Abstract
In this report, we will review the various clinical and laboratory approaches to diagnosing immune thrombocytopenia (ITP), with a focus on its laboratory diagnosis. We will also summarize the results from a number of laboratories that have applied techniques to detect anti-platelet autoantibodies as diagnostic tests for ITP. Although there is considerable variability in methods among laboratories, there is general agreement that platelet autoantibody testing has a high specificity but low sensitivity. This suggests several possibilities: (1) the ideal test for ITP has yet to be developed, (2) current test methods need to be improved, or (3) ITP is the clinical expression of a variety of thrombocytopenic disorders with different underlying mechanisms. Even the clinical diagnosis of ITP is complex, and experienced clinicians do not always agree on whether a particular patient has ITP. Improvements in the diagnostic approach to ITP are necessary to improve the management of this disorder.
Learning Objectives
Review the clinical diagnosis of immune thrombocytopenia
Review laboratory testing for the diagnosis of immune thrombocytopenia, with an emphasis on glycoprotein-specific autoantibody assays
Introduction
To paraphrase the Victorian scientist and mathematician, Lord Kelvin, “we know what we can measure.” Beginning in the 19th century, the ability of physicians to biopsy, sample, and collect human tissues rapidly led to a better understanding of diseases. This nosological approach to the diagnosis and classification of disease continues to be used today. Red blood cells were some of the first patient materials to be sampled by physicians, and the techniques used to study red cell disorders, particularly immune hemolysis, were quickly applied to immune platelet disorders. Hence, in trying to understand how to diagnose immune thrombocytopenia (ITP), it is informative to review the approaches used to diagnose autoimmune hemolytic anemia (AIHA).
AIHA as a prototype for ITP
The morphological examination of red cells by early hematologists helped classify a variety of anemias. In the mid-1940s, Coombs and others were developing tools to permit red cell transfusions; these techniques were also applied to the study of red cell destructive disorders. The Coombs test measures immunoglobulin and/or complement on the surface of red blood cells (direct) or in the plasma or serum (indirect).1,2 Investigations by Coombs and others identified an autoimmune disorder, termed AIHA, which was caused by premature red cell destruction by immunoglobulin or complement.3 Initial reports suggested that many patients with AIHA had a positive direct Coombs test, now termed the direct antiglobulin test (DAT).4 Over the ensuing decades, DAT testing moved from specialized hematology laboratories to general laboratories and is now a routine diagnostic test.2 Progressive refinements in the DAT using modifications to target red cells (eg, enzyme treatment, concentrating the red cell eluate) or to test methods (flow cytometry)5 have resulted in an exceptionally high sensitivity and specificity for the diagnosis in patients with suspected AIHA. Today, these laboratory tests are essential in making the diagnosis of AIHA.2
AIHA is characterized by a reduction in the red cell number (anemia) and evidence of increased red cell destruction, including an elevated bilirubin, and elevated red cell lactate dehydrogenase. The anemia in turn leads to an increase in red cell production measured by increased reticulocytes.6 As noted, the vast majority (>95%) of all patients with AIHA have detectable anti-red cell antibodies using sophisticated techniques.4 Although there are many similarities between AIHA and ITP, one important difference is that the application of direct and indirect testing for the detection of platelet autoantibodies has proven to be much more challenging in ITP.
