Abstract
Allogeneic hematopoietic stem-cell transplantation remains the only curative treatment for patients with acquired severe aplastic anemia (SAA). When a matched sibling is not available, one can search for a matched unrelated donor or a cord blood unit (CB) in the international registries or, more recently, for an HLA haploidentical (HAPLO) family member. International guidelines call for a course of antithymocyte globulin (ATG) and cyclosporine before a patient with SAA receives a transplant from a donor other than an HLA identical sibling, but whether this is necessary for patients age <20 years is less clear. Here I will examine the rapid increase in HAPLO transplantations for SAA, showing encouraging early results both in children and young adults. Graft-versus-host disease prophylaxis remains of primary importance in patients with SAA, and in vivo T-cell depletion with either ATG or alemtuzumab offers a significant survival advantage. Finally, I will discuss the strong age effect, which is particularly evident at >40 and 50 years of age for reasons not entirely clear and which should be taken into account when designing a treatment strategy for a given patient.
Learning Objective
Understand the place of allogeneic transplantation using alternative donors in acquired SAA, how to select patients for this curative treatment, and the current most effective transplantation platforms
Introduction
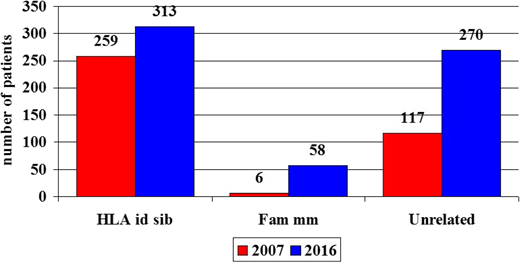
Unrelated donor (UD) transplantations have become increasingly popular for patients with acquired severe aplastic anemia (SAA); this is possibly due to larger donor pools in the international registries and better HLA matching between donor and recipient but also may be due to improved survival. As a consequence, the number of transplantations has increased. In the last decade (2007-2016), the number of UD grafts for SAA increased from 117 in year 2007 to 270 in 2016 (131% increase; Figure 1).1,2 In the same period, the number of HLA identical sibling grafts went from 259 to 313 (21% increase), with the largest change being the increase in the use if familial mismatched grafts, which increased from 6 per year to 58 per year (866% increase; Figure 1).1,2 The larger number of UD grafts is the result of improved outcome, as documented by several reports,3-8 and transplantation physicians in Europe have become more confident in allocating a patient with SAA to an alternative donor transplantation, both in children5,6 and older adults. In a recent report, the number of patients age >40 years receiving allografts from UDs in Europe increased from 94 overall in the period of 2001 to 2009 (10 per year) to 227 in the period of 2010 to 2015 (37 per year).9
Number of patients with acquired SAA allografted in Europe in 2007 (red bars) and 2016 (blue bars). Shown are the numbers of grafts from HLA-identical siblings (HLA id sibs), family mismatched donors (Fam mm), and UDs (Unrelated).
Number of patients with acquired SAA allografted in Europe in 2007 (red bars) and 2016 (blue bars). Shown are the numbers of grafts from HLA-identical siblings (HLA id sibs), family mismatched donors (Fam mm), and UDs (Unrelated).
UD transplantations
When should we look for a UD?
Acquired SAA remains a difficult disease, with significant morbidity and mortality. The patient can present with severe or very severe cytopenia and associated infections and/or hemorrhages; in other cases, the course is more insidious, with declining counts until pancytopenia develops. In both cases, the search for an HLA identical sibling should be 1 of the first interventions for SAA, and medically suitable family members should also be HLA typed. If an identical sibling is identified, and the patient is age <40 years, the international guidelines recommend transplantation first line.10,11 If the patient is older, or in the absence of an identical sibling, the patient should receive a first course of antithymocyte globulin (ATG) and cyclosporin (CsA), and the search for a UD should start at the same time (Table 1).
