeding also likely influence decisions to withhold OACs in eligible patients and to use inappropriately low doses of DOACs for which net clinical benefit is not established.14-16 Over the next 50 years, the number of patients with indications for OACs is expected to increase ≥2.5-fold, making OAC-related bleeding complications a growing major health issue.17
The anticoagulant effect of DOACs is reversible in bleeding patients
Dabigatran
Dabigatran is the only oral direct thrombin inhibitor approved for prevention of stroke and systemic embolism in atrial fibrillation and for treatment and prevention of venous thromboembolism. Idarucizumab, a monoclonal antibody with high affinity for dabigatran, specifically attenuates its anticoagulant effect as measured by correction of abnormal laboratory indices and reduction of unbound (active) dabigatran (Table 1).18-20 It is the only approved agent in the United States, Europe, and Canada for reversal of dabigatran for major bleeding or urgent surgery, based on the results of the prospective, single-cohort, open-label REVERSE-AD (Reversal Effects of Idarucizumab on Active Dabigatran) study.20 In REVERSE-AD, idarucizumab was administered (as two 2.5-g intravenous boluses) to 503 dabigatran-treated patients for acute bleeding (n = 301) or urgent surgery (n = 202), the majority of whom were treated for atrial fibrillation (96%). Gastrointestinal bleeds were the most common (46%), followed by intracranial (33%) and trauma-related (26%) bleeds. Among 461 patients (92%) with prolonged dilute thrombin time or ecarin clotting time at baseline, the median maximum percentage of reversal within 4 hours of administration of idarucizumab was 100% (95% confidence interval [CI], 100-100). The median concentration of unbound dabigatran decreased to <20 ng/mL after idarucizumab administration, an effect that was sustained for 24 hours in the majority of patients, although levels >20 ng/mL recurred in 23% of patients. Hemostasis was judged by treating clinicians. Among the 203 patients who were evaluable for assessment of hemostasis, 68% experienced bleeding cessation within 24 hours, and the median time to hemostasis was 2.5 hours (95% CI, 2.2-3.9). Normal periprocedural hemostasis was achieved in 93% of surgical patients, as determined by treating physicians. Although idarucizumab attenuates the anticoagulant effect of dabigatran, the methodological limitations of a single-cohort observational study (lacking a control group) preclude definitive conclusions regarding whether dabigatran confers incremental clinical benefit with respect to hemostasis in addition to withdrawal of dabigatran, drug clearance (ie, short half-life), and supportive measures.
Summary of specific DOAC reversal agent characteristics and key clinical findings
| . | Idarucizumab . | Andexanet alfa . | |
|---|---|---|---|
| Structure | Humanized monoclonal antibody fragment against dabigatran | Human recombinant factor Xa variant that lacks catalytic and membrane binding activity | |
| Target | Dabigatran | Direct and indirect FXa inhibitors | |
| Binding | Noncompetitive | Competitive | |
| Dosage form | Supplied as 5 g boxed kit with two separate ready-to-use vials each containing 2.5 g/50 mL | 200-mg vials of lyophilized powder for reconstitution | |
| Dosing regimen studied | Total 5 g (two 2.5 g/50 mL vials) | Low dose: 400 mg IV bolus followed by continuous infusion of 4 mg/min up to 120 min (480 mg) for rivaroxaban or apixaban taken more than 8 h before | |
| High dose: 800 mg IV bolus followed by continuous infusion of 8 mg/min (960 mg) for rivaroxaban within last 8 h or unknown time, edoxaban, enoxaparin | |||
| Dosing as per US Food and Drug | Total 5 g (two 2.5 g/50 mL vials) | Dosing based on type of factor Xa inhibitor and timing of last dose: | |
| Administration package insert | Rivaroxaban | ||
| Last dose ≤10 mg: low dose | |||
| Last dose >10 mg or unknown: if <8 h, high dose, if ≥8 h, low dose | |||
| Apixaban | |||
| Last dose ≤ 5 mg: low dose | |||
| Last dose >5 mg or unknown: if <8 h, high dose, if ≥8 h, low dose | |||