Challenges with the clinical diagnosis of ITP
Hematologists quickly discovered that there was no parallel DAT test for platelets; therefore, ITP became a diagnosis of exclusion.7 But the weakness of such a tautological approach for the diagnosis of ITP is the challenge of confirming a negative. Consequently, over the past decades, several working groups have attempted to further refine the diagnosis. The designation of “ITP” continues to be used, although the previous designation of “idiopathic” has been replaced by immune, and “purpura” is no longer used, recognizing that many patients do not have any bleeding symptoms.8-10 ITP can be defined as a platelet count <100 × 109/L with other causes of thrombocytopenia excluded. ITP is further differentiated into primary ITP or secondary ITP, which indicates immune-mediated thrombocytopenia associated with a variety of disorders such as chronic viral infections or autoimmune rheumatological disorders such as systemic lupus erythematosus. That differentiation is important because many of the treatments of secondary ITP target the underlying disorder. Primary ITP is separated into newly diagnosed (<3 months from diagnosis), persistent (3-12 months from diagnosis), or chronic (>12 months since diagnosis).8
Difficulties in establishing the clinical diagnosis of ITP
Thrombocytopenia is a common hematological presentation with a variety of potential causes. Establishing the diagnosis can be difficult, and the correct identification of the underlying cause is important to make appropriate management decisions for thrombocytopenic patients.11,12 In a recent study based on data from the McMaster ITP Registry, 1 in 7 patients diagnosed with primary ITP at presentation or on follow-up were reclassified as additional investigations were performed (Table 1). These patients were usually male, with a milder thrombocytopenia and a fewer number of severe bleeding episodes.13
The laboratory investigation of a suspected ITP patient
| American Society of Hematology Guidelines9 . | International Consensus Report10 . | McMaster ITP Registry13 . |
|---|---|---|
| CBC | CBC, reticulocytes | CBC, reticulocytes |
| Blood film | Blood film | Blood film |
| HIV | HIV | HIV |
| HCV | HCV | HCV |
| HBV before rituximab | HBV before rituximab | HBV |
| Further tests depend on history, CBC | Quantitative immunoglobulins | Quantitative immunoglobulins |
| DAT | DAT | |
| H pylori (in adults) | H pylori (in adults) | |
| Bone marrow (age ≥60 years) | Bone marrow* | |
| Blood group (Rh) if considering anti-D | Platelet size | |
| Further tests depend on history and CBC | Electrolytes | |
| Creatinine | ||
| Liver enzymes | ||
| Serum protein electrophoresis | ||
| TSH | ||
| ANA | ||
| ACA | ||
| NSI | ||
| Abdominal ultrasound |
| American Society of Hematology Guidelines9 . | International Consensus Report10 . | McMaster ITP Registry13 . |
|---|---|---|
| CBC | CBC, reticulocytes | CBC, reticulocytes |
| Blood film | Blood film | Blood film |
| HIV | HIV | HIV |
| HCV | HCV | HCV |
| HBV before rituximab | HBV before rituximab | HBV |
| Further tests depend on history, CBC | Quantitative immunoglobulins | Quantitative immunoglobulins |
| DAT | DAT | |
| H pylori (in adults) | H pylori (in adults) | |
| Bone marrow (age ≥60 years) | Bone marrow* | |
| Blood group (Rh) if considering anti-D | Platelet size | |
| Further tests depend on history and CBC | Electrolytes | |
| Creatinine | ||
| Liver enzymes | ||
| Serum protein electrophoresis | ||
| TSH | ||
| ANA | ||
| ACA | ||
| NSI | ||
| Abdominal ultrasound |
CBC, complete blood count; HBV, hepatitis B virus; HCV, hepatitis C virus; NSI, nonspecific inhibitor; TSH, thyrotropin.
We perform a bone marrow test if there are unexplained hematological findings, such as macrocytosis, or if the patient has had a poor response to standard therapy.
In another recent study,14 a panel of 3 hematologists provided their assessment for the diagnosis of thrombocytopenic patients based on medical records. In this study, the 3 hematologists occasionally did not agree with each other or with the working diagnosis of ITP. Thus, even with the standardization of diagnostic criteria8 and the current consensus guidelines for ITP,9 it remains difficult to diagnose ITP in practice. In the study conducted by Salib et al, there was improved agreement about the diagnosis when 2 criteria were met: (1) the patient had a very low platelet nadir (<20 × 109/L) and (2) the platelet count increased following treatment with intravenous immunoglobulin (IVIG), corticosteroids, or treatment of the underlying cause of secondary ITP.14 Consequently, we suggest that these criteria can be used to enhance the clinical diagnosis of ITP.
