Abstract
Mechanical circulatory support (MCS) is the overarching term that encompasses the temporary and durable devices used in patients with severe heart failure. MCS disturbs the hematologic and coagulation system, leading to platelet activation, activation of the contact pathway of coagulation, and acquired von Willebrand syndrome. Ischemic stroke and major hemorrhage occur in up to 30% of patients. Hematologists are an essential part of the MCS team because they understand the delicate balance between bleeding and clotting and alteration of hemostasis with antithrombotic therapy. However, prior to this important collaborative role, learning the terminology used in the field and types of MCS devices allows improved communication with the MCS team and best patient care. Understanding which antithromobotic therapies are used at baseline is also required to provide recommendations if hemorrhage or thrombosis occurs. Additional challenging consultations in MCS patients include the influence of thrombophilia on the risk for thrombosis and management of heparin-induced thrombocytopenia. This narrative review will provide a foundation to understand MCS devices how to prevent, diagnose, and manage MCS thrombosis for the practicing hematologist.
Learning Objectives
Understand the terminology used to describe mechanical circulatory devices to allow communication with mechanical circulatory support (MCS) experts
Describe the indications for MCS and the specific devices, their duration, and type of blood flow
Outline the impact of MCS on the hematologic system
Describe challenging consultations including influence of thrombophilia on risk for thrombosis and treatment of heparin-induced thrombocytopenia
Introduction
More than 6.5 million adults in the United States have heart failure, and the annual cost of care is expected to be $70 billion by 2030.1 Heart failure causes ∼14 000 hospital admissions for children, leading to $1 billion in hospital charges annually. Despite the increasing incidence of heart failure, the number of curative heart transplants has not changed because of the limited availability of organs. Mechanical circulatory support (MCS) devices replace the function of the failing heart in patients ineligible for a heart transplant or until an organ becomes available. However, potentially life-threatening hemostatic events, including pump thrombosis, stroke, and bleeding, occur (Table 1).2 Anticoagulation and antiplatelet medications are used to prevent thrombotic complications but may contribute to the risk for bleeding. Acquired von Willebrand syndrome and other hematologic changes may also influence hemorrhage risk. The ongoing evolution of devices, antithrombotic regimens, and management of adverse events necessitates that hematologists are integral members of the MCS management team. The expert in hemostasis must have an understanding of the terminology used in the MCS field, the device types, and prevention, diagnosis, and management of MCS thrombosis. Challenging questions, including influence of thrombophilia on thrombosis risk and management of heparin-induced thrombocytopenia (HIT), will also be discussed. Management of hemorrhage in MCS patients is covered in a subsequent chapter by Karkouti and Ho.3 Overall, this review will provide the practicing hematologist with a solid foundation to provide consultation for MCS patients and significantly impact their care.
MCS options for children and adults
| Device . | Type of flow . | Max pump rate (max flow) . | Used in children . | Used in adults . | Survival . | Major hemorrhage . | Ischemic stroke . |
|---|---|---|---|---|---|---|---|
| Temporary | |||||||
| VA-ECMO pumps4 | Continuous | (10 L/min) | Yes | Yes | 38-68% of ECLS | 7-10%*,† | 4% |
| Impella 2.5/CP/5.09,10 | Continuous | 51 000 rpm (2.5 L/min); 46 000 rpm (4.0 L/min); 33 000 rpm (5.0 L/min) | Yes | Yes | 25-46% at 1 mo (adults) | 8% (adults) | 4% (adults) |
| 68% at 1 mo (pediatrics) | 5% (pediatrics) | 2% (pediatrics) | |||||
| TandemHeart8 | Continuous | 3 000-7 000 rpm (5 L/min) | Yes | Yes | 43-47% at 1 mo | 42-90% | 0% |
| Long-term | |||||||
| EXCOR14 | Pulsatile | 30-120 bpm (3-7.2 L/min depending on pump size) | Yes | No | Median survival 144-174 d | 28%-50%†,‡ | 6-29% |
| HeartMate II6 | Continuous, axial | 8 000-15 000 rpm (3-10 L/min) | Yes, adolescents | Yes | 90% at 6 mo | 14-15% at 6 mo | 6% at 6 mo |
| HVAD16 | Continuous, centrifugal | 2 400-3 200 rpm (10 L/min) | Yes, adolescents | Yes | 96% at 6 mo | 10-14% at 6 mo | 7% at 6 mo |
| HeartMate 36 | Continuous, centrifugal | 3 000-9 000 rpm (10 L/min) | NR | Yes | 91% at 6 mo | 10-16% at 6 mo | 5% at 6 mo |
| Syncardia TAH12 | Pulsatile | 125 bpm (7-9 L/min) | Yes, adolescents | Yes | 68% to transplant | 25% at 1 mo | 5% at 1 mo |
| Device . | Type of flow . | Max pump rate (max flow) . | Used in children . | Used in adults . | Survival . | Major hemorrhage . | Ischemic stroke . |
|---|---|---|---|---|---|---|---|
| Temporary | |||||||
| VA-ECMO pumps4 | Continuous | (10 L/min) | Yes | Yes | 38-68% of ECLS | 7-10%*,† | 4% |
| Impella 2.5/CP/5.09,10 | Continuous | 51 000 rpm (2.5 L/min); 46 000 rpm (4.0 L/min); 33 000 rpm (5.0 L/min) | Yes | Yes | 25-46% at 1 mo (adults) | 8% (adults) | 4% (adults) |
| 68% at 1 mo (pediatrics) | 5% (pediatrics) | 2% (pediatrics) | |||||
| TandemHeart8 | Continuous | 3 000-7 000 rpm (5 L/min) | Yes | Yes | 43-47% at 1 mo | 42-90% | 0% |
| Long-term | |||||||
| EXCOR14 | Pulsatile | 30-120 bpm (3-7.2 L/min depending on pump size) | Yes | No | Median survival 144-174 d | 28%-50%†,‡ | 6-29% |
| HeartMate II6 | Continuous, axial | 8 000-15 000 rpm (3-10 L/min) | Yes, adolescents | Yes | 90% at 6 mo | 14-15% at 6 mo | 6% at 6 mo |
| HVAD16 | Continuous, centrifugal | 2 400-3 200 rpm (10 L/min) | Yes, adolescents | Yes | 96% at 6 mo | 10-14% at 6 mo | 7% at 6 mo |
| HeartMate 36 | Continuous, centrifugal | 3 000-9 000 rpm (10 L/min) | NR | Yes | 91% at 6 mo | 10-16% at 6 mo | 5% at 6 mo |
| Syncardia TAH12 | Pulsatile | 125 bpm (7-9 L/min) | Yes, adolescents | Yes | 68% to transplant | 25% at 1 mo | 5% at 1 mo |
bpm, beats per minute; ECLS, extracorporeal life support; ECMO, extracorporeal membrane oxygenation; Max, maximum; NR, not reported; rpm, revolutions per minute.
