Abstract
We are several years into the “postdiscovery” era in acute myeloid leukemia (AML) thanks to extensive work involving the sequencing of genomes and exomes of countless patients, which has led to routine comprehensive targeted sequencing in clinical care. The ability to unlock the molecular underpinnings of each patient’s disease was supposed to usher in a new treatment era in which each patient was assigned, based on her mutational profile, a personalized cocktail of targeted therapies that would snuff the disease into submission with minimal toxicity. Whether we have fully realized the promise of personalized therapy in AML is unclear. Here, I review those new drugs that have been inspired by genomics, discuss others that might be possible and their potential roles, and consider whether the ability to target genomic mutations in a personalized manner constitutes the future of AML therapeutics or is representative of an era that has already passed.
Learning Objectives
Learn about the new drugs that have been inspired by genomics and the clinical impact, with respect to response rates and survival, that they have had on acute myeloid leukemia (AML)
Learn about the advantages and disadvantages of strategies to personalize AML therapy based on genomically targeted therapies
Understand how the field has arrived at a point where genomically targeted therapies are possible, and consider where this field is headed
Introduction
In 10 years, acute myeloid leukemia (AML) has gone from a disease so mysterious that it was largely classified by hematopathologists based on the morphologic appearance of blasts1 to being one of the most well-understood human diseases classified based on relevant biological features2 and brimming with databases of thousands of patient samples profiled in countless ways.3-7 This rapid advance would be akin to introducing the Internet to a Bronze Age village, and just as that plot for a time travel movie would be expected to jumpstart a society, it was widely assumed that these advances in understanding the underpinnings of AML would lead to stunning improvements in clinical outcomes for this moribund disease. Because the most accessible and easily interpretable data involved a determination of the somatic mutations that defined each patients’ disease, it was perhaps inevitable that drug development would revolve around efforts to target these mutations, and this approach has permeated the conventional wisdom for the personalized manner in which we will treat AML. However, roughly 10 years after the first AML genome was fully sequenced, the list of drugs inspired by genomics is small, the pipeline for the development of other such targeted therapies is lean, the best clinical scenarios for use of these drugs is uncertain, and clinical outcomes today are not very different compared with our Bronze Age, when each patient’s disease was a mystery that we could not hope to understand. This review will focus on the genomically focused drugs that we have and when to use them, those that we may reasonably expect to hope for, and how our era distinguished by a sophisticated understanding of each patients’ disease biology may (or may not) contribute to improved outcomes for patients.
New drugs inspired by genomics
There have been clear responses to the genomic era from the drug development field, which are best articulated by attempts to exploit the targets Fms-like tyrosine kinase 3 (FLT3) and isocitrate dehydrogenase (IDH).
FLT3 is one of the most commonly recurrently mutated genes in patients with AML8 ; although not associated with decreased response rates, FLT3 mutations confer a poor prognosis mainly due to the accompanied increased relapse risk and their association with proliferative disease.9 Because FLT3 was identified years ago as a recurrent mutation10 and because therapeutic strategies are possible to target cells with mutant copies of this gene, FLT3 inhibition has been a hopeful strategy for a subset of AML patients.11 When administered with intensive chemotherapy in younger, newly diagnosed FLT3-positive patients fit for induction, the multikinase and FLT3 inhibitor midostaurin results in a survival benefit (a median of 74.7 months compared with 25.6 months for patients who received chemotherapy alone [P = .009]12 ) and is now the standard of care for this population. Intriguingly, this survival benefit remains even when censoring patients who proceeded to allogeneic stem cell transplantation,12 suggesting that the initial treatments used to achieve remissions are relevant and that transplantation is not the great equalizer that it is often believed to be.
Relapsed FLT3-positive AML is a significant clinical challenge; in this setting, the use of more specific FLT3 inhibitors as single agents, such as quizartinib, crenolanib, and gilteritinib, can allow for relatively high response rates between 50% and 66%, but the duration of responses and survival are typically brief, with patients living just 20 to 33 weeks.13-18 If, in the appropriate setting, these brief pauses can be successfully traded in for curative allogeneic stem cell transplantations when it is otherwise impossible to achieve the requisite disease response for this intervention, these responses can represent a very significant benefit. In fact, 19% of relapsed FLT3+ patients who received gilteritinib proceeded to transplant,19 and 2 recent reports of single-agent quizartinib for patients with relapsed FLT3+ AML reported that 23% to 37% of patients successfully bridged to transplant.17,18 One of these studies reported that patients who proceeded to transplant lived longer.18 Therefore, single-agent FLT3-targeted therapies as a means to an end for relapsed patients may have clear value. However, without transplantation as an endgame, it is perhaps only in combination with other agents that inhibiting FLT3 in the relapsed setting will allow for more clinically meaningful responses.