The difficulty in establishing an accurate diagnosis of ITP has implications for the management of ITP because patients can be exposed to treatment-related toxicities without achieving a platelet count response and because of the generalizability of results from clinical trials. Furthermore, this diagnostic uncertainty leads to fundamental challenges in the investigation of the pathophysiology of ITP and in the assessment of laboratory tests.
Our approach to the evaluation of a patient with suspected ITP
For some patients who present with ITP, the diagnosis is not difficult. For example, the young woman who presents with severe thrombocytopenia, a platelet count >10 × 109/L and who promptly responds to IVIG almost certainly has ITP. But for many patients, the diagnosis is more obscure. Consequently, the hematologist who is managing a patient who might have ITP needs to investigate other causes of thrombocytopenia as well as secondary causes of ITP. Table 1 illustrates guidelines that have been used during the investigation and management of ITP. The International Consensus Report10 is more extensive than the American Society of Hematology Guidelines.9 In addition, the International Consensus Report identifies a number of tests of potential utility in the diagnosis and management of a thrombocytopenic patient.10 These include testing for glycoprotein-specific antibodies, antiphospholipid antibodies, antithyroid antibodies and thyroid function, antinuclear antibodies, viral tests for parvovirus and cytomegalovirus, and pregnancy tests, if indicated. Our group chose to prospectively evaluate whether a more expensive series of laboratory investigations including those described in the International Consensus Report was useful. We have initiated a prospective study of thrombocytopenic patients within the McMaster ITP Registry13 ; these tests are shown in Table 1. The rationale for the performance of some of these tests will be described subsequently.
The history and physical examination are focused on estimating the duration of the thrombocytopenia and the exclusion of other thrombocytopenic and secondary immune thrombocytopenic disorders, including exposure to drugs, associated disorders, or infections. The patient is questioned about evidence of hemostatic impairment and precipitating causes such as infection and alcohol, among others. Particular attention during the physical examination relates to evidence of bleeding, particularly mucous membrane bleeding in the mouth as well as examination for lymphadenopathy, and palpation of the liver and spleen. Increasingly, we are using abdominal ultrasound to evaluate the size of the spleen to exclude splenomegaly.15 Not infrequently, a fatty liver and some degree of splenomegaly are found, which indicates a diagnosis other than ITP.16,17
The International Consensus Report and the approach we are evaluating in the McMaster ITP Registry are intended to operationalize a strategy to diagnose ITP by exclusion. Tests include the antinuclear antibody (ANA), anticardiolipin antibody (ACA), nonspecific inhibitor (NSI), thyrotropin, quantitative immunoglobulins, serum electrophoresis, hepatitis B and C screening, and serological testing of adults for Helicobacter pylori (Table 1). The rationale of some of these additional tests are as follows. The ANA, ACA, and NSI provide information about secondary ITP associated with systemic lupus erythematosus18 or antiphospholipid syndrome,19 and may inform the baseline risk of thrombosis. In a systematic review, ITP patients who have antiphospholipid antibodies have an increased risk of thrombotic complications,20 which may be higher when they are given certain ITP treatments such as thrombopoietin receptor agonist medications. For ITP patients at risk of thrombotic complications, there is limited evidence for the use of anticoagulation, but we have found that in some patients with ITP, stopping anticoagulation can result in more harmful outcomes than continuing anticoagulation.21 There is an increased prevalence of hyperthyroidism in patients with ITP compared with the rest of the population22 ; between 8% and 14% of ITP patients develop thyroid dysfunction during their lives.23 H pylori testing is a more contentious issue because a positive test is common with increasing age and the prevalence of pathogenic strains can depend upon the country of birth. The Maastricht V/Florence Consensus Report recommends that, in ITP patients, H pylori should be sought and eradicated.24 We use H pylori serology as a screen test and the C14 urea breath test to confirm active infection. H pylori eradication has been reported to be associated with a platelet count rise in up to 50% of H pylori–positive patients with ITP based on studies that were mostly conducted in Japan.25,26 The response rate is lower in our experience from our registry, although we have managed patients with H pylori eradication therapy to raise their platelet counts to safer levels.