Treatment strategies in patients without an HLA identical sibling
| Treatment . | <20 y . | 20-40 y . | 40-60 y . | >60 y . |
|---|---|---|---|---|
| First line | hATG + CsA or UD Tx | hATG + CsA and UD search | hATG + CsA and UD search | hATG + CsA |
| Second line | UD Tx | UD Tx | UD Tx or rATG + CsA | hATG + CsA or EPAG |
| Other treatment | HAPLO Tx | HAPLO Tx | +EPAG | +EPAG |
| +Androgens | +Androgens | |||
| +Clinical trials | +Clinical trials |
| Treatment . | <20 y . | 20-40 y . | 40-60 y . | >60 y . |
|---|---|---|---|---|
| First line | hATG + CsA or UD Tx | hATG + CsA and UD search | hATG + CsA and UD search | hATG + CsA |
| Second line | UD Tx | UD Tx | UD Tx or rATG + CsA | hATG + CsA or EPAG |
| Other treatment | HAPLO Tx | HAPLO Tx | +EPAG | +EPAG |
| +Androgens | +Androgens | |||
| +Clinical trials | +Clinical trials |
CsA, cyclosporine; EPAG, eltrombopag; HAPLO Tx, related HLA haploidentical transplant; hATG, horse anti-thymocyte globulin; rATG, rabbit ATG; UD Tx, unrelated donor transplant.
Who should receive a UD transplant?
As shown in Table 1, young patients age <20 years may be grafted upfront, as first-line therapy, from a matched UD. This option is based on reports of 90% survival in children grafted upfront from a UD, showing outcomes comparable to those in children grafted from matched siblings.5,6 First-line treatment with ATG plus CsA is recommended for all other age groups and also remains an option in children (Table 1). Patients should then be followed for at least 3 to 4 months after their first course of ATG plus CsA to assess a hematologic response,12 in which time an HLA-A, -B, -C, or -DRB1 identical (8/8 matched) donor may be identified. For patients who do not respond to first-line therapy and have an identical UD, the choice for second-line treatment may differ according to the age of the patient; patients under 40 years of age will proceed to a UD graft, but the situation is less clear for patients in the age group of 40 to 60 years, for whom a second course of ATG plus CsA may also be an option (Table 1). For this age group, one will need to consider other factors such as neutrophil count, performance status, comorbidities, and infections. A 45-year-old patient with a neutrophil count of 0.2 × 109/L and a lung infection but a good performance status should proceed to a transplantation using an 8/8-matched UD; in contrast, a 58-year-old patient with a neutrophil count of 0.8 × 109/L, no infections, and comorbidities will probably be offered a second course of immunosuppression, possibly with the addition of eltrombopag (Table 1).13 For patients age >60 years, the search for a UD may not even have been started, and a second course of ATG plus CsA or eltrombopag14 may be preferable; however, in selected cases of very fit patients or patients with very severe disease, the possibility of transplantation upfront as first-line therapy could be considered.
HLA matching and UD transplantations
A study from the Center for International Blood and Marrow Transplant Research (CIBMTR)15 compared the outcome of 8/8-, 7/8-, and 6/8-matched UDs in nonmalignant disorders; the outcome of <8/8-matched UD transplantations was significantly inferior to that of 8/8-matched grafts, with the major problem being graft failure. In a multicenter study of patients with SAA, Deeg et al16 also reported superior results with 8/8-matched UDs as compared with <8/8-matched donors. A European Blood and Marrow Transplant (EBMT) analysis examined 100 patients grafted with a homogeneous conditioning regimen17 ; there were 75 UD transplantations with full HLA typing: 46 were classified as HLA matched (reported as 8/8 or 10/10 allele matched) and 29 were mismatched, the donor being ≥1 allele mismatched with the recipient. The crude mortality of HLA-matched and HLA-mismatched transplantations was 17% vs 34% (P = .1); with a median follow-up of >2000 days, the 10-year actuarial survival for matched UD transplantations is 80% vs 65% for patients grafted from donor <8/8 matched. Therefore, the aim of the UD search is an 8/8 high-resolution HLA-A–, -B–, -C–, or -DRB1–matched donor. Whether a 7/8 donor is also acceptable will depend on the age of the patient and his or her hematologic status.
Conditioning regimens for UD transplantations
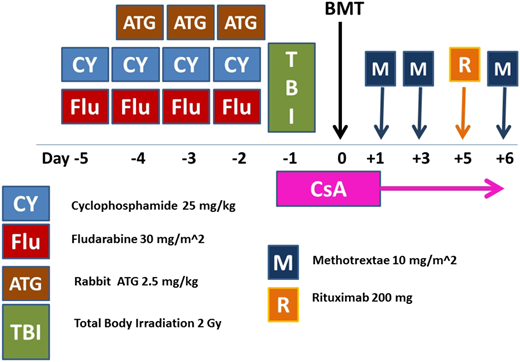
The combination of fludarabine (FLU) and cyclophosphamide (CY; FC), originally introduced by the Houston group into the transplantation arena,18 is currently the basis of the preparative regimens for patients with SAA undergoing a UD transplantation17,19-22 (Figure 2). There are 3 components of this regimen that deserve attention: the dose of CY, the dose of total-body irradiation (TBI), and the dose and brand of ATG.