Percentage of reported events.
Gastrointestinal and intracranial hemorrhage.
Chest reexploration and intracranial.
Terminology used to describe MCS
MCS is the overarching term used to describe any mechanical device used to pump blood. The term extracorporeal life support encompasses circuits used to replace cardiac and/or respiratory function. Different terms for extracorporeal life support are used depending upon the purpose, duration, and cannulation procedures (where blood is taken from and returned). Cardiopulmonary bypass (CPB) provides hours of heart and lung function during cardiac surgery via intrathoracic cannulas. Extracorporeal membrane oxygenation (ECMO) provides days of respiratory (veno-venous ECMO [VV-ECMO]) or respiratory and cardiac support (veno-arterial ECMO [VA-ECMO]) (Figure 1). ECMO cannulas are placed under local anesthesia in the extrathoracic circulation, commonly in the jugular or femoral arteries and veins.4 Extracorporeal lung assist and extracorporeal CO2 removal describe extrathoracic cannulation for respiratory support. Emergent use of ECMO after cardiac arrest has been called extracorporeal cardiopulmonary resuscitation. Despite the different names, these circuits are composed of vascular access catheters, a pump, an artificial lung (often called an oxygenator), a heat exchanger, filters, tubing, and connectors (Figure 1).3
(A) Schematic of ECMO systems using a roller or centrifugal pump. Some centrifugal pumps can be used without the membrane lung as percutaneous VADs. (B) The Impella heart pumps are catheter-based temporary MCS devices. Purge fluid flows retrograde through the catheter in the femoral artery, past the motor housing to cool the rotor, and into the circulation. Originally published in Laliberte and Reed11 © [2017], American Society of Health-System Pharmacists, Inc. All rights reserved. Reprinted with permission (R1804).
(A) Schematic of ECMO systems using a roller or centrifugal pump. Some centrifugal pumps can be used without the membrane lung as percutaneous VADs. (B) The Impella heart pumps are catheter-based temporary MCS devices. Purge fluid flows retrograde through the catheter in the femoral artery, past the motor housing to cool the rotor, and into the circulation. Originally published in Laliberte and Reed11 © [2017], American Society of Health-System Pharmacists, Inc. All rights reserved. Reprinted with permission (R1804).
Many different words are also used to describe MCS devices, type of flow, and indication for device implantation (Table 2). The terminology is also evolving, which can lead to confusion for physicians outside the fields of cardiology, critical care medicine, or cardiothoracic surgery. Devices are described as full support if the flow replaces the entire cardiac output and partial support if the device flow only augments the heart. As the Latin roots suggest, pumps located outside of the body are called extracorporeal, whereas intracorporeal pumps are completely implanted within the body. The new heart allocation system from the United Network for Organ Sharing and Organ Procurement and Transplantation Network has separated devices into those that require hospitalization (nondischargeable) and devices with which a patient can leave the hospital (dischargeable).5 MCS devices are also described based upon the type of flow. Pulsatile devices drive blood through a flexible membrane that creates a pulse similar to the native heart. Patients with nonpulsatile or continuous flow devices do not have a pulse because blood constantly flows from the left ventricle to the aorta. Axial and centrifugal devices are types of continuous flow devices. Axial devices have inlets and outlets parallel to each other, and blood flow is created via a rotor like an Archimedes screw (Figure 2). In contrast, the inlet of a centrifugal ventricular assist device (VAD) is perpendicular to the outlet. The rotor creates blood flow by centrifugal force (Figure 2). The last descriptions for MCS devices apply to VADs and denote the purpose for implantation. Previous terminology included device implantation prior to heart transplant (bridge to transplant), to stabilize patients to determine whether they were eligible for transplant (bridge to candidacy), or until improvement in myocardial function (bridge to recovery). With the U.S. Food and Drug Administration (FDA) approval of the HeartMate 3 system, the “bridging” terms have transitioned to “short term.”6 Destination therapy used to describe the indication for VAD placement in patients who are ineligible for heart transplant, which is now transitioned to “long-term” therapy.