The laboratory diagnosis of ITP
Surrogate tests for increased platelet turnover
An elevated reticulocyte count in AIHA is evidence for increased red cell production. Reticulated platelets (RPs) and the immature platelet fraction have been suggested as the platelet equivalent of red cell reticulocytes.27 Reticulated platelets and the immature platelet fraction can be detected by flow cytometry and automated hematological analyzers. Several studies have used thiazole orange to measure reticulated platelets in ITP, with variable findings. Reports on the percentage of reticulated platelets found in ITP patients range from 2.5% to 24% and from 1% to 9% in other thrombocytopenic patients.28,29 In disorders in which thrombocytopenia is caused by platelet underproduction, the RP percentage is often low, whereas in disorders of increased platelet turnover, the RP percentage is often elevated11,27 ; however, the wide range of these results suggest that these tests have a limited usefulness.
Overview of techniques used for measuring platelet-bound immunoglobulin
Soon after Coombs applied his techniques to the investigation of AIHA, similar techniques were applied to platelets; however, platelets are much more difficult to isolate, wash, and investigate in comparison with red cells. For example, the proportional number of platelets compared with red cells is fewer and the ability to separate platelets into a platelet-pure preparation is technically more difficult. Although many physicians had suspected that ITP was an autoimmune disorder similar to AIHA, it took the bold experiments of Harrington to demonstrate that plasma from patients with ITP carried a “platelet destructive factor,” which caused thrombocytopenia in healthy volunteers (including Harrington himself).30 These studies provided insight into the pathogenesis of ITP, and perhaps more important, provided a strong impetus for the development of serological (indirect) assays for ITP.
In this manuscript, we will use the definitions that relate to those used for red cell serology. Direct assays measure immunoglobulins (or complement) on the surface of washed patient platelets. Indirect assays use patient serum (or plasma) mixed with test platelets or platelet glycoproteins (GPs).31 After a wash step, the immunoglobulins (or complement) bound to the test platelets are measured. Unlike red cells, platelets add an additional “degree of difficulty” because trace amounts of thrombin within test serum can activate or aggregate the test platelets unless certain steps are taken.
Serological tests for ITP
The observations by Harrington and coworkers documented that ITP was often caused by a plasma factor.30 These investigators reported an abrupt fall in the platelet count in healthy individuals following the infusion of ITP plasma. These observations led to the development of a variety of assays designed to measure this factor. These assays, as summarized previously,32 were all dependent upon a platelet end point, which ranged from platelet aggregation to platelet secretion. Today, except for a number of specialized diagnostic tests, such as the serotonin release assay for heparin-induced thrombocytopenia,33,34 assays that use functional platelet end points are no longer used.