ATG-based conditioning regimen (FCA-TBI) for patients with acquired SAA undergoing unrelated donor transplantation. BMT, bone marrow transplantation; CsA, cyclosporine; CY, cyclophosphamide; FLU, fludarabine; M, methotrexate; R, rituximab 200 mg fixed dose; TBI, total body irradiation.
ATG-based conditioning regimen (FCA-TBI) for patients with acquired SAA undergoing unrelated donor transplantation. BMT, bone marrow transplantation; CsA, cyclosporine; CY, cyclophosphamide; FLU, fludarabine; M, methotrexate; R, rituximab 200 mg fixed dose; TBI, total body irradiation.
CY.
Anderlini et al7 completed a prospective dose-finding study on CY in combination with FLU and low-dose TBI (2 Gy). Major regimen-related toxicity was seen in 11% of patients receiving CY 50 mg/kg vs 22% in patients receiving Cy 100 mg/kg; the proportions of patients engrafted and alive were 92% and 85%, respectively7 ; interestingly, rejection was similar in both cohorts of patients (8% vs 15%, respectively). The authors previously excluded CY 150 mg/kg as too toxic. In conclusion, both CY 50 mg/kg (12.5 mg/kg per day ×4) and CY 100 mg/kg (25 mg/kg per day ×4) can be considered adequate doses of CY to add to the combination of FLU and low-dose TBI.
TBI.
Deeg et al16 performed a deescalation study on the optimal dose of TBI to be used for UD transplantations in SAA. TBI was started at 3 fractions of 2 Gy, to be escalated or deescalated in steps of 2 Gy dependent upon graft failure/toxicity. The dose-limiting toxicity was diffuse pulmonary injury. The optimum TBI dose was found to be 2 Gy.16 Other studies have confirmed that a dose of TBI between 2 and 3 Gy is safe and effective in patients with SAA.17,19,20
ATG.
ATG has been part of the conditioning regimen since the early 1970s,23 when transplantations were performed only with HLA identical siblings, and is associated with improved survival both in the identical sibling and UD settings.24 The dose of ATG depends on the ATG brand: horse ATG (ATGAM) is administered at 40 mg/kg per day ×3, rabbit ATG (Thymoglobulin) is administered at 2.5 mg/kg per day ×3, and rabbit ATG (Fresenius) is administered at 10 mg/kg per day ×3.
A recent registry study from the CIBMTR compared the outcome of transplantation in SAA using either horse or rabbit ATG.25 The study included 833 SAA transplantations using HLA-matched siblings (n = 546) or UDs (n = 287). Acute and chronic graft-versus-host disease (GVHD) was significantly more frequent in recipients of horse ATG as compared with rabbit ATG, both in sibling as well as UD grafts. In patients grafted from matched siblings, 3-year survival was similar for horse (87%) and rabbit ATG (92%), but in UD transplantations, 3-year survival was superior when using rabbit ATG (83%) as compared with horse ATG (75%; P = .02).25
Of interest in the CIBMTR study on ATG25 is the incidence of Epstein-Barr virus (EBV) lymphoproliferative disorders (LPDs); the rates were reported to be low in recipients of identical sibling grafts (0.3% for horse and 2.2% for rabbit ATG), but in patients grafted from UDs, the rates were 2.5% with horse and 10.4% with rabbit ATG. The authors refer to this rate as an uncommon event, although a 10% incidence with the preferred source of ATG (rabbit) is not really uncommon and is clearly increased in patients receiving ATG in the conditioning regimen.26 EBV LPDs can be life-threatening complications and are currently managed with weekly monitoring of EBV viral load and preemptive treatment with rituximab. However, another option for patients receiving an ATG-based conditioning regimen is prophylaxis with 200 mg of rituximab27 early after transplantation on day +5, which almost abrogates the problem of EBV reactivation and seems to reduce acute GVHD.27
Alemtuzumab-based conditioning regimens.