Terminology used to describe MCS devices or indications for use
| Term . | Definition . | Device examples . |
|---|---|---|
| Description of device | ||
| Extracorporeal | Pump for device located outside of the body | ECMO, CPB, EXCOR |
| Intracorporeal | Pump for device located with the body | HeartMate 3, HVAD |
| Temporary | Device intended for limited duration of support (hours-days) | Impella 2.5, Impella CP, ECMO |
| Durable | Device with capability of support for months-years | HeartMate 3, HVAD |
| Partial support | Device provides less flow than complete cardiac output | Impella 2.5 |
| Full support | Device provides complete cardiac output | ECMO, EXCOR, HeartMate 3, HVAD |
| Dischargeable | Device does not require hospitalization for continued support | HeartMate 3, HVAD |
| Type of flow | ||
| Pulsatile | Device with flexible membranes that creates intermittent flow and a pulse | Total artificial heart, EXCOR |
| Nonpulsatile/continuous flow | Device that provides constant blood flow | HeartMate 3, HVAD |
| Axial | Device with inlet and outflow in the same axis and flow produced by rotating impeller | HeartMate II, Jarvik 2000 |
| Centrifugal | Device with inlet perpendicular to outflow tracts. Flow produced by centrifugal force. | HeartMate 3, HVAD |
| Indication for MCS use | ||
| Bridge to transplant | Device implanted to support patient until a heart transplant | HeartMate II, HVAD |
| Bridge to candidacy | Device implanted to support patient until can determine whether eligible for a heart transplant | HeartMate II, HVAD |
| Bridge to recovery | Device implanted until myocardial recovery from injury | HeartMate II, HVAD |
| Destination therapy | Device implanted to support patients who are ineligible for heart transplant | HeartMate II, HVAD |
| Short term | New term to describe device implanted with intention for months-years of support (replaces bridge to transplant or recovery) | HeartMate 3 |
| Long term | New term to describe device implanted with the intention for years of support (replaces destination therapy) | HeartMate II, HVAD |
| Term . | Definition . | Device examples . |
|---|---|---|
| Description of device | ||
| Extracorporeal | Pump for device located outside of the body | ECMO, CPB, EXCOR |
| Intracorporeal | Pump for device located with the body | HeartMate 3, HVAD |
| Temporary | Device intended for limited duration of support (hours-days) | Impella 2.5, Impella CP, ECMO |
| Durable | Device with capability of support for months-years | HeartMate 3, HVAD |
| Partial support | Device provides less flow than complete cardiac output | Impella 2.5 |
| Full support | Device provides complete cardiac output | ECMO, EXCOR, HeartMate 3, HVAD |
| Dischargeable | Device does not require hospitalization for continued support | HeartMate 3, HVAD |
| Type of flow | ||
| Pulsatile | Device with flexible membranes that creates intermittent flow and a pulse | Total artificial heart, EXCOR |
| Nonpulsatile/continuous flow | Device that provides constant blood flow | HeartMate 3, HVAD |
| Axial | Device with inlet and outflow in the same axis and flow produced by rotating impeller | HeartMate II, Jarvik 2000 |
| Centrifugal | Device with inlet perpendicular to outflow tracts. Flow produced by centrifugal force. | HeartMate 3, HVAD |
| Indication for MCS use | ||
| Bridge to transplant | Device implanted to support patient until a heart transplant | HeartMate II, HVAD |
| Bridge to candidacy | Device implanted to support patient until can determine whether eligible for a heart transplant | HeartMate II, HVAD |
| Bridge to recovery | Device implanted until myocardial recovery from injury | HeartMate II, HVAD |
| Destination therapy | Device implanted to support patients who are ineligible for heart transplant | HeartMate II, HVAD |
| Short term | New term to describe device implanted with intention for months-years of support (replaces bridge to transplant or recovery) | HeartMate 3 |
| Long term | New term to describe device implanted with the intention for years of support (replaces destination therapy) | HeartMate II, HVAD |
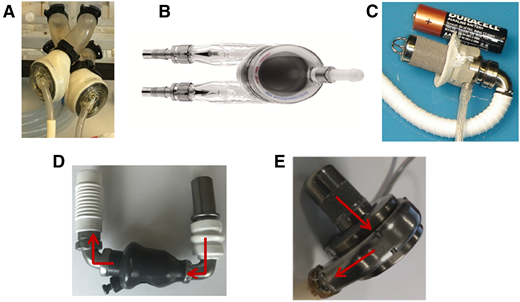
Pictures of durable MCS devices. (A) Total artificial heart. (B) EXCOR pulsatile VAD. (C) Jarvik 2015 pediatric VAD. (D) HeartMate II continuous-flow axial VAD. (E) HVAD continuous-flow centrifugal VAD.
Pictures of durable MCS devices. (A) Total artificial heart. (B) EXCOR pulsatile VAD. (C) Jarvik 2015 pediatric VAD. (D) HeartMate II continuous-flow axial VAD. (E) HVAD continuous-flow centrifugal VAD.
MCS device mechanics
Temporary devices
ECMO.