| Storage | Refrigeration of intact vials (2°C-8°C) and protection from light | Refrigeration of intact vials (2°C-8°C) | |
| Registration study | REVERSE-AD (n = 503) | ANNEXA-4 (n = 352) | |
| Population | Group A: Dabigatran-treated patients with acute bleeding (n = 301) | Acute major bleeding on apixaban, rivaroxaban, edoxaban, enoxaparin | |
| Group B: Dabigatran-treated patients needing urgent surgery (n = 202) | |||
| Efficacy | |||
| Reversal of anticoagulant effect | Median maximum reversal of prolonged dTT or ECT within 4 h: 100% (95%CI 100-100) | Reduction of anti-Xa activity from baseline | |
| Rivaroxaban: 92% (95% CI, 88-94) | |||
| Apixaban: 92% (95% CI, 91-93) | |||
| Hemostasis, n (%) | Group A: 134/201 (68) | Excellent/good: 208 (82) | |
| Group B: 184/197 (93) | |||
| Safety | |||
| Thrombotic events, n (%) | |||
| 30 days | Group A: 14 (5) | 34 (10) | |
| Group B: 10 (5) | — | ||
| 90 days | Group A: 19 (6) | ||
| Group B: 15 (7) | |||
| Mortality, n (%) | |||
| 30 days | Group A: 39 (13) | 49 (14) | |
| Group B: 26 (13) | |||
| 90 days | Group A: 57 (19) | — | |
| Group B: 38 (19) | |||
| Restart of antithrombotics, n (%) | |||
| 30 days | — | Any† 220 (62), oral 100 (28) | |
| 90 days | Group A: 220 (73)* | — | |
| Group B: 181 (90)* | |||
| . | Idarucizumab . | Andexanet alfa . | |
|---|---|---|---|
| Structure | Humanized monoclonal antibody fragment against dabigatran | Human recombinant factor Xa variant that lacks catalytic and membrane binding activity | |
| Target | Dabigatran | Direct and indirect FXa inhibitors | |
| Binding | Noncompetitive | Competitive | |
| Dosage form | Supplied as 5 g boxed kit with two separate ready-to-use vials each containing 2.5 g/50 mL | 200-mg vials of lyophilized powder for reconstitution | |
| Dosing regimen studied | Total 5 g (two 2.5 g/50 mL vials) | Low dose: 400 mg IV bolus followed by continuous infusion of 4 mg/min up to 120 min (480 mg) for rivaroxaban or apixaban taken more than 8 h before | |
| High dose: 800 mg IV bolus followed by continuous infusion of 8 mg/min (960 mg) for rivaroxaban within last 8 h or unknown time, edoxaban, enoxaparin | |||
| Dosing as per US Food and Drug | Total 5 g (two 2.5 g/50 mL vials) | Dosing based on type of factor Xa inhibitor and timing of last dose: | |
| Administration package insert | Rivaroxaban | ||
| Last dose ≤10 mg: low dose | |||
| Last dose >10 mg or unknown: if <8 h, high dose, if ≥8 h, low dose | |||
| Apixaban | |||
| Last dose ≤ 5 mg: low dose | |||
| Last dose >5 mg or unknown: if <8 h, high dose, if ≥8 h, low dose | |||
| Storage | Refrigeration of intact vials (2°C-8°C) and protection from light | Refrigeration of intact vials (2°C-8°C) | |
| Registration study | REVERSE-AD (n = 503) | ANNEXA-4 (n = 352) | |
| Population | Group A: Dabigatran-treated patients with acute bleeding (n = 301) | Acute major bleeding on apixaban, rivaroxaban, edoxaban, enoxaparin | |
| Group B: Dabigatran-treated patients needing urgent surgery (n = 202) | |||
| Efficacy | |||
| Reversal of anticoagulant effect | Median maximum reversal of prolonged dTT or ECT within 4 h: 100% (95%CI 100-100) | Reduction of anti-Xa activity from baseline | |
| Rivaroxaban: 92% (95% CI, 88-94) | |||
| Apixaban: 92% (95% CI, 91-93) | |||
| Hemostasis, n (%) | Group A: 134/201 (68) | Excellent/good: 208 (82) | |
| Group B: 184/197 (93) | |||
| Safety | |||
| Thrombotic events, n (%) | |||
| 30 days | Group A: 14 (5) | 34 (10) | |
| Group B: 10 (5) | — | ||
| 90 days | Group A: 19 (6) | ||
| Group B: 15 (7) | |||
| Mortality, n (%) | |||
| 30 days | Group A: 39 (13) | 49 (14) | |
| Group B: 26 (13) | |||
| 90 days | Group A: 57 (19) | — | |
| Group B: 38 (19) | |||
| Restart of antithrombotics, n (%) | |||
| 30 days | — | Any† 220 (62), oral 100 (28) | |
| 90 days | Group A: 220 (73)* | — | |
| Group B: 181 (90)* | |||
ANNEXA-4, Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor Who Have Acute Major Bleeding; CI, confidence interval; dTT, dilute thrombin time; ECT, ecarin clotting time; REVERSE-AD, Reversal Effects of Idarucizumab on Active Dabigatran study.
