Learning Objectives
Learn about the diagnostic and prognostic workup for young, newly diagnosed multiple myeloma patients, including the role of novel imaging and the emerging role of genomic studies
Learn about the role of optimal induction, transplant, consolidation, and maintenance in young, newly diagnosed multiple myeloma patients
Introduction
Mrs. K is a 62-year-old woman who notes a 6-month history of worsening midthoracic back pain with marginal relief from increasing doses of ibuprofen. She is a school librarian working in a rural community and married, with 3 children out of the house. An X-ray of the thoracic spine reveals small lytic lesions, which are confirmed on subsequent magnetic resonance imaging (MRI) scans of the thoracic spine and lumbar spine. Blood studies revealed elevated globulin levels prompting the following workup on referral to the medical oncologist: normal serum calcium level, normal serum creatinine levels (0.8 mg/dL), hemoglobin 10.8 g/dL, serum albumin 3.2 g/dL, immunoglobulin G (IgG) κ isotype by serum immunofixation, serum electrophoresis shows an M-protein spike of 3.8 g/dL, total serum IgG 4.7 g/dL, low IgM/IgA, serum free κ light-chain levels 2550 mg/L, serum free λ light-chain levels 21 mg/L, free λ/κ ratio >100, and serum β-2–microglobulin 4.2 mg/dL. A 24-hour urine protein electrophoresis showed 55 mg proteinuria, with 30% urine M protein. Skeletal survey revealed >20 small lytic lesions and no impending fractures in weight-bearing areas. Bone marrow biopsy revealed 50% cellularity and 70% atypical plasma cells with κ restriction consistent with myeloma, and fluorescence in situ hybridization (FISH) testing revealed hyperdiploidy in 55% of cells. Mrs. K is informed of the diagnosis of IgG κ multiple myeloma (MM) revised international staging system (ISS) stage II disease and the need for therapy.1 She requests consultations with 2 of the leading world experts; however, she wants to return to her rural community for initial therapy and is refusing clinical trial participation. Would you advise any other testing here?
It is important to highlight some of the recent changes in the imaging recommendations from the International Myeloma Working Group (IMWG).2-4 There has been an increased use of novel imaging to assess both bone disease burden and the disease response in clinical trials,5-7 and these data do eventually impact clinical practice. We now recognize, based on recent studies looking at prospective comparison of skeletal surveys with positron emission-computed tomography (PET-CT) and whole-body MRIs,8,9 that skeletal surveys do not reveal lytic bone disease until 70% of the bone has decalcified and that damage is detected much earlier with both PET-CT and MRI techniques. A patient may appear to have no bone lesions on skeletal survey but will have bone damage detected by novel imaging qualifying them for a symptomatic disease and active therapy, thus preventing morbidity and debility from bone fractures.
PET-CT have shown prognostic value as well, and it has been shown in several prospective trials10 that having 3 or more 18F-fluorodeoxyglucose avid lesions at the time of diagnosis confers shorter progression-free survival (PFS) as well as poor overall survival (OS) for newly diagnosed, transplant-eligible myeloma patients. Additionally, posttransplant suppression (complete suppression of those lesions) confers superior OS, even in patients who have complete response. Based on these observations, clinical trials are utilizing PET-CTs for newly diagnosed patients at baseline as well as postinduction and posttransplantation. PET-CT negativity has been incorporated in the updated IMWG response criteria.11 PET-CTs also help us recognize extramedullary disease, which is an aggressive phenotype of high-risk myeloma, and if we are not doing PET-CTs, we will not recognize these patients at baseline.6 The IFM2009 clinical trial12 data have also shown us that minimal residual disease (MRD) assessments by flow cytometry and PET-CT serve as surrogate markers for improved survival outcome, but not all patients are negative with both techniques. This trial has also shown that patients may be MRD negative by flow cytometry and still positive on PET-CTs, showing us the heterogeneous nature of the disease when it comes to skeletal involvement. It is important to recognize that the MRD flow only represents sampling from the pelvic bone, and the disease may be active in other areas of involvement (vertebral bones, skull, long bones, etc.); we will not necessarily find that just by doing MRD flow cytometry on the pelvic bone. Therefore, PET-CTs are becoming more important.