Patients with cardiac or pulmonary failure refractory to medical therapy may be treated with ECMO as a bridge to recovery or organ transplant. ECMO allows replacement of lung, as well as right and left heart, function. ECMO provides nonpulmonary gas exchange through passage of blood over a membrane that removes CO2 and oxygenates the blood (Figure 1). A pump is included in the circuit, which augments or replaces heart function.4 The Rotaflow (Maquet, Hirrlingen, Germany), CentriMag (Abbott, Lake Bluff, IL), and Biomedicus pump (Medtronic, Minneapolis, MN) are extracorporeal centrifugal pumps used independently in ECMO or as a component of the heart–lung machine for CPB. The use of roller pumps has declined substantially since 2005 due to potential increased hemolysis. The circuit also contains vascular access catheters, a heat exchanger, tubing, and interface for gas exchange and oxygenation in VV-ECMO. Accessing the vasculature, known as cannulation, is performed with 21F to 28F cannulas for adults and smaller sizes in newborns and children. In adults, the venous cannula is positioned in the right internal jugular vein or right atrium with the return cannula placed in the femoral vein (VV-ECMO) or the femoral artery (VA-ECMO). In newborns, the right internal jugular vein and right common carotid artery are cannulated for VA-ECMO. The right atrium and the right internal jugular vein are cannulated for VV-ECMO in infants. In children older than 2 years or >10 kg, femoral vessels are most often used. Central cannulation into the right atrium and ascending aorta can be done if there is failure to wean from CBP after surgery.7
ECMO results in serious complications in all patients after 10 to 20 days of support, including vasomotor instability, capillary leak syndrome, bleeding, and multisystem organ failure. Relative contraindications to ECMO include end-stage primary disease, severe neurologic injury or intracranial bleeding, uncontrolled visceral bleeding, prematurity (<34 weeks of gestation), low weight (<2 kg), and family or patient directive limiting ECMO use.7
TandemHeart
TandemHeart (CardiacAssist, Pittsburgh, PA) is an extracorporeal centrifugal peripherally inserted VAD that can support patients up to 2 weeks (Table 2).8 The inflow cannula is inserted through the femoral vein and into the left atrium via a transseptal puncture. Oxygenated blood is returned to the femoral artery. TandemHeart supplies blood flow of up to 5 L/min, depending on the catheter size. TandemHeart can be used with an oxygenator in an ECMO system (TandemLife).
Impella.
The Impella pumps (Abiomed, Danvers, MA) are temporary percutaneous continuous flow axillary devices that are approved for support of patients undergoing high-risk percutaneous coronary intervention or patients in cardiogenic shock. A limited number of children have been treated with an Impella.9 The catheter traverses the aortic and mitral valves to pull blood from the left ventricle and expel blood into the aorta (Figure 1).10,11 The Impella 2.5 and CP provide partial support and are placed into the left ventricle via an endovascular approach with access from the femoral artery. The larger pumps, Impella 5.0 and Impella LD, require a femoral cut-down or surgical placement into the axillary artery and can provide up to 5 L/min of blood flow. Typical duration of support is hours to days. Fluid flows through the catheter (purge fluid) to cool and prevent blood from entering by the motor.11
Durable devices
Total artificial heart.
The Syncardia total artificial heart (TAH; Tucson, AZ) is a pulsatile system that surgically replaces both heart ventricles with the mechanical pumps and the heart valves (Figure 2). The ventricles are pneumatically driven through an external driver. The Syncardia TAH is FDA approved as a bridge to transplant, and ongoing clinical trials are evaluating long-term use.12 TAH has been implanted in larger children (>2 years of age) with need for biventricular support.13
EXCOR.
The EXCOR VAD (Berlin Heart, Berlin, Germany) is an extracorporeal pneumatically driven pulsatile pump (Figure 2).14 Silicone cannulas connect the pump to the atrium or ventricle and to the great arteries. Pump sizes of 10, 15, 25, 30, 50, 60, and 80 mL are available to support infants ≥3 kg and children of all sizes. The Ikus driver is connected to the pump through driving tubes and provides alternating air pressure to move the pump membrane by creating suction to fill and empty the pump. The presence of valves (3-leaflet polyurethane valves) at the inlet and outlet positions of the blood pump connector stubs ensures unidirectional flow. The blood chamber and the polyurethane connectors are transparent, which allows visual detection of clots and monitoring of filling and emptying of the pump. Children must remain hospitalized during EXCOR support because the device is not fully implantable and requires a large cumbersome driver.
Axial devices.
HeartMate II (Abbott) is an intracorporeal continuous flow axial device that can fully support left ventricular function in patients weighing >30 kg (Figure 2). The pump is implanted in an intraperitoneal pocket, with an inflow cannula in the left ventricle and an outflow cannula in the ascending aorta.6 The benefit of axial flow is the ability to miniaturize the rotor for use in pediatric patients (Jarvik 2015). All surgically implanted left VADs (LVADs) are powered through an external driveline connected to a battery, which is a constant source for infection. The LVADs in the development pipeline have used axial flow, which limits the power needed to run the pump. This could allow implantation of the device and a transcutaneously rechargeable battery to eliminate the driveline.
The Jarvik 2015 is an intracorporeal axial pump that has been approved by the FDA for clinical study in children (Figure 2). The device is a redesign of the Jarvik 2000, which resulted in unacceptable levels of hemolysis (plasma-free hemoglobin > 40 mg/dL). Animal studies confirmed low rates of hemolysis in the Jarvik 2015.15 A feasibility study of patients with and without complex congenital heart disease using the Jarvik 2015 has received FDA approval.
Centrifugal devices.