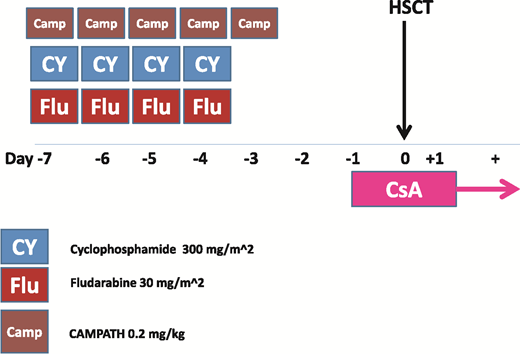
Alemtuzumab (Campath) can be used in combination with FC (FCC) instead of ATG in combination with FC (FCA).28 The FCC conditioning regimen is summarized in Figure 3. One clear advantage is that this regimen is TBI free, which is relevant for transplantation in a nonmalignant disease; in fact, radiation therapy has been reported to be one of the major determinants of second tumors in patients with SAA,29 and efforts to eliminate radiation therapy are always welcome. The second advantage is a low incidence of chronic GVHD, which can only be detrimental in a nonmalignant disorder.28 In contrast, the transplantation outcome is quite unique in patients prepared with FCC, especially in terms of chimerism; FCC patients usually exhibit a mixed chimerism early after transplantation, and donor engraftment gradually picks up after several months. Mixed chimerism, both in myeloid as well as in CD3+ peripheral blood (PB) lympohcytes, can persist long term. A retrospective comparison of FCA and FCC in UD transplantations for SAA has been reported30 ; failure to engraft was comparable (11% vs 9%), but the risk of chronic GVHD was lower for FCC (11%) compared with FCA (26%; P = .03). In a multivariate analysis, the use of bone marrow (BM) and younger age were positive predictors of survival.30
Campath-based conditioning regimen (FCC) for patients with acquired SAA undergoing unrelated donor transplantation. HSCT, hematopoietic stem cell transplantation; Camp, Campath; CsA, cyclosporine; CY, cyclophosphamide; FLU, fludarabine.
Campath-based conditioning regimen (FCC) for patients with acquired SAA undergoing unrelated donor transplantation. HSCT, hematopoietic stem cell transplantation; Camp, Campath; CsA, cyclosporine; CY, cyclophosphamide; FLU, fludarabine.
ATG or alemtuzumab?
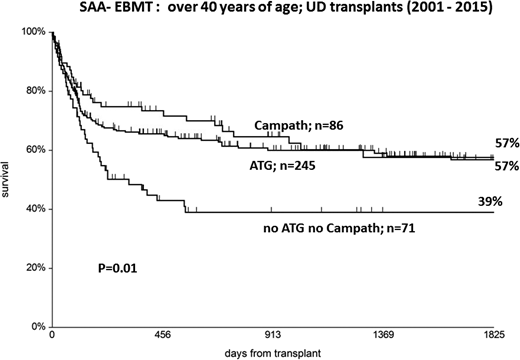
ATG is probably the most common form of in vivo T-cell depletion in Europe and the United States, whereas alemtuzumab is largely used in the United Kingdom. Whatever the choice, it is important to know that the use of either ATG or alemtuzumab results in a significant survival advantage for patients with SAA undergoing UD transplantations, as shown in the EBMT analysis.24 Further support for the use of either ATG or alemtuzumab comes from a recent EBMT analysis of patients age >40 years; the use of either alemtuzumab or ATG (n = 564) resulted in 5-year survival of 63%, compared with 48% in patients who did not receive ATG nor alemtuzumab (n = 161).9 In the same data set, when looking at UD transplantations only, the 5-year survival was 57% for patients receiving either alemtuzumab or ATG and 39% for patients not receiving alemtuzumab or ATG in the conditioning regimen (Figure 4).
UD transplants over the age of 40: a significant survival advantage is seen when ATG or Campath is added in the conditioning regimen (EBMT data from Ref. 9 ).
UD transplants over the age of 40: a significant survival advantage is seen when ATG or Campath is added in the conditioning regimen (EBMT data from Ref. 9 ).
Therefore, the conditioning regimen for UD transplantations in SAA should be based on a combination of FC, probably with the addition of low-dose TBI; the use of either ATG (especially rabbit ATG) or alemtuzumab seems to be crucial in reducing the risk of chronic GVHD and improving survival. When using alemtuzumab (FCC), the radiation therapy may be omitted, but mixed chimerism will likely occur.