Included prophylactic or therapeutic dose anticoagulant or antiplatelet therapy.
Included prophylactic or therapeutic dose of anticoagulant (parenteral or oral).
Oral direct factor Xa inhibitors
Oral direct factor Xa (FXa) inhibitors include apixaban, rivaroxaban, edoxaban, and betrixaban. Andexanet alfa is a recombinant, catalytically inactive recombinant FXa molecule that lacks membrane-binding activity but retains affinity for FXa inhibitors. It reduces anti-FXa activity and unbound drug levels in individuals treated with FXa inhibitors.10,21,22 It is the only approved agent in the United States and Europe for reversal of apixaban or rivaroxaban for life-threatening or uncontrolled bleeding, based on the prospective, single-cohort, open-label ANNEXA-4 registration study (Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor Who Have Acute Major Bleeding).10 In ANNEXA-4, 352 individuals presenting with acute major bleeding within 18 hours of apixaban (n = 194), rivaroxaban (n = 128), enoxaparin (n = 20), or edoxaban (n = 10) were treated with andexanet alfa administered as an intravenous bolus, followed by a 2-hour infusion dosed according to the type of FXa inhibitor and timing of the last FXa inhibitor dose (Table 1). All patients who received a dose of andexanet comprised the safety population (n = 352), and those who retrospectively had baseline anti-FXa activity of ≥75 ng/mL and major bleeding at presentation as adjudicated by committee comprised the efficacy population (n = 254). FXa inhibitors were prescribed for atrial fibrillation in 80%, and the majority of bleeding events were intracranial (64%) and gastrointestinal (26%). Hemostatic efficacy was adjudicated by an independent committee using predefined criteria. At the end of the andexanet bolus, anti-FXa activity was reduced from baseline by 92% (95% CI, 91-93) in apixaban-treated patients and by 92% (95% CI, 88-94) in rivaroxaban-treated patients. This effect persisted for the duration of the infusion, followed by a subsequent increase in anti-FXa activity seen 4 hours after discontinuation, the implications of which are uncertain because hemostasis was achieved in the majority of patients. Of 254 evaluable patients, 82% were adjudicated as having good/excellent hemostasis at 12 hours (95% CI, 77-87), with similar results for gastrointestinal (85%; 95% CI, 76-94) and intracranial (80%; 95% CI, 74-86) bleeding. Overall, there was no relationship found between hemostatic efficacy and reduction in anti-FXa activity by receiver operating characteristic curve analysis. However, the magnitude of the reduction in anti-FXa activity was a predictor of hemostatic efficacy in patients with intracranial hemorrhage (area under curve, 0.64; 95% CI, 0.52-0.74). Andexanet has not been studied in patients requiring urgent surgery and is not approved for this indication.