To implant the centrifugal LVADs (HVAD; Medtronic, Minneapolis, MN and HeartMate 3; Abbott), a core of ventricular tissue is excised, and the pump is sown onto the ventricle (Figure 2).6,16 The larger rotor diameter in centrifugal devices allows lower rotational speeds (2000-3000 revolutions per minute), less shear stress, and hemolysis.17
Effects of MCS on the hematologic system
The biomaterials and shear forces created by MCS pumps cause multiple changes in the hematologic system. Blood contact with biomaterials results in activation of the complement and contact pathway and production of inflammatory cytokines.18 Decreased contact pathway proteins (factor XI and XII) have been reported in patients implanted with pulsatile VADs.19 Activation of the contact pathway of coagulation by foreign materials, including ECMO circuits and mechanical valves, provides potential new targets for anticoagulant therapies.20
Modeling studies suggest that axial LVADs create mean shear stress levels ∼ 100 times higher than normal arterioles.21 Hemolysis is used during device development as a marker of blood trauma due to shear stress but is also a sign of device thrombosis in patients. Baseline levels of hemolysis are higher in patients with temporary and permanent axial flow devices or ECMO roller pumps compared with intracorporeal or extracorporeal centrifugal flow devices.17 Hemoglobin levels after TAH implantation range from 5 to 7 g/dL due to hemolysis, and patients require transfusion support.12 Hemolysis is defined by plasma-free hemoglobin > 40 mg/dL, but increased lactate dehydrogenase (LDH) may identify thrombotic events earlier than elevated levels of plasma-free hemoglobin.22 Plasma-free hemoglobin may play a role in VAD-related thrombosis by interacting with the von Willebrand factor (vWF) A2 domain and promoting increased platelet adhesion to fibrinogen (adherent to the pump) through vWF A1 domain.23
The degree of platelet activation caused by MCS is debated depending upon the method used to detect activation. Shedding of glycoprotein Ibα, GPVI, and α2bβ3 has been reported in patients with continuous-flow VADs and ECMO and associated with increased markers of platelet oxidative stress.24
MCS causes supraphysiologic shear stress, leading to unfolding of vWF and cleavage of vWF multimers ADAMTS-13. A second potential mechanism of acquired von Willebrand syndrome (AvWS) could include consumption of high molecular weight multimers by binding to platelets.21 With the exception of the Syncardia TAH, all MCS devices have been reported to cause AvWS, and the loss of high molecular weight multimers mimics type 2A von Willebrand disease.21 Although higher baseline levels of hemolysis have been reported with the HeartMate II compared with the HVAD,17 the degree of AvWS is similar. AvWS occurs within hours of MCS implantation, and the severity remains stable over time. Although patients treated with the HeartMate 3 showed less high molecular weight vWF multimer loss compared with HeartMate II patients, the functional testing of vWF remained similar.25
Antithrombotic therapy to prevent thrombosis
Stroke and/or pump thrombosis are the most devastating life-threatening effects of MCS and can occur in up to 30% of patients (Table 1). Antithrombotic therapy is used in all MCS devices to prevent thrombosis.
ECMO
There are no prospective studies determining safety and efficacy of antithrombotic therapy regimens for patients supported by ECMO. An international survey on antithrombotic therapy practices in centers belonging to the Extracorporeal Life Support Organization determined that 100% of centers used unfractionated heparin (UFH) at varying infusion rates.26 At that time, only 8% of centers reported the use of direct thrombin inhibitors (argatroban and bivalirudin) even though half of the centers had access to the medications. Adjunct therapies were also used to control coagulation (antiplatelet agents: acetylsalicylic acid and prostacyclin; anticoagulant agents: warfarin) and/or bleeding (ε-aminocaproic acid, tranexamic acid, recombinant human factor VIIa, aprotinin, and serine proteases like aprotinin). Monitoring of antithrombotic therapy was carried out using activated clotting time (ACT) in 97% of centers with variable treatment ranges. Testing antithrombin levels occurred in 82% of centers, with antithrombin supplementation occurring at various triggers.26
The Extracorporeal Life Support Organization Anticoagulation Management Expert Consensus Guidelines were developed as a result of the variability among centers.27 A 50- to 100-U/kg bolus and 7.5 to 20 U/kg per hour infusion of UFH are recommended to achieve an ACT of 180 to 220 seconds. Comparison between monitoring strategies is covered in a subsequent chapter.28
Impella
The Impella catheter has a channel through which purge fluid flows at >300 mm Hg to prevent blood from entering the motor chamber. The manufacturer recommends a 5% dextrose solution with 50 IU/mL UFH is the recommended purge solution. Additional heparin is used systemically, because the heparin in the purge solution often is not sufficient to reach the recommended ACT of 160 to 180 seconds or correlated activated partial thromboplastin time (aPTT).29
EXCOR
The EXCOR was evaluated in a prospective study with comparison with a historical group of age-matched children receiving ECMO30 Standardized antithrombotic therapy guidelines were developed for the study and referred to as the Edmonton protocol (Table 3).30, 31 antithrombin supplementation is recommended if the activity is <70%.30 Gastrointestinal bleeding and intracranial hemorrhage occurred in 11% to 43% of children treated with EXCOR, and ischemic stroke affected 9% to 38% of patients (Table 2).30 Analysis of the EXCOR studies suggested that decreased stroke and bleeding events could result from earlier initiation of aspirin and more rapid upward dose titration, consideration of steroid use, and ensuring hemostasis was optimized prior to initiating anticoagulation31 These revisions were incorporated into the Stanford protocol, which resulted in 84% decreased stroke (2.4 vs 14.7 events per 100 patient months of support) and decreased bleeding (25.8 vs 56.4 events per 100 patient months of support) (Table 3)32 Low molecular weight heparin and/or warfarin is recommended depending upon patient age. A target international normalized ratio (INR) of 2.7 to 3.5 is recommended in the Edmonton and Stanford protocols.