BM or PB
Several studies from the CIBMTR and EBMT have shown that BM is the preferred stem-cell source as compared with granulocyte colony-stimulating factor (G-CSF)–mobilized PB, both in matched-sibling as well as in UD transplantations, in all age groups.31-33 Although PB grafts were expected to reduce the risk of rejection, this has not been the case,31-33 and these grafts have come at a cost of higher risk of chronic GVHD. Despite these registry-based data showing a survival advantage for BM graft recipients, many centers are still using PB as a stem source, especially when the donor is unrelated or when GVHD prophylaxis includes alemtuzumab.28 Unmanipulated BM should remain the stem-cell source of choice in patients with acquired SAA undergoing a first allogeneic transplantation, either from a sibling or a UD.
Unrelated cord blood transplantations
In a study of unrelated cord blood (UCB) transplantations in SAA, patients received either a single UCB transplant (n = 57; 80%) or double UCB transplants (n = 14; 20%) between 1996 and 2009. Most patients (69%) received FLU-based reduced-intensity conditioning regimens. The cumulative incidence of neutrophil recovery at day 60 was 51% ± 6%, with a median time of 25 days (range, 6-91 days). In multivariate analysis, the only factor associated with shorter time to engraftment and higher probability of engraftment was the prefreezing total nucleated cell (TNC) dose (>3.9 × 107/kg; P < .05). Grades 2 to 4 GVHD was seen in 20% of patients, and 11 of 34 at risk developed chronic GVHD. The estimated probability of 3-year overall survival was 38% ± 6%. The main cause of death was graft failure associated with infections. This is the largest cohort of UCB grafts in patients with acquired SAA, and the message is very clear: a TNC dose of >4 × 107/kg is required both for engraftment and overall survival.34 If a CB unit containing this number can be identified, a single-unit CB transplantation should be planned; on the contrary, if this number of TNCs cannot be secured with 1 CB unit, it may be reasonable to opt for a double-unit CB graft.
The conditioning regimen for CB transplantations is identical to the regimen proposed for UD transplantations (Figure 2). It is customary to omit methotrexate from the GVHD prophylaxis of UCB grafts; 1 dose of rituximab 150 mg is recommended on day +5 to prevent EBV LPDs.34
Should we consider CB transplantations for SAA? They should be considered only after failure of a first course of immunosuppression, if a matched UD is not available, or probably if a program for HAPLO transplantations is not active at the center, as suggested by results with family haploidentical donors, discussed in the next section.
Haploidentical transplantations
With the rapidly increasing use of unmanipulated HAPLO transplantations for patients with leukemia,2 it became clear that experienced centers would start testing HAPLO donors for patients with acquired SAA as well.35-42
Table 2 summarizes recent publications of results for a total of 277 patients, most of whom were prepared with nonmyeloablative regimens, including FLU, low-dose CY, and low-dose TBI. The stem-cell source was mostly a combination of G-CSF–mobilized BM combined with G-CSF–mobilized PB, as pioneered by Chinese centers, in 1 case with the addition of mesenchymal stem cells. GVHD prophylaxis included ATG-based regimens, posttransplantation CY-based programs, and ex vivo CD3 depletion. It should be noted that most of these studies included a large proportion of pediatric patients, and the overall median age was 27 years. The median overall engraftment rate was 92%, median risk of grade 2 to 4 GVHD was 12%, and median survival at 1 year was 85%.