The second issue to highlight is the genomic heterogeneity of MM biology, which is more complex than just conventional cytogenetics and FISH differences. The field is moving quickly toward getting more comprehensive biologic information on MM patients by way of gene expression profiling (GEP) as well gene mutational panel studies (RNA sequencing and whole-exome sequencing), and the MMRF CoMMpass study13,14 is a big example of that. We can recognize different molecular subgroups of myeloma with different clinical outcomes, no longer leaving one to wonder why 2 ISS I patients with “normal” cytogenetics behave so differently in regard to survival outcomes. GEP studies have already identified at least 7 different molecular subgroups of myeloma patients with different prognoses, and they help us to identify the high-risk patients in a broader category. There are 2 GEP signatures that were identified by the Arkansas group15 as well as the HOVON group16 that can identify 20% patient at very high risk of early relapse/death. There are clinical trials evaluating alternative strategies for high-risk patients; GEP-based high-risk patients can be offered such options.
Given the findings, what is the preferred induction therapy for Mrs. K, who is a previously untreated, relatively young woman?
The combination of lenalidomide, bortezomib, and dexamethasone (RVd) is the most preferred regimen for induction based on the SWOG-0777 phase 3 trial that compared RVd with lenalidomide-dexamethasone.17 However, there is a caveat regarding patients who are presenting with renal insufficiency, because lenalidomide may not be readily available for those patients because of the Food and Drug Administration (FDA)–required patient registration process. There is also a potential for toxicities in the setting of renal insufficiency, requiring lenalidomide dose adjustments. Patients may get started on the combination of cyclophosphamide, bortezomib, and dexamethasone (CyBorD) initially,18-20 and as the renal insufficiency improves, they can be transitioned to RVd in the outpatient setting. Patients may continue to receive CyBorD as the induction of choice.
The other emerging second generation proteasome inhibitor induction regimen, carfilzomib-lenalidomide-dexamethasone (KRd), seems to be quite promising. We have 3 separate and complimentary phase 1/2 experiences21-23 that have shown that KRd induction can actually lead to very high levels of complete responses postinduction and sustained PFS with at least 2 years of follow-up. The responses seem to be similar in depth and duration for both standard-risk patients as well as high-risk patients. There have been concerns around the carfilzomib-related cardiopulmonary side effects and whether they may impact the decision to use KRd across the board. The KRd regimen is being compared with RVd as frontline induction in a prospective, randomized phase 3 trial by the Eastern Cooperative Oncology Group, but the earliest readout may still be 3 to 4 years away.
The anti-CD38 monoclonal antibody daratumumab is making its way into the frontline setting; the first frontline FDA approval in May 2018 was of daratumumab in combination with bortezomib-melphalan-prednisone for transplant-ineligible patients.24 This combination does not apply to Mrs. K, but there are 3 other clinical trials in the frontline setting that will likely impact the practice in the United States. Daratumumab has been combined with bortezomib-thalidomide-dexamethasone in a prospective phase 3 study in Europe for transplant-eligible patients (clinicaltrials.gov #NCT02541383), and there are randomized phase 2 trials of daratumumab with RVd25 and CyBorD (clinicaltrials.gov #NCT02951819) in the frontline setting. All 3 trials have accrued, and we are eagerly awaiting data.
Do you incorporate cytogenetic risk categorization in your decision of what induction you give?
RVd remains the standard of care induction for both the standard-risk patient and the high-risk patient. However, I am starting to lean more toward KRd for the young high-risk or ultrahigh-risk26 patients based on the early data in both the early relapse and newly diagnosed settings. The ASPIRE trial,27 which compared KRd with Rd for early relapse MM (1-3 prior lines), showed that the median PFS for standard-risk patients on KRd was about 28 months, and it was close to 23 months for the high-risk patients. However, if you looked at the standard of care arm, the high-risk patients had a dismal PFS. KRd phase 2 experience23 at the University of Chicago shows similar 1- and 2-year survival outcomes, with both standard- and high-risk patients with KRd induction doing about the same.23
Are there any data on optimal induction cycles before transplant?