Prothrombin complex concentrates (PCCs) contain plasma-derived inactive vitamin K–dependent coagulation factors, with 3-factor (factors II, IX, and X) and 4-factor (factors II, VII, IX, and X) formulations available. The effect of PCCs on laboratory indices of DOAC anticoagulant effect has been studied in in vivo/ex vivo experiments, animal models, and human volunteers, showing conflicting and, at best, modest effects (reviewed elsewhere).23 Although PCCs do not appear to correct abnormal laboratory indices of hemostasis per se, they may have utility as hemostatic agents for management of major bleeding (Table 2). In a Swedish cohort study, 4-factor PCC (weight <65 kg, 1500 units; weight >65 kg, 2000 units) was administered to 84 prospectively enrolled patients with major bleeding who were receiving apixaban or rivaroxaban.24 Hemostatic effectiveness was determined using guidance from the International Society on Thrombosis and Haemostasis on the basis of a review of clinical notes in the medical record. The majority of patients were treated for atrial fibrillation (75%) and experienced intracranial bleeding (70%). The median time from FXa inhibitor administration to PCC treatment was 12 hours (interquartile range [IQR], 9-16). Hemostasis was classified as effective in 69% of patients. In a Canadian cohort study, 4-factor PCC (fixed dose of 2000 units) was administered to 66 prospectively enrolled patients with apixaban- or rivaroxaban-related major bleeding.25 The primary outcome was good effectiveness of PCC as assessed by treating physicians with the aid of an assessment guide. The majority of patients were treated for atrial fibrillation (82%) and experienced intracranial bleeding (55%) or gastrointestinal bleeding (24%). The median time from last dose of FXa inhibitor to PCC administration was 17 hours (IQR, 12-21). Hemostasis was judged as good in 65%, moderate in 20%, and poor/none in 15% of patients.
Prothrombin complex concentrate for management of major bleeding in patients receiving apixaban or rivaroxaban
| . | Majeed et al24 (N = 84) . | Schulman et al25 (N = 66) . |
|---|---|---|
| Design | Cohort, prospective enrollment | Cohort, prospective enrollment |
| Treatment | 4-Factor PCC | 4-Factor PCC |
| <65 kg, 1500 units | 2000 units | |
| >65 kg, 2000 units | ||
| Time from factor Xa inhibitor, median (IQR) | 12 h (9–16) | 18 h (12–21) |
| Hemostatic effectiveness, n (%) | 58 (69) | — |
| Good | — | 43 (65) |
| Moderate | — | 13 (20) |
| Poor/none | — | 10 (15) |
| Thromboembolism, n (%) | 3 (4) | 5 (8) |
| Death, n (%) | 27 (32) | 9 (14) |
| Restart of oral anticoagulants, n (%) | NA | 41 (62)* |
| . | Majeed et al24 (N = 84) . | Schulman et al25 (N = 66) . |
|---|---|---|
| Design | Cohort, prospective enrollment | Cohort, prospective enrollment |
| Treatment | 4-Factor PCC | 4-Factor PCC |
| <65 kg, 1500 units | 2000 units | |
| >65 kg, 2000 units | ||
| Time from factor Xa inhibitor, median (IQR) | 12 h (9–16) | 18 h (12–21) |
| Hemostatic effectiveness, n (%) | 58 (69) | — |
| Good | — | 43 (65) |
| Moderate | — | 13 (20) |
| Poor/none | — | 10 (15) |
| Thromboembolism, n (%) | 3 (4) | 5 (8) |
| Death, n (%) | 27 (32) | 9 (14) |
| Restart of oral anticoagulants, n (%) | NA | 41 (62)* |
IQR, interquartile range; NA, not available; PCC, prothrombin complex concentrate.
Parenteral or oral anticoagulants (unknown dosing).
Similarly to idarucizumab, andexanet rapidly attenuates the anticoagulant effect of FXa inhibitors. However, there is uncertainty with respect to incremental clinical benefit of both andexanet and PCCs in studies lacking control groups of patients treated without these agents. A randomized trial is currently underway to assess the efficacy and safety of andexanet compared with usual care in patients with intracranial bleeding receiving FXa inhibitors (Trial of Andexanet in ICH Patients Receiving an Oral FXa Inhibitor; registered at www.clinicaltrials.gov as #NCT03661528).