Results of HAPLO transplants in SAA
| Reference . | No. of patients . | Age, y . | Conditioning . | GVHD Proph . | SC source . | Engraftment . | GVHD 2-4 . | Alive at 1 y . |
|---|---|---|---|---|---|---|---|---|
| 36 | 26 | 30 | RIC | ATG CsA | BM | 92% | 10% | 84% |
| 37 | 21 | 14 | NMA | CD3 dep | PB | 96% | 30% | 94% |
| 38 | 8 | 30 | NMA | PTCY, FK, MMF | GPregimen; B | 75% | ||
| 39 | 17 | 19 | NMA | ATG, Basilix, CsA | GBM + GPB | 90% | 25% | 65% |
| 40 | 26 | 30 | NMA | ATG, CsA, MTX, MMF | GBM+GPB | 92% | 12% | 84% |
| 41 | 77 | 8 | NMA | ATG, CsA, MTX, MMF + MSC | GBM+GPB | 92% | 12% | 93% |
| 42 | 13 | 30 | RIC | PTCY, FK, MMF | BM | 100% | 10% | 100% |
| 43 | 89 | 25 | RIC | ATG, CsA, MTX, MMF | GBM+GPB | 97% | 30% | 86% |
| Total | 277 | 27 | 92% | 12% | 85% |
| Reference . | No. of patients . | Age, y . | Conditioning . | GVHD Proph . | SC source . | Engraftment . | GVHD 2-4 . | Alive at 1 y . |
|---|---|---|---|---|---|---|---|---|
| 36 | 26 | 30 | RIC | ATG CsA | BM | 92% | 10% | 84% |
| 37 | 21 | 14 | NMA | CD3 dep | PB | 96% | 30% | 94% |
| 38 | 8 | 30 | NMA | PTCY, FK, MMF | GPregimen; B | 75% | ||
| 39 | 17 | 19 | NMA | ATG, Basilix, CsA | GBM + GPB | 90% | 25% | 65% |
| 40 | 26 | 30 | NMA | ATG, CsA, MTX, MMF | GBM+GPB | 92% | 12% | 84% |
| 41 | 77 | 8 | NMA | ATG, CsA, MTX, MMF + MSC | GBM+GPB | 92% | 12% | 93% |
| 42 | 13 | 30 | RIC | PTCY, FK, MMF | BM | 100% | 10% | 100% |
| 43 | 89 | 25 | RIC | ATG, CsA, MTX, MMF | GBM+GPB | 97% | 30% | 86% |
| Total | 277 | 27 | 92% | 12% | 85% |
BM, bone marrow; FK, tacrolimus; GVHD Proph, GVHD prophylaxis; MMF, mycophenolate; NMA, nonmyeloablative regimen; PB, peripheral blood; PTCY, high-dose posttransplant cyclophosphamide; RIC, reduced intensity conditioning regimen; SC source, stem cell source.
For most of the patients reported in these studies, a first course of ATG plus CsA had failed, but 1 report compared upfront transplantation using HLA-identical siblings (n = 69) with upfront transplantation using HAPLO family donors (n = 89) in children with SAA.42 There was more acute GVHD in HAPLO grafts, but engraftment, chronic GVHD, and 3-year survival (86% vs 91%) were comparable.
Should we consider a HAPLO donor for patients with acquired SAA? The currently available data seem to suggest a favorable outcome, at least in the pediatric age group, and with the combination of G-CSF–mobilized BM and G-CSF–mobilized PB; however, early results are available for adults as well, and one should probably consider this option in a patient not responding to immunosuppression and lacking a suitable matched UD.
Rejection and graft failure
The risk of rejection in acquired SAA is high when using alternative donors, and this is why a small dose of TBI is usually combined with immunosuppression (FC).- Chimerism studies are helpful to identify patients who are at risk of graft failure.43 In an EBMT study, it was possible to identify 5 different patterns of serial chimerisms in patients with SAA: full donor chimera,1 transient mixed chimera,2 stable mixed chimera,3 progressive mixed chimera,4 and autologous reconstitution with rejection.5,43 As expected, progressive mixed chimeras were at high risk of graft rejection, whereas complete donor chimeras had the highest rate of GVHD. This study emphasized the difficulty of finding the right balance between engraftment and GVHD in patients with SAA with a nontoxic conditioning regimen, a relatively small cell dose at transplantation, and the indication of not using PB as a stem-cell source. Should we intervene in patients with different chimerism patterns? Full donor chimerism will require careful monitoring of immunosuppressive therapy to prevent chronic GVHD. Stable mixed chimeras are the rule and can remain such for decades; no intervention is necessary. Progressive mixed chimeras, especially if combined with declining PB counts, may call for the use of donor lymphocyte infusion to tip the balance in favor of donor T cells. When progressive mixed chimerism is followed by rejection, a second transplantation is mandatory. Two studies recently addressed the issue of graft rejection and second transplantations in SAA.44,45 In the CIBMTR study,44 166 second transplantations were analyzed, and worse survival could be predicted by shorter interval between the first and second transplantations and poor performance score at second transplantation. The authors were unable to show an effect of different conditioning regimens, most combining CY with low-dose radiation therapy.44 In the second EBMT study of 162 patients,45 survival was again predicted by performance status; interestingly, survival was similar, if not identical, when the donor for the second transplantation was a matched sibling or a UD.45 Most patients received PB grafts for the second transplantation, but this did not translate into a survival advantage when compared with patients receiving a second BM graft.45 In conclusion, a second transplantation is feasible, with survival in excess of 60%, but can we prevent graft failure?