The only data that we have are from a retrospective look from Mayo Clinic showing that there is no difference in outcomes.20 In my clinical practice, the number of induction cycle varies between 4 and 6 cycles. If there is no response plateau after 4 cycles of induction, it may be reasonable to give 2 additional cycles of induction before planning stem cell mobilization and autologous stem cell collection.
Mrs. K opts for RVd—lenalidomide, bortezomib subcutaneously, and dexamethasone. After 4 monthly cycles, she is tolerating the regimen well and having a nice response. Reevaluation reveals that M spike is now 0.3 g/dL and that urine M is undetected. Thus, Mrs. K is in a very good partial response by the IMWG response criteria.
What would be the next step? Consolidation therapy with autotransplant now, later, or never?
For young transplant-eligible patients, the weight of the data is on side of early transplantation (the EMN/HOVON-95 trial, the IFM2009 trial), and by delaying stem cell transplant for eligible patients, there are portions of patients (∼20%) who do not end up getting an autologous stem cell transplant (ASCT) at the time of relapse because of performance status of other comorbidities.27,28 By choosing a “delayed” approach, these patients may lose the opportunity of benefiting from that intervention all together.
It is important to understand why the IFM2009 trial is only showing PFS benefit and no OS difference at the 3-year follow-up mark. Whether one looks at the MM transplant studies from the 1990s or the 2000s, which compared standard of care with transplantation for transplant-eligible patients, or even the SWOG0777 study,17 which showed OS advantage in favor of RVd induction vs Rd induction, the OS benefit does seem to emerge after at least 5 years of follow-up. Therefore, it might be a little early to draw that conclusion from the IFM2009 trial regarding lack of OS benefit.
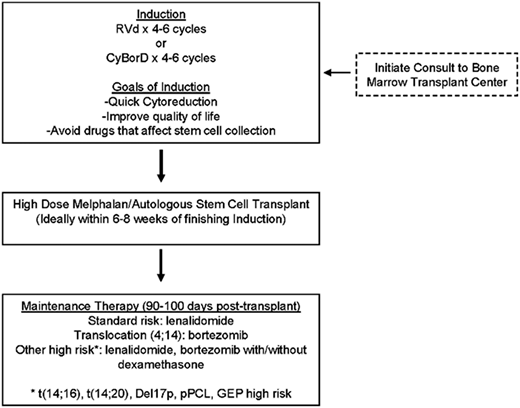
Common questions from the practicing hematologist/oncologist are as follows: how are we utilizing MRD in clinical practice, and how do we use this information? The IFM2009 study did show that a significantly higher proportion of patients in the early transplant arm achieved MRD negativity than those randomized to delayed transplant. MRD testing has now shown that it can distinguish patients who are going to have better PFS and OS.29 There have been at least 3 studies that have shown that you can tease out, even within complete response patients, who is going to relapse early vs have better PFS and OS—the difference is achievement of MRD negativity. ASCT deepens the response postinduction and puts more patients into MRD negativity.12,29 Inclusion of early/frontline ASCT would be the right approach in the year 2018 for a young transplant-eligible patient like Mrs. K (Figure 1).
Approach to transplant-eligible newly diagnosed MM. pPCL, primary plasma cell leukemia.
Approach to transplant-eligible newly diagnosed MM. pPCL, primary plasma cell leukemia.
What is your take on the role of consolidation and tandem transplantation today?
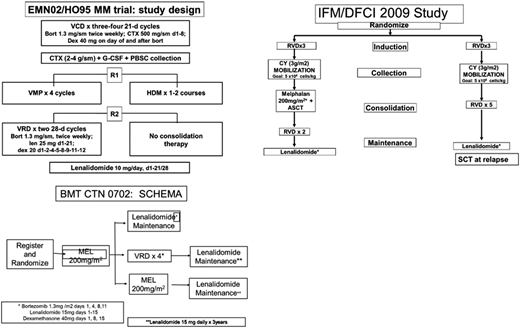
The BMT-CTN 070230 trial compared single ASCT, tandem ASCT, and single ASCT followed by RVd consolidation, and it showed no difference in PFS after a 38-month median follow-up. These results were different from those of the EMN02/HOVON95 trial (Figure 2), which showed benefit in favor of the tandem ASCT arm, especially for the high-risk MM patients. From the US practice standpoint, the EMN02/HOVON95 trial was perhaps a little handicapped, because lenalidomide was not part of the induction therapy in that clinical trial. The utilization of RVd induction in the United States perhaps takes away part of the PFS benefit observed in the EMN02/HOVON-95 trial. The practice is not going to shift toward tandem transplants in general, even for high-risk patients, at least at this point unless we start seeing that subset of patients benefiting from tandem ASCT in a BMT-CTN 0702 long-term follow-up. It also remains to be seen if RVd consolidation, like the Intergroupe Francophone du Myélome uses for their clinical trials posttransplantation, confers the same survival benefit as a tandem transplant. This information may also emerge with long-term follow-up from the BMT-CTN 0702 trial.