Patients treated for DOAC-related bleeding are at substantial short-term risk of thrombosis and death
The 30-day rates of thrombotic events after major bleeding in the REVERSE-AD and ANNEXA-4 studies were 5% and 10%, respectively (Table 1).10,20 Similarly, the 30-day rates of thrombosis in the cohort studies evaluating PCC for FXa inhibitor–associated bleeding were 4% and 8%, respectively (Table 2).10,20 In a randomized trial of PCC compared with plasma for warfarin-associated major bleeding, the 30-day rates of thrombosis were 8% in the PCC group and 6% in the plasma group.26 The majority of thromboembolic events occurred in patients in whom oral anticoagulants had not been resumed, although most received some form of antithrombotic therapy including prophylactic or therapeutic dose anticoagulants or antiplatelet therapies (Table 1). Because of the methodological limitations of single-cohort studies lacking control groups, the possible incremental harm associated with use of specific reversal or hemostatic agents remains uncertain for these older, highly comorbid patients with elevated thrombotic risk at baseline in whom anticoagulants have been discontinued. A potential prothrombotic mechanism unique to andexanet is its interaction with tissue factor pathway inhibitor (TFPI), which normally inhibits the tissue factor–factor VIIa complex. Binding of andexanet to TFPI decreases circulating concentrations of TFPI, which may increase thrombin generation. Although not directly comparable, the reported annual risks of ischemic stroke in atrial fibrillation (≈5%) and recurrence after unprovoked venous thromboembolism (5%-10%) in non-anticoagulated patients are useful to illustrate that the post-bleed setting is a high-risk time for thrombosis.27-29
Although the degree of morbidity and mortality associated with major bleeds is typically attributed to intracranial bleeding, patients with extracranial major bleeding also have a significant risk of death, as shown in a substudy of ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) in which such patients had a high mortality rate of 9.2% within 30 days and a 12-fold increased risk of all-cause death in comparison with those without such bleeding.30 In REVERSE-AD and ANNEXA-4, the overall 30-day mortality rates were 13% and 14%, respectively.10,20 Indeed, among patients with dabigatran-associated major gastrointestinal bleeding treated with idarucizumab, the 30- and 90-day mortality rates were 11% and 15%, respectively, despite normalization of hemostasis.31 Although it is tempting to speculate that OAC withdrawal is the main contributor, post-bleed thrombosis does not entirely explain the excess mortality seen.32 For example, among 137 patients treated for gastrointestinal bleeding in REVERSE-AD, 6 patients (4.4%) experienced adjudicated thromboembolic events (including myocardial infarction, deep vein thrombosis, pulmonary embolism, and ischemic stroke) within 90 days, which were implicated as the cause of death in 4 patients. In addition to OAC discontinuation, bleeding leads to hospitalization, procedures, transfusion, anemia, and end-organ damage, all of which are associated with poor outcomes, especially in elderly, highly comorbid patients.
Managing bleeding is about more than anticoagulant reversal
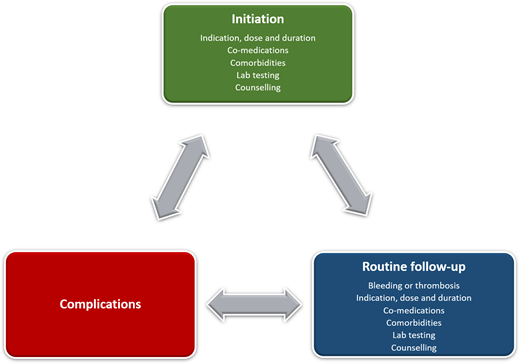
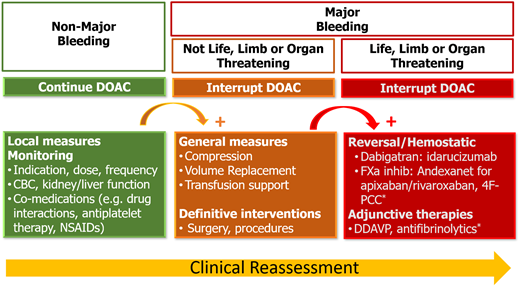
OACs are prescribed to patients for whom the clinical benefit for the treatment or prevention of thromboembolism outweighs the risk of bleeding, which represents a source of iatrogenic harm; decisions to initiate and continue OACs require ongoing reassessment and shared decision making. Reversal is one component of a global strategy to minimize the harms of OAC therapy that starts with prescribing the right OAC (including dose and duration) with attention to comorbidities, comedications, and patient values and preferences. Patients receiving long-term OAC therapy require ongoing monitoring for changes in their clinical status over time (Figure 1). Acute management of patients with OAC-related bleeding depends on the severity of bleeding and includes general measures (eg, local therapies, procedures, transfusion support), with administration of reversal or hemostatic agents reserved for those with severe or