A small dose of TBI for UD transplantations is probably necessary to ensure a robust engraftment, together with ATG pretransplantation (or alemtuzumab). CsA should not be discontinued before 6 months and never abruptly. Using PB will not reduce the risk of graft failure; it will only increase the risk of chronic GVHD. After transplantation, chimerism should be assessed on unfractionated BM cells and on CD3+ selected PB cells, and the patient’s chimerism pattern should be identified. Progressive mixed chimerism with falling counts should alert physicians to the risk of rejection and might call for the use of donor lymphocyte infusion. If counts continue to fall and donor chimerism does not improve, a second transplantation should be planned. Unfortunately, we cannot recommend any specific conditioning regimen for a second transplantation; it may include a dose of radiation and again ATG or alemtuzumab. In these patients, a small dose of rituximab should be considered on day +5 because of the risk of EBV LPDs. It should be pointed out that transplantation programs including alemtuzumab can also lead to successful engraftment in the absence of radiation therapy.30
Alternative donor transplantations in patients age >40 years
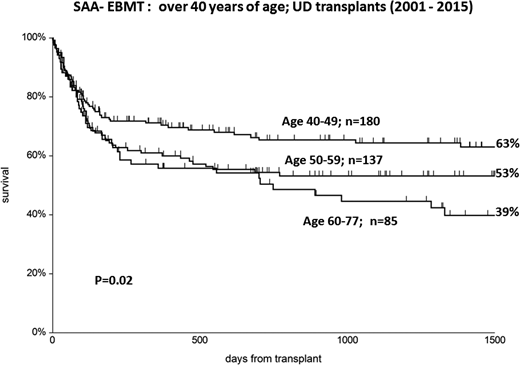
Age remains a major predictor of outcome in patients undergoing an alternative-donor hematopoietic stem-cell transplantation for SAA, as recently shown in an EBMT survey.46 Ten-year survival was 85%, 77%, 66%, and 49%, respectively, for patients age 1 to 10, 11 to 30, 31 to 40, and >40 years, and this should be considered when discussing treatment strategies. The age effect also remains beyond age 40 years, as shown in Figure 5; actuarial survival at 5 years is 63%, 53%, and 39%, respectively, in patients age 40 to 49, 50 to 59, and >60 years, suggesting that mortality is in the order of 40% in younger patients as well. In the EBMT study of older patients with SAA, a strong center effect was also identified,9 with 5-year survival of 65% in experienced centers, compared with 48% in less experienced centers. For these reasons, alternative-donor transplantations in patients age >40 years should be planned only after a course of ATG has failed, and they are probably best in experienced centers.
UD transplants over the age of 40: significant survival advantage for younger patients (40-49) compared with older patients (EBMT data from Ref 9 ).
UD transplants over the age of 40: significant survival advantage for younger patients (40-49) compared with older patients (EBMT data from Ref 9 ).
Conclusions
In conclusion, significant progress has been made with UD transplantations in SAA, such that survival is now almost comparable to that with sibling grafts.24 For selected pediatric patients, upfront UD transplantations have been performed and shown results comparable to those with sibling grafts and superior to UD grafts after failed immunosuppression.6 There are important details in conditioning regimens, GVHD prophylaxis, the choice of the stem-cell source, and the prevention of EBV LPDs. In the absence of a well-matched UD, a CB unit with a cell dose > 4 × 107/kg can be used, according to the French and EBMT study. Unmanipulated HAPLO transplantations are increasingly used, both with ATG- and/or posttransplantation CY-based platforms, and may be considered if a suitable UD or CB unit is not available. Even in the last decade, survival beyond the age of 40 years has remained inferior to expectations,9 which calls for first-line ATG in this older age group when an identical sibling is lacking.
Correspondence
Andrea Bacigalupo, Department of Hematology, Universita’ Cattolica del Sacro Cuore, Fondazione Policlinico Universitario Gemelli IRCCS, Largo Agostino Gemelli 1, 00168 Rome, Italy; e-mail: apbacigalupo@yahoo.com.
References
Competing Interests
Conflict-of-interest disclosure: A.B. declares no competing financial interests.
Author notes
Off-label drug use: None disclosed.