Important transplant trials. CTX, cytoxan; CY, cyclophosphamide; G-CSF, granulocyte colony-stimulating factor; HDM, high-dose melphalan; MEL, melphalan; PBSC, peripheral blood stem cells; SCT, stem cell transplant; VCD, bortezomib-cyclophosphamide-dexamethasone; VMP, bortezomib-melphalan-prednisone; VRD, bortezomib-lenalidomide-dexamethasone.
Important transplant trials. CTX, cytoxan; CY, cyclophosphamide; G-CSF, granulocyte colony-stimulating factor; HDM, high-dose melphalan; MEL, melphalan; PBSC, peripheral blood stem cells; SCT, stem cell transplant; VCD, bortezomib-cyclophosphamide-dexamethasone; VMP, bortezomib-melphalan-prednisone; VRD, bortezomib-lenalidomide-dexamethasone.
The next question that we are frequently asked is the role of maintenance therapy
For standard-risk patients, lenalidomide maintenance is the standard of care based on the recent meta-analysis,31 and it showed almost 2.5-year OS benefit. However, there are data supporting the utility of bortezomib as maintenance, especially for translocation (4;14) patients. We have data from the HOVON group27 as well as the Arkansas group32 supporting the utility of bortezomib for both translation (4;14) and del17p patients. The Emory retrospective high-risk MM experience was published a little over 4 years ago,33 and it showed that high-risk patients, especially those with del17p, seemed to have PFS/OS benefit from the dual maintenance strategy. Based on the best available evidence, I would recommend maintenance based on disease risk (Figure 1).
Are you using MRD in any role after transplant?
Beyond the prognostic implications of being MRD negative or positive, there are no data supporting the role of treatment modifications based on those results. Escalation or deescalation of therapy based on MRD assay results is not recommended in general. The only caveat would be an ultrahigh-risk patient who becomes MRD positive after having achieved MRD negativity on a previous reading. Although such patients may not show serological evidence of relapse, the MRD positivity will likely serve as a harbinger of adverse clinical outcome. I would be watching that patient very carefully and would change therapy with even modest rises in MM markers (ie, early biochemical relapse) in my practice. Outside of such very specific scenarios, I am not utilizing MRD testing to change, escalate, or deescalate therapy in patients.
Mrs. K opts for maintenance with lenalidomide at 15 mg daily for 21 of 28 days. She remains in remission for 2.5 years but has gradually raising M protein. Clarithromycin is added, and the doses are raised to 25 mg with only a transient benefit.
What is the treatment of choice for relapsing disease?
Mrs. K, at this point, is lenalidomide refractory and will dictate our choices (Figure 3). She has had over 3 years of PFS benefit from her first-line treatment, and to our knowledge, she did not develop any neuropathy with bortezomib. The questions to ask and consider would be as follows. Can we use bortezomib as part of the triplet regimen as we reinduce her? Should we be considering a second ASCT for her?
Treatment approaches in first relapse. Dara-Rd, daratumumab-lenalidomide-dexamethasone; Dara-Vd, daratumumab-bortezomib-dexamethasone; Elo-Rd, elotuzumab-lenalidomide-dexamethasone; IMiD, immunomodulatory drugs; Ixa-Rd, ixazomib-lenalidomide-dexamethasone; Kd, carfilzomib-dexamethasone; PI, proteasome inhibitors; Pom-Vd, pomalidomide-bortezomib-dexamethasone.