Platelet-associated immunoglobulin testing
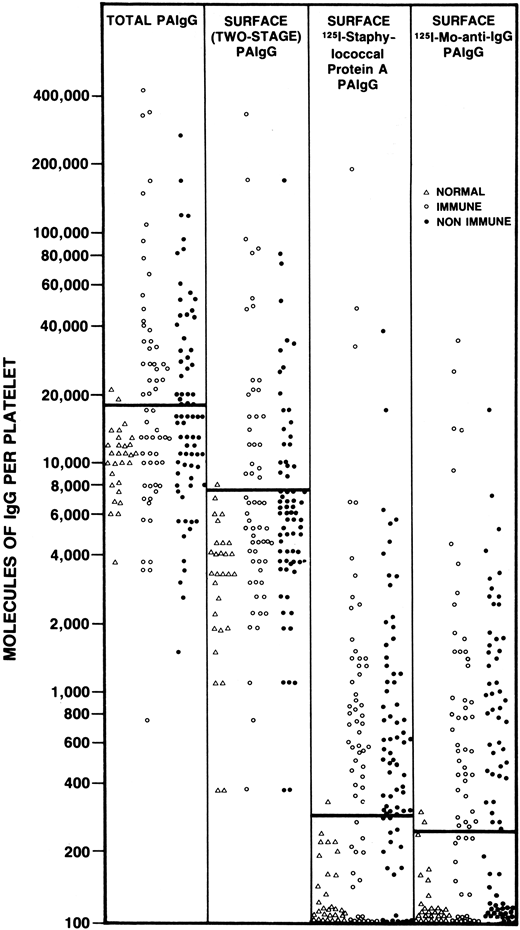
The next group of assays that were used to study patients with ITP included the direct measurement of platelet-bound IgG. Through a variety of techniques, the direct binding of a labeled antibody probe (either polyclonal or monoclonal) was detected using radioisotopes, flow cytometry, or other methods. Together, these assays provided new insights into the complexity of measuring immunoglobulin on the surface of red cells and platelets. For example, we showed that washed red cells from healthy controls carried, on average, 50 molecules of IgG per red cell and a patient with AIHA might have 10-fold more.35 In comparison, washed normal platelets typically carry 100 to several thousand molecules of IgG, with ITP patients having twofold or threefold higher levels.36 Additionally, it has been shown that the amount of platelet-bound IgG correlated with the amount of platelet-bound albumin37 and the amount of IgG within platelets was many fold higher than on the platelet surface, which suggested that megakaryocytes incorporate plasma proteins into developing α-granules.38 It is now assumed that much of the IgG on the platelet surface is not pathological. Techniques for measuring platelet associated IgG fell out of favor when it was shown that a similar rate of positivity was noted in almost all immune and non-immune thrombocytopenic disorders39 (Figure 1).
The results of PAIgG assays (expressed as molecules of IgG per platelet) determined using 1 of 4 different assays for PAIgG. The assays used included a technique for measuring “total” PAIgG (first vertical column); a 2-stage assay for surface PAIgG (second vertical column); a direct binding surface assay using 125I-staphylococcal protein A (third vertical column); and a direct binding surface assay using 125I-monoclonal anti-IgG (fourth vertical column). Each point represents a different patient or control tested using each technique on the same day. Healthy nonthrombocytopenic controls (∆), patients with acute or convalescent immune thrombocytopenia (○), and patients with non-immune thrombocytopenia (●). Solid bar across each column, upper limits of normal, defined as 2 standard deviations above the mean PAIgG for the 29 healthy, non-thrombocytopenic controls. PAIgG, platelet-associated immunoglobulin. With permission of authors and publishers.36
The results of PAIgG assays (expressed as molecules of IgG per platelet) determined using 1 of 4 different assays for PAIgG. The assays used included a technique for measuring “total” PAIgG (first vertical column); a 2-stage assay for surface PAIgG (second vertical column); a direct binding surface assay using 125I-staphylococcal protein A (third vertical column); and a direct binding surface assay using 125I-monoclonal anti-IgG (fourth vertical column). Each point represents a different patient or control tested using each technique on the same day. Healthy nonthrombocytopenic controls (∆), patients with acute or convalescent immune thrombocytopenia (○), and patients with non-immune thrombocytopenia (●). Solid bar across each column, upper limits of normal, defined as 2 standard deviations above the mean PAIgG for the 29 healthy, non-thrombocytopenic controls. PAIgG, platelet-associated immunoglobulin. With permission of authors and publishers.36
Tests that measure immunoglobulin bound to platelet glycoproteins
In 1982, van Leeuwen applied the platelet suspension immunofluorescence test developed by von dem Borne40 to show that antibodies obtained from some ITP platelet eluates or serum samples were not able to bind to platelets from patients with Glanzmann thrombasthenia because these platelets lack GPIIb/IIIa.41 These studies were analogous to the demonstration that autoantibodies from patients with AIHA do not react to Rhesus null cells. This observation suggested that at least some patients with ITP have autoantibodies against GPIIb/IIIa, the most abundant platelet glycoprotein with ∼80 000 copies per cell.42 Subsequently, a variety of assays to detect anti-platelet autoantibodies were developed; some are still in use today.