Comparison between Edmonton antithrombotic guidelines and Stanford protocol for management of children with the Berlin Heart EXCOR pediatric VAD
| Medication . | Edmonton protocol . | Stanford protocol . | ||
|---|---|---|---|---|
| Initiation parameters . | Goal . | Initiation parameters . | Goal . | |
| Perioperative | ||||
| Antithrombin concentrate or plasma | Antithrombin activity < 70% | Antithrombin activity ≥ 70% | Antithrombin activity < 70% | Antithrombin activity ≥ 70% |
| Protamine | Completion of CPB | Complete heparin reversal (institution-dependent parameters) | Completion of CBP | Complete heparin reversal (institution-dependent parameters) |
| Postoperative | ||||
| UFH | 24 h postimplantation, platelets > 20 000/µL, normal TEG Platelet Mapping, TEG MAcKH > 46 mm, TEG RcKH < 10 | Anti-factor Xa 0.35-0.5 U/mL, TEG R 8.0-15.0 | 12-24 h postimplantation, platelets > 40 000/μL, no bleeding | Anti-factor Xa 0.35-0.5 U/mL |
| Antiplatelet | ||||
| Dipyridamole | 48 h postimplantation, platelets > 40 000/µL, TEG MAcKH > 56 mm, Platelet Mapping: net ADP G ≥ 4, AA inhibition < 70% | Platelet Mapping: net ADP G 4-8, AA inhibition > 70% | 8 d after implant, add after max dose of asa reached and no bleeding | Titrated to a weight-based dose of 15 mg/kg/d |
| Aspirin | 4 d postimplantation, TEG MAcKH > 72 mm, net ADP G > 2 | Platelet Mapping: net ADP G 4-8, AA inhibition > 70% | 3 d postimplantation, no bleeding | Titrated to a weight-based dose of 30 mg/kg/d (max dose 2000 mg/d) |
| Clopidogrel | No recommendation | 11 d postimplantation, after max dose of aspirin and dipyridamole reached and no bleeding | Titrated to weight-based dose of 0.2 mg/kg/d, max dose 1 mg/kg/d | |
| Long-term anticoagulant | ||||
| Enoxaparin | Age < 1 y, >48 h postimplantation, normal creatinine | Anti-factor Xa 0.6-1.0 U/mL | Age ≤ 2 y, or if unstable INR | Anti-factor Xa 0.6-1.0 U/mL |
| Warfarin | Age ≥ 1 y, full oral diet | INR 2.7-3.5 | Age > 2 y, full oral diet | INR 2.7-3.5 |
| Anti-inflammatory | ||||
| Prednisone | No recommendation | As needed for fibrinogen > 600 mg/dL or other signs of inflammation (fever, rise in CRP), no other signs of sepsis | Methylprednisolone should be initiated at a dose of 2 mg/kg/d IV (or PO equivalent) divided BID, discontinue when fibrinogen ≤ 400 mg/dL | |
| Medication . | Edmonton protocol . | Stanford protocol . | ||
|---|---|---|---|---|
| Initiation parameters . | Goal . | Initiation parameters . | Goal . | |
| Perioperative | ||||
| Antithrombin concentrate or plasma | Antithrombin activity < 70% | Antithrombin activity ≥ 70% | Antithrombin activity < 70% | Antithrombin activity ≥ 70% |
| Protamine | Completion of CPB | Complete heparin reversal (institution-dependent parameters) | Completion of CBP | Complete heparin reversal (institution-dependent parameters) |
| Postoperative | ||||
| UFH | 24 h postimplantation, platelets > 20 000/µL, normal TEG Platelet Mapping, TEG MAcKH > 46 mm, TEG RcKH < 10 | Anti-factor Xa 0.35-0.5 U/mL, TEG R 8.0-15.0 | 12-24 h postimplantation, platelets > 40 000/μL, no bleeding | Anti-factor Xa 0.35-0.5 U/mL |
| Antiplatelet | ||||
| Dipyridamole | 48 h postimplantation, platelets > 40 000/µL, TEG MAcKH > 56 mm, Platelet Mapping: net ADP G ≥ 4, AA inhibition < 70% | Platelet Mapping: net ADP G 4-8, AA inhibition > 70% | 8 d after implant, add after max dose of asa reached and no bleeding | Titrated to a weight-based dose of 15 mg/kg/d |
| Aspirin | 4 d postimplantation, TEG MAcKH > 72 mm, net ADP G > 2 | Platelet Mapping: net ADP G 4-8, AA inhibition > 70% | 3 d postimplantation, no bleeding | Titrated to a weight-based dose of 30 mg/kg/d (max dose 2000 mg/d) |
| Clopidogrel | No recommendation | 11 d postimplantation, after max dose of aspirin and dipyridamole reached and no bleeding | Titrated to weight-based dose of 0.2 mg/kg/d, max dose 1 mg/kg/d | |
| Long-term anticoagulant | ||||
| Enoxaparin | Age < 1 y, >48 h postimplantation, normal creatinine | Anti-factor Xa 0.6-1.0 U/mL | Age ≤ 2 y, or if unstable INR | Anti-factor Xa 0.6-1.0 U/mL |
| Warfarin | Age ≥ 1 y, full oral diet | INR 2.7-3.5 | Age > 2 y, full oral diet | INR 2.7-3.5 |
| Anti-inflammatory | ||||
| Prednisone | No recommendation | As needed for fibrinogen > 600 mg/dL or other signs of inflammation (fever, rise in CRP), no other signs of sepsis | Methylprednisolone should be initiated at a dose of 2 mg/kg/d IV (or PO equivalent) divided BID, discontinue when fibrinogen ≤ 400 mg/dL | |
Net ADP G = [(100 − %ADP inhibition) × GCKH]/100.
AA, arachidonic acid; ADP, adenosine diphosphate; asa, aspirin; BID, twice daily; CRP, C-reactive protein; IV, intravenous; MA, maximum amplitude; MAcK, maximum amplitude citrate kaolin; MAcKH, maximum amplitude citrate kaolin heparinase; max, maximum; PO, by mouth; RCK, reaction time citrate kaolin; RcKH, reaction time citrate kaolin heparinase; TEG, thomboelastogram.
The anticoagulation protocol for the Jarvik 2015 study incorporates standardized antithrombotic therapy guidelines considering lessons learned from the EXCOR study and adult guidelines.