Compared with FLT3, a late and promiscuous event, mutations in IDH1 and IDH2, which occur in approximately 20% of AML patients, are earlier and more stable events.5,20 Both IDH isoforms are thought to contribute to leukemogenesis through the overproduction of an oncometabolite, 2-hydroxyglutarate (2HG).21 Enasidenib is a Food and Drug Administration (FDA)–approved therapy for relapsed and refractory IDH2+ AML patients, and it results in a 20% complete remission (CR)/complete remission with incomplete count recovery (CRi) rate, with an additional 20% of patients achieving a clinical response that did not meet criteria for a CR/CRi; the median overall survival (OS) was 9.3 months, and patients who attained CR or a response short of a CR had OS rates of 19.7 and 13.8 months, respectively.22,23 There is some experience using enasidenib as a single agent for previously untreated patients; in a small study of 39 such patients, the overall response rate was 31%. Median OS was 11.3 months for the entire cohort, with the median not reached after 8 months of follow-up for responding patients.24 Other studies involving enasidenib combined with intensive chemotherapy or hypomethylating agents in newly diagnosed patients have shown early promise and are ongoing.
IDH1 is also a promising target. Ivosidenib targets cells with mutant copies of IDH1 and results in a 30% CR/CRi rate, 40% overall response rate, and 8.8 months of median OS25 ; patients who achieved a CR had a median OS of 19 months. Based on this data, ivosidenib is also newly FDA approved for relapsed and refractory IDH1+ AML patients, and like enasidenib, it is being explored in the upfront treatment setting as a single agent and in combination with other therapies. It should be noted that both enasidenib and ivosidenib were granted full approval based on single-arm, uncontrolled studies; although outcomes seem superior to historical controls, the data to fully prove this in the relapsed/refractory setting do not exist. Furthermore, like FLT3 inhibitors, IDH inhibition may serve as a bridge to a transplantation for appropriate patients; roughly 10% of patients proceeded to transplantation.23,25 Data on posttransplantation outcomes for these patients are not yet available.
Given the fact that a minority of patients respond but that responders have prolonged responses and OS, biomarkers to help predict those most likely to respond to IDH inhibitors would be helpful. 2HG levels are not useful biomarkers, because they decrease equally in responders and nonresponders.23,25 There are some emerging data to suggest that patients with RAS pathway mutations or mutations in genes that control cell signaling may respond less well to IDH inhibition,24 but more work is needed in this area.
IDH inhibitors have a unique mechanism of action in that they promote immature cells to differentiate.26 This can be associated with the clinical adverse event differentiation syndrome, which can have a disparate clinical presentation that may include effusions, fevers, and rash.27 The incidence of this complication is around 10%, and it is managed conservatively with corticosteroids, diuretics, hydroxyurea, and potentially, temporary drug interruptions.23,25,27 Patients who experience this complication, which can occur later after initiation of therapy than is typically seen with acute promyelocytic leukemia, can still derive clinical benefit from treatment, and therefore, it is generally recommended that they continue therapy during or after management of the differentiation syndrome.
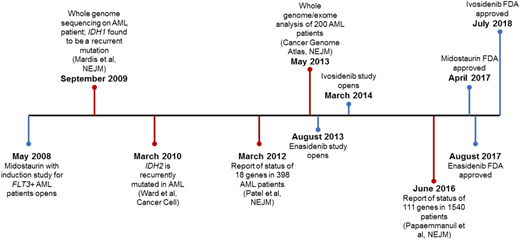
Other IDH1 inhibitors and dual IDH1/2 inhibitors are also in development, and the use of these agents in combination with other novel therapies and in the upfront treatment setting, both alone and in combination with standard backbone therapies, is being explored.28 However, to date, IDH is the only postwhole–genome sequencing era target that has been successfully exploited to result in a new approved therapy for AML (Figure 1). Table 1 has a summary of genomically inspired targeted therapies and their relevant publications.