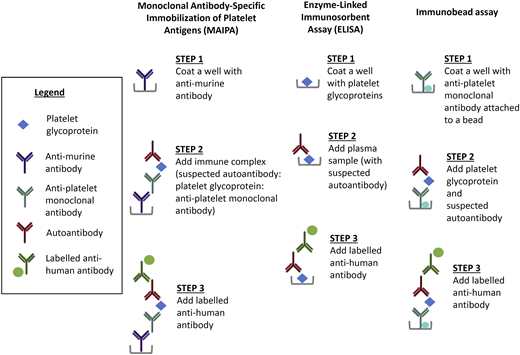
There are 2 general types of assays that have been used to measure the binding of autoantibodies to individual glycoproteins. The first general type is the antigen capture and includes the monoclonal antibody-specific immobilization of platelet antigens (MAIPA), the enzyme-linked immunosorbent assay (ELISA), and the immunobead assay, as illustrated in Figure 2. All antigen capture assays use monoclonal antibodies against individual platelet proteins to isolate the antibody of interest.
The MAIPA assay can detect autoantibodies directly on the platelet surface or indirectly in the plasma. In the first step of MAIPA, a plate is coated with anti-murine antibody. In the direct assay, a patient’s platelet lysate containing the glycoprotein bound by the suspected autoantibody is mixed with an anti-platelet monoclonal antibody. For indirect MAIPA, normal platelets are mixed with patient test plasma to allow the autoantibody to bind. In the third step, the presence of an autoantibody is detected with a labeled anti-human antibody. The ELISA is an indirect assay in which a plate is coated with platelet glycoprotein followed by the patient test plasma sample. As in the MAIPA, the presence of an autoantibody is detected with a labeled anti-human antibody. The Immunobead assay can be indirect or direct, and it is a type of antigen capture assay similar to the MAIPA; the difference is that the glycoprotein is captured on the well by an anti-platelet monoclonal antibody conjugated to a bead.
The MAIPA assay can detect autoantibodies directly on the platelet surface or indirectly in the plasma. In the first step of MAIPA, a plate is coated with anti-murine antibody. In the direct assay, a patient’s platelet lysate containing the glycoprotein bound by the suspected autoantibody is mixed with an anti-platelet monoclonal antibody. For indirect MAIPA, normal platelets are mixed with patient test plasma to allow the autoantibody to bind. In the third step, the presence of an autoantibody is detected with a labeled anti-human antibody. The ELISA is an indirect assay in which a plate is coated with platelet glycoprotein followed by the patient test plasma sample. As in the MAIPA, the presence of an autoantibody is detected with a labeled anti-human antibody. The Immunobead assay can be indirect or direct, and it is a type of antigen capture assay similar to the MAIPA; the difference is that the glycoprotein is captured on the well by an anti-platelet monoclonal antibody conjugated to a bead.
In 1984, Woods et al used a microtiter well assay in which 5 of 56 ITP patient plasma samples reacted against GPIIb/IIIa43 and 3 of 106 ITP patients reacted to GPIb.44 In 1987, Kiefel et al used MAIPA, which can be used to study both patient platelets (direct) and plasma (indirect).45 One advantage of the MAIPA is that it preserves epitopes on platelet glycoproteins. A disadvantage of all antigen capture assays is the possibility of steric hindrance between the monoclonal antibodies and patient autoantibodies, which can be overcome by using a mixture of monoclonal antibodies. Another disadvantage of these assays is that the detection of novel platelet autoantigens is not possible.