Continuous flow ventricular support
Variability exists among institutions in the management of antithrombotic therapy for continuous-flow ventricular devices. In the early experience with the HeartMate II, investigators questioned whether UFH was needed postoperatively, but most centers returned to bridging therapy after increased thrombosis rates occurred.22 The International Society for Heart and Lung Transplantation Consensus Guidelines recommend a step-wise increase in the targeted range for UFH.33 Usually, warfarin is started after the chest tubes have been removed.33 Long-term anticoagulation with dabigatran was examined in a randomized pilot study. The trial was stopped early because of a significant incidence of thromboembolic complication (50% of patients treated with dabigatran vs 13% of patients treated with warfarin)34 Therefore, warfarin remains the standard long-term anticoagulant after continuous-flow VAD implantation.33 The most common INR target is 2.0 to 3.0, but variable INR goals have been reported without an ability to compare the study outcomes.33,35
Whether aspirin is needed for patients with continuous-flow VADs depends upon the device and center experience. Patients with the HeartMate II in several European centers are not treated with aspirin and have been found to have similar rates of hemorrhagic and thrombotic complications.36 The PREVENT II study is evaluating the need for aspirin with a placebo-controlled trial of patients after HeartMate II implantation (NCT02836652). The protocols for the most recent clinical trials of the HVAD and HeartMate 3 devices have recommended aspirin with doses between 81 and 325 mg.6 Older children receiving a continuous-flow VAD are treated similarly to adults with anticoagulant and aspirin.35
TAH
The antithrombotic therapy protocol used for TAH was developed at La Pitié and has been adjusted by several groups over time.12,37 When the chest tube output is <30 mL/h for 4 hours, UFH is started with infusion rates adjusted to provide a normal coagulation index on thomboelastogram (Haemonetics Corporation, Braintree, MA). Long-term anticoagulation with warfarin is used with a target INR between 2.5 and 3.5 because of the 4 mechanical valves in the TAH. Antiplatelet therapy with aspirin (81 mg/d) and dipyridamole (100 mg every 8 hours to 250 mg every 6 hours) is started when the platelet count recovers to >50 000. Antiplatelet therapy doses are adjusted to provide >50% inhibition of aggregation to arachidonic acid and adenosine diphosphate stimulation while preserving the response to collagen. Pentoxifylline is given to reduce blood viscosity at doses between 200 and 800 mg every 8 hours. In patients with signs of hemolysis, pentoxifylline is started immediately after implantation.12,37
Diagnosis and management of thrombosis
In both ECMO and the EXCOR, device components other than the cannulas are external to the body and are composed of transparent synthetic material.7,30 This allows easy visualization of device-associated thrombus using a high-intensity flashlight. In ECMO, thrombus formation most often occurs at the connectors and the bridge between the venous and arterial lines.4 In a patient with the EXCOR, thrombus can occur within the blood pump, cannula, or oxygenator. Management of thrombosis depends upon the size, color, location, and thrombus mobility. Potential management options include removal of a piece of the ECMO circuit or replacement of the entire circuit attempting to avoid stroke (Table 1). Often, streaks of white clot will be observed that indicate fibrin deposition and are usually not associated with clinical events. However, areas with large red clot will be replaced because of the risk for embolization and ischemic stroke. The EXCOR pump can be exchanged at the bedside, but cannula replacement requires surgical intervention.
The development of thrombus in any type of MCS should prompt urgent assessment of antithrombotic therapy to ensure adequate intensity is present; if not, escalation of therapy should be considered. Material coatings and alternative design of the circuit connectors are being tested to decrease the rates of circuit thrombosis. After ischemic stroke, the risk for hemorrhagic transformation will determine whether the antithrombotic therapy is continued, modified, or temporarily held.30
Indirect markers must be used to diagnose thrombosis in continuous-flow VADs because they are intracorporeal and made of metal, which limits imaging of clot within the device. Changes in shear force occur in the presence of a thrombus, which leads to hemolysis. The Interagency Registry for Mechanically Assisted Circulatory Support defines minor hemolysis as LDH > 2.5 times the upper limit of normal and plasma-free hemoglobin > 20 mg/dL without anemia or pump dysfunction. A hemolytic episode is defined as plasma-free hemoglobin > 40 mg/dL with symptoms of heart failure, anemia, or pump dysfunction.22 A physician will set the VAD speed, and the controller will show the power required to run the pump. Blood flow is then estimated. Power spikes in the axial devices suggest pump thrombosis. The Jarvik 2015, a newly developed continuous-flow device for children, will use similar definitions for hemolysis suggestive of thrombosis. Analysis of the HVAD log files, a record of the pump power, rotor speed, and estimated flow can assist with identification of an increase in pump power before changes in flow are detected.38 Surgical VAD exchange is recommended for severe hemolysis (LDH > 1000 IU/L) or significant pump dysfunction. Case series have reported treatment with heparin, thrombolysis, and intravenous direct thrombin inhibitors. A systematic review of case reports and series noted that intravenous heparin alone resolved thrombosis in only 23% of reported cases. Treatment with bivalirudin or argatroban resolved clot in 56% of patients and was associated with an 11% incidence of major hemorrhage. Thrombolysis had similar rates of clot resolution and risk for hemorrhage increased to 33% if thrombolysis was used after ≥2 other treatments.39
Continuous-flow VADs are being implanted in teenagers and younger children. Device malfunction, including pump thrombosis, occurred in 16% of children (3.2 per 100 patient months of support) implanted with a continuous-flow device, approximately double the rate in adults.40 Neurologic dysfunction, including stroke, was similar to adults (4.1 events per 100 patient months of support).40 Factors that could influence the incidence of pump thrombosis in children include anatomic differences (eg, small right ventricle, a morphologic right ventricle serving as the systemic ventricle in corrected transposition of the great arteries, and dextrocardia where the outflow graft is reversed), heterogeneity in the causes of heart failure, and developmental differences in hemostasis.