Treatment approaches in first relapse. Dara-Rd, daratumumab-lenalidomide-dexamethasone; Dara-Vd, daratumumab-bortezomib-dexamethasone; Elo-Rd, elotuzumab-lenalidomide-dexamethasone; IMiD, immunomodulatory drugs; Ixa-Rd, ixazomib-lenalidomide-dexamethasone; Kd, carfilzomib-dexamethasone; PI, proteasome inhibitors; Pom-Vd, pomalidomide-bortezomib-dexamethasone.
For MM patients in first relapse, there are several lenalidomide-based combinations as well lenalidomide-free novel combinations available for consideration.34 In Mrs. K’s case, one would consider a lenalidomide-free combination—my choice would be daratumumab-bortezomib-dexamethasone (Dara-Vd) based on the CASTOR trial34 data. Mrs. K is now 65 years of age and utilizing an ASCT as salvage after achieving the best depth of response with Dara-Vd reinduction. Patients would technically be getting maybe 50% to 70% of the PFS benefit from the second high-dose melphalan (HDM)/ASCT compared with the first HDM/ASCT.35
Now thinking about the lenalidomide-free combinations for lenalidomide refractory patients, there were 2 presentations of note at American Society of Clinical Oncology 2018. The first was on the combination of pomalidomide, bortezomib, and dexamethasone (PVd), which showed superior median PFS and depth of response compared with bortezomib and dexamethasone.36 The second study was a single-arm experience of daratumumab, carfilzomib, and dexamethasone showing almost 90% overall response rate and median PFS of about 20 months37 ; a randomized phase 3 trial (carfilzomib-dexamethasone ± daratumumab) is ongoing (clinicaltrials.gov #NCT03158688). The PVd combination may get FDA approval this year and would be another good option for Mrs. K.
What are your views about allogeneic transplant?
For advanced relapsed/refractory MM patients, my preference is to try to find clinical trials for the patients with novel mechanisms of action. We have a lot of clinical trial activity in terms of early development with new immunotherapy approaches when it comes to monoclonal antibody–based therapies with antibody-drug conjugates or bispecific monoclonal antibodies as well as vaccines and T cell–engineered strategies, including chimeric antigen receptor T cells (CAR-Ts), but there are other things coming down the pike as well. B-cell maturation antigen (BCMA) is proving to be an excellent target for such strategies; there are several BCMA CAR-T early-phase clinical trials that have been reported at international meetings.38-40 Perhaps the Bluebird bb2121 product is the farthest ahead in clinical development, showing an overall response rate of 95.5% in 22 evaluable patients (8 median prior lines) who had received cell dose of >150 × 106 T cells and median PFS of about 11.7 months.40
With the availability of these options in clinical trials, I find the utility of allogeneic stem cell transplantation (allo-SCT) quite infrequent in relapsed/refractory myeloma patients outside of clinical trials. Although there are anecdotal examples of long-term survivorship with allo-SCT, we are not sure whether those anecdotes exist because of true graft-versus-myeloma effect or just the effects of the myeloablative preparative regimen. There are several MM trials that have shown OS benefit (RVd vs lenalidomide-dexamethasone induction, Len maintenance, bisphosphonates, etc.), but the ASCT/allo-SCT vs tandem ASCT approach and the ASCT vs allo-SCT approach have not shown OS benefit.35
Acknowledgments
S.Z.U. is funded by the Leukemia Lymphoma Society, the Heinemann Foundation of Charlotte, the Freeman Fund, the Carolinas Myeloma Research Fund, and the National Cancer Institute (grant 5R01CA201634).
Correspondence
Saad Z. Usmani, Plasma Cell Disorders Section, Department of Hematologic Oncology & Blood Disorders, Levine Cancer Institute/Atrium Health, Suite 500, 1021 Morehead Medical Dr, Charlotte, NC 28204; e-mail: saad.usmani@carolinashealthcare.org.
References
Competing Interests
Conflict-of-interest disclosure: S.Z.U. has received research funding from Amgen, Array Biopharma, BMS, Celgene, Janssen, Pharmacyclics, Sanofi, and Takeda; consulted for Abbvie, Amgen, BMS, Celgene, Janssen, Takeda, Sanofi, and SkylineDx; and been affiliated with the Speaker’s Bureau for Amgen, Celgene, Janssen, and Takeda.
Author notes
Off-label drug use: None disclosed.