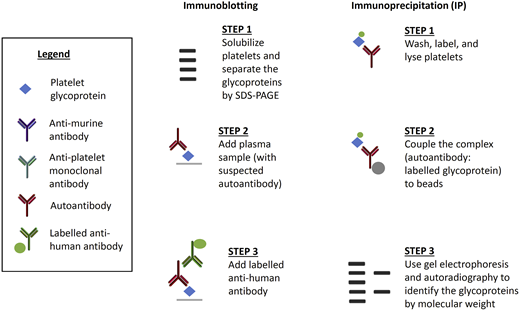
The second general type of assay to measure antibodies on individual platelet glycoproteins include the immunoblot and the radioimmunoprecipitation assays (Figure 3). Immunoblotting is an indirect technique that separates individual platelet proteins, followed by binding of the autoantibody. This technique often distorts platelet antigens and is rarely used today. Immunoprecipitation is a direct or indirect technique, first used by Tomiyama et al,46 involving the binding of the proteins and the autoantibody followed by separation of the platelet proteins. Immunoprecipitation has the advantage of preserving platelet antigens and avoids the issue of steric hindrance. The disadvantages of immunoprecipitation include its complexity and the requirement for a radioactive probe. Nonetheless, our group continues to use immunoprecipitation to study novel platelet autoantigens.
Less common assays used to detect anti-platelet glycoprotein autoantibodies in patients with ITP. Immunoblotting is an indirect assay in which normal platelet proteins are separated and then mixed with patient test plasma. Labeled anti-human antibody detects the autoantibody and the molecular weight of the glycoprotein determines the specificity of the autoantibody. Immunoprecipitation can be direct or indirect. For direct immunoprecipitation, patient test platelets are labeled and lysed, and the suspected autoantibody is bound to the labeled glycoprotein. In the indirect assay, normal platelets are sensitized with patient test plasma before lysis. Autoantibody–glycoprotein complexes are immunoprecipitated, separated, and autoradiographed to identify the glycoproteins by molecular weight.
Less common assays used to detect anti-platelet glycoprotein autoantibodies in patients with ITP. Immunoblotting is an indirect assay in which normal platelet proteins are separated and then mixed with patient test plasma. Labeled anti-human antibody detects the autoantibody and the molecular weight of the glycoprotein determines the specificity of the autoantibody. Immunoprecipitation can be direct or indirect. For direct immunoprecipitation, patient test platelets are labeled and lysed, and the suspected autoantibody is bound to the labeled glycoprotein. In the indirect assay, normal platelets are sensitized with patient test plasma before lysis. Autoantibody–glycoprotein complexes are immunoprecipitated, separated, and autoradiographed to identify the glycoproteins by molecular weight.