Challenging consultations
Influence of thrombophilia on management of MCS patients
There is limited literature to understand whether inherited thrombophilia affects the risk for thrombosis in MCS patients. Retrospective studies have combined the outcomes of patients with congenital thrombophilia, anti-phospholipid antibody syndrome, and HIT, which makes the reported increase in deep vein thrombosis and bleeding outcomes in patients with thrombophilia difficult to attribute.41,42 One group increased the INR target to 2.5 to 3.5 in patients with heterozygous factor V Leiden or prothrombin gene mutations and found 3 times the incidence of gastrointestinal bleeding and 5 times the incidence of hemorrhagic stroke compared with contemporary controls.43 The current literature does not support routine thrombophilia testing in patients prior to MCS implantation or exclusion of patients from MCS support based upon thrombophilia testing without a history of thrombosis.
HIT
Antibody development to the platelet factor 4 (PF4) and heparin complex occurs in 60% of adults undergoing cardiac surgery, with 1% to 2% going on to develop the clinical syndrome of HIT.44 The incidence of HIT in children is likely overestimated, with reported incidences ranging between 0% and 1.7% in neonates and between 1.3% and 52% in children. Four established risk factors for HIT include female gender, duration of heparin therapy, type of heparin exposure (highest risk with UFH compared with low molecular weight heparin or fondaparinux), and patient type (highest risk in postsurgical patients compared with medical, obstetric, or pediatric patients).45 The diagnosis of HIT during MCS is difficult because thrombocytopenia can occur as the result of multiple factors, including postsurgical consumption, bleeding, or infection. Because of the high incidence of false-positive PF4 enzyme-linked immunosorbent assays, functional testing for heparin-induced platelet activation with the serotonin-release assay is required if the clinical scenario is suggestive of HIT.44
Alternative anticoagulation with an intravenous direct thrombin inhibitor is required for HIT during MCS.45 Dosing regimens for bivalirudin or argatroban are based upon small case and case series. A recent systematic review of treatment with bivalirudin during ECMO reported significant variation in the 3 studies and 5 case reports in the literature (n = 58, 24 children). Bivalirudin infusion rates varied from 0.1 to 0.2 mg/kg per hour without a loading dose to 0.5 mg/kg per hour with a loading dose.46 Monitoring strategies also varied from targeting an aPTT of 1.5 to 2.5 times normal to using ACT (goal 180-220 seconds) or thromboelastography. In children with a VAD, case series suggest using higher doses of bivalirudin (0.3-0.5 mg/kg/h) and titrated to an aPTT of 70 to 100 seconds.47 HIT during TAH has been treated with 0.05 to 0.2 mg/kg per hour of bivalirudin and monitored with thromboelastography.37 Argatroban infusions in patients on ECMO have been initiated at 0.1 to 0.2 μg/kg per minute and increased by 0.05 μg/kg per minute to obtain PTT of 1.3 to 3 times baseline.48 In patients with suspected HIT while supported with an Impella, use of argatroban in the purge fluid at 0.05 to 0.1 mg/mL can provide adequate systemic anticoagulation.11 For patients who require urgent CPB, plasma exchange and treatment with intravenous immunoglobulin have been used to remove PF4 antibodies and decrease platelet activation, respectively, to allow use of UFH.49,50 Lastly, an ECMO circuit exchange is needed with HIT if heparin-bonded circuits are used.
Conclusions
The field of MCS can be confusing because of the number of terms used to describe extracorporeal circuits and implanted devices. Antithrombotic therapy management to prevent or treat thrombosis remains complex, with significant variation among institutions. Input from hematologists with development of evidence-based antithrombotic strategies has the potential to significantly improve the outcomes for patients treated with MCS.
Correspondence
Lisa Baumann Kreuziger, Blood Research Institute, BloodCenter of Wisconsin, and Division of Hematology and Oncology, Medical College of Wisconsin, 8733 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: Lisa.BaumannKreuziger@bcw.edu.
References
Competing Interests
Conflict-of-interest disclosures: L.B.K. declares no competing financial interests. M.P.M. has consulted for the National Institutes of Health/National Heart, Lung, and Blood Institute PumpKIN study: Pumps in children, infants, and neonates.
Author notes
Off-label drug use: Off-label drug use includes aspirin, dipyridamole, warfarin, and argatroban for thromboprophylaxis of ventricular assist devices, and direct thrombin inhibitors and thrombolytic therapy for device thrombosis.

![Figure 1. (A) Schematic of ECMO systems using a roller or centrifugal pump. Some centrifugal pumps can be used without the membrane lung as percutaneous VADs. (B) The Impella heart pumps are catheter-based temporary MCS devices. Purge fluid flows retrograde through the catheter in the femoral artery, past the motor housing to cool the rotor, and into the circulation. Originally published in Laliberte and Reed11© [2017], American Society of Health-System Pharmacists, Inc. All rights reserved. Reprinted with permission (R1804).](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2018/1/10.1182_asheducation-2018.1.507/2/m_hem01868f1.png?Expires=1765899733&Signature=NNjrHVY3RAQ7zu7092~UW0wu1V8qCKsJm~0kI2tT1vsDFAP0OkdwjQgOxtrv1dLsQ~4kiKPNN8wKIlyD~lQdAX-9IVWr1Q5vsv~r2orhZqASaXL8oycaKbAtQQFuz4R-wY4BbPma6jgm3f2qccuzQt7iQrnE-HHqjRHY1j8MF-kolcAuicZhI5NWlcpSxXVLq-3b5lCdtCZ1oT3ZOlOiUPAY8~0Rzuqwb4~qWK4044VndroEK8xjwcCW97ZUtWDX-NOf~7-ffR5csd6mIbv~EFFm815rYnsXed~87PYaF9oyuLjCusjqIJMfd2RAh9eg8BCQmyl~~HKKslKxO2Kd1w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)