Timeline of 10 years of genomic profiling in AML patients accompanied by major breakthroughs in genomically inspired targeted therapies. IDH1 and IDH2 are the only genes to have major drug development advancements in the era since the first AML genome was sequenced. Blue lines represent drug development milestones, and red lines represent genomic advances.
Timeline of 10 years of genomic profiling in AML patients accompanied by major breakthroughs in genomically inspired targeted therapies. IDH1 and IDH2 are the only genes to have major drug development advancements in the era since the first AML genome was sequenced. Blue lines represent drug development milestones, and red lines represent genomic advances.
Key findings and publications related to specific genomically defined targeted therapies for AML patients
| Target . | Therapy . | Key finding or NCTN number if trial is in progress . | Reference . |
|---|---|---|---|
| FLT3 | Midostaurin | Midostaurin with induction chemotherapy results in a survival benefit for chemotherapy-eligible patients with an FLT3 mutation compared with patients who only receive chemotherapy | 12 |
| FLT3 | Quizartinib | Quizartinib as a single agent in relapsed and refractory FLT3+ AML results in a 50% remission rate | 17 |
| FLT3 | Gilteritinib | Gilteritinib as a single agent in relapsed and refractory FLT3+ AML results in a 37% remission rate | 19 |
| FLT3 | Crenolanib | Induction chemotherapy with midostaurin vs crenolanib in FLT3+ AML patients; NCT03258931 | N/A |
| IDH2 | Enasidenib | Enasidenib as a single agent in IDH2+ patients results in a 40% overall response rate with a median OS of 9 mo | 23 |
| IDH1 | Ivosidenib | Ivosidenib as a single agent in IDH1+ patients results in a 40% overall response rate with a median OS of 9 mo | 25 |
| KIT | Dasatinib | Chemotherapy with dasatinib for core binding factor AML patients was tolerated and may improve efficacy; a randomized trial is ongoing (NCT02013648) | 34 |
| Splice gene mutations | H3B-8800 | NCT02841540 | N/A |
| Target . | Therapy . | Key finding or NCTN number if trial is in progress . | Reference . |
|---|---|---|---|
| FLT3 | Midostaurin | Midostaurin with induction chemotherapy results in a survival benefit for chemotherapy-eligible patients with an FLT3 mutation compared with patients who only receive chemotherapy | 12 |
| FLT3 | Quizartinib | Quizartinib as a single agent in relapsed and refractory FLT3+ AML results in a 50% remission rate | 17 |
| FLT3 | Gilteritinib | Gilteritinib as a single agent in relapsed and refractory FLT3+ AML results in a 37% remission rate | 19 |
| FLT3 | Crenolanib | Induction chemotherapy with midostaurin vs crenolanib in FLT3+ AML patients; NCT03258931 | N/A |
| IDH2 | Enasidenib | Enasidenib as a single agent in IDH2+ patients results in a 40% overall response rate with a median OS of 9 mo | 23 |
| IDH1 | Ivosidenib | Ivosidenib as a single agent in IDH1+ patients results in a 40% overall response rate with a median OS of 9 mo | 25 |
| KIT | Dasatinib | Chemotherapy with dasatinib for core binding factor AML patients was tolerated and may improve efficacy; a randomized trial is ongoing (NCT02013648) | 34 |
| Splice gene mutations | H3B-8800 | NCT02841540 | N/A |
N/A, not available; NCTN, National Clinical Trials Network.
Old drugs repurposed by genomics
“Targeted” therapies with off-target effects are appealing ways to repurpose drugs for rare diseases. Unlike the specific FLT3 inhibitors discussed above that are engineered to target mutant copies of FLT3, sorafenib and ponatinib have other functions outside of FLT3 inhibition. Clinical data for the use of sorafenib as an FLT3 inhibitor exist,29 with significant experience in combination with azacitidine and a CR/CRi rate of 43% in that setting.30 Limited data for ponatinib as a single agent show a CR/CRi rate of 17% in the relapsed setting.31 The advantage of these agents is their potential for off-label use in patients who may not qualify for a clinical trial.