The diagnostic utility of anti-platelet autoantibody assays
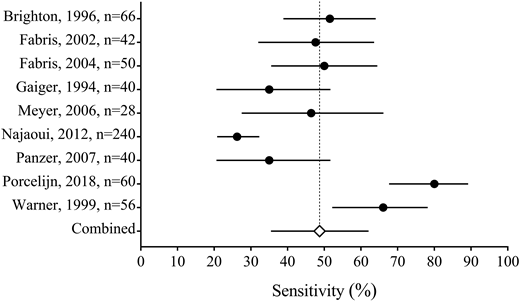
Recently, we systematically reviewed the literature on the serological investigation of ITP patients (Figure 4) (J.R.V., J. Moore, D.M.A., J.G.K., I. Nazy, manuscript submitted, August 2018). We found that the MAIPA and ELISA are the most common methods used to detect platelet GPIIb/IIIa and/or GPIb/IX-specific autoantibodies in ITP among various laboratories. Our systematic review included the results from glycoprotein-specific platelet autoantibody testing in 1170 ITP patients, and 225 non-immune thrombocytopenic controls. We found that the pooled estimates for the sensitivity of glycoprotein-specific autoantibody detection assays were low (<50%), whereas the specificity estimates were higher (>90%). The specificity estimates did not include the results from healthy controls. We also found that direct assays have a better sensitivity than indirect assays. Together, these results suggest that serological investigations for ITP have a high specificity but low sensitivity, meaning that these assays are useful for ruling in, but not for ruling out ITP. This is in contrast to the results found in patients with suspected AIHA, in which the sensitivity and specificity are both high. Our interpretation for the low sensitivity yet high specificity in ITP is that either: (1) a proportion of ITP patients have autoantibodies against other non-platelet target antigens such as thrombopoietin or its receptor c-Mpl,47 (2) the autoantibodies are undetectable in some patients (because of low titer or sequestration), or (3) other pathological immune mechanisms exist that are independent of platelet autoantibodies, such as cytotoxic T cells.48 These results suggest that ITP is a heterogenous group of disorders caused by multiple mechanisms including, but not limited to, anti-platelet autoantibodies.
Forest plot of the sensitivity of direct autoantibody testing (either anti-GPIIb/IIIa or anti-GPIb/IX). The sensitivity is reported from each study with 95% confidence intervals (solid lines); the pooled estimate (open diamond with dashed line) is also reported (J.R.V., J. Moore, D.M.A., J.G.K., I. Nazy, manuscript submitted, August 2018).
Forest plot of the sensitivity of direct autoantibody testing (either anti-GPIIb/IIIa or anti-GPIb/IX). The sensitivity is reported from each study with 95% confidence intervals (solid lines); the pooled estimate (open diamond with dashed line) is also reported (J.R.V., J. Moore, D.M.A., J.G.K., I. Nazy, manuscript submitted, August 2018).
Some investigations have shown that autoantibody testing results correlate with the response to certain therapies. For example, a positive anti-GPIb/IX test may predict the lack of a response to corticosteroids49 and IVIG.50,51 These few studies require confirmation. Predicting the response to therapy has been variable; for example, there is a lack of agreement about whether detectable autoantibodies predict a response to rituximab. We found that the presence of an autoantibody before treatment did not predict the response to treatment.52 Another group noted that the absence of an autoantibody is associated with a lack of response to rituximab.53
The utility of the assays used to detect autoantibodies in ITP is further complicated by the high degree of variability in the diagnostic test characteristics (sensitivity and specificity) of these assays, which has limited their use. These different results may be at least partially explained by the optical density used, or by the autoantigen targets tested.
Toward a more precise diagnosis of ITP
In the absence of a biomarker or gold standard test, it is our opinion that a more accurate diagnosis of ITP should rely on both clinical and laboratory indicators. These would include: (1) a platelet count <100 × 109/L, with the exclusion of other causes of thrombocytopenia; (2) a low platelet count nadir (<20 × 109/L); (3) a platelet count response to therapy (corticosteroids, IVIG, or treatment of the underlying secondary cause); and (4) a positive anti-platelet autoantibody test. This approach maintains the criteria outlined by the international working group8 and incorporates new findings.13,14 The criterion based on a positive autoantibody test relates to the high degree of specificity for these tests. Additional investigations can help exclude other secondary causes and assess the risks of comorbidities such as thrombosis.
Correspondence
John G. Kelton, 1280 Main St West, Hamilton, ON L8S 4K1, Canada; e-mail: keltonj@mcmaster.ca.
References
Competing Interests
Conflict-of-interest disclosure: D.M.A. received research funding from Amgen and Novartis and is a consultant for Amgen, Novartis, Rigel, and VCB. J.G.K. and J R.V. declare no conflicts of interest.
Author notes
Off-label drug use: None disclosed.