life-threatening bleeds (Figure 2). Because practicing clinicians are unlikely to have access to DOAC-specific assays to measure drug levels in urgent situations, clinical judgment about whether clinically significant DOAC levels are likely to be present is essential and should incorporate (1) the timing of the last DOAC dose, (2) DOAC half-life, (3) concurrent medications that could increase drug levels, and (4) renal and liver function. The potential benefits, harms, and uncertainties of reversal and hemostatic therapies should be discussed with patients and caregivers. The main clinical conundrum after bleeding complications is whether, when, and how to reinitiate OACs safely to reduce the risk of thrombosis and death and to minimize rebleeding. There is substantial uncertainty in this setting, with no randomized trials to guide clinical decision making. OACs are permanently discontinued in up to 50% of patients who experience OAC-related bleeding.13,33 Observational data suggest that resumption of OACs after intracranial and gastrointestinal bleeding is associated with a reduced risk of thrombosis and death but an increased risk of rebleeding.13,33 However, the likelihood of bias in these studies precludes firm conclusions regarding post-bleed management of OACs in general and patient subgroups for whom the net clinical benefit favors resumption or discontinuation. The uncertainty about benefit and harm necessitates individual treatment plans based on shared decision making between patients and providers.34
Approach to management of direct oral anticoagulant–related bleeding.
Although the availability of reversal and/or hemostatic therapies within health systems and hospitals may be impacted by cost ($3 500-$4 200 for 5-g idarucizumab kit, $27 500 for low-dose and $49 500 for high-dose andexanet [$5 500 per 200-mg vial], $1.60-$2.77 per unit for 4-factor PCC), standardized multidisciplinary protocols for DOAC reversal can promote the judicious use of these agents.35 Several published documents provide practical guidance for prevention and management of DOAC-related bleeding.34-36
Case resolution
The patient received 2 units of packed red blood cells, and his post-transfusion hemoglobin was 7.8 g/dL. Repeat vital sign measurements after resuscitation showed blood pressure of 98/64 mm Hg and heart rate of 110 beats per minute. Owing to the life-threatening nature of the bleed and prolonged exposure to rivaroxaban (recent ingestion, abnormal renal function), 4-factor PCC (2000 units) was approved as a hemostatic agent as per institutional protocol for FXa inhibitor–associated major bleeding because andexanet alfa was not on the hospital formulary. The patient underwent urgent angioembolization by interventional radiology, and a repeat computed tomographic scan showed no contrast extravasation or hematoma expansion. His rivaroxaban was temporarily discontinued to be reassessed before discharge, and he was advised to avoid nonsteroidal anti-inflammatory drugs while receiving oral anticoagulant therapy.
Conclusions
DOAC-related bleeding is a source of iatrogenic harm. Minimizing the occurrence and severity of bleeding is paramount. Specific reversal agents reduce the anticoagulant effect of dabigatran and FXa inhibitors, although there is uncertainty regarding the incremental benefits and harms of therapy in bleeding patients. Further studies are needed for unbiased assessments of efficacy and safety. In the interim, decisions to use reversal or hemostatic agents for major bleeds should be undertaken in conjunction with clinical protocols that promote the judicious use of these agents for the patients most likely to benefit.
Correspondence
Deborah M. Siegal, Population Health Research Institute, Thrombosis and Atherosclerosis Research Institute, Department of Medicine, McMaster University, 20 Copeland Ave, C3-120, Hamilton, ON L8L 2X2, Canada; e-mail: siegald@mcmaster.ca.
References
Competing Interests
Conflict-of-interest disclosure: D.M.S. reports receiving honoraria for attending consultant meetings for Bayer, Novartis, Portola Pharmaceuticals, Leo Pharma, and Aspen Pharma.
Author notes
Off-label drug use: Off-label use of prothrombin complex concentrate (PCC) for management of direct factor Xa inhibitor–related major bleeding will be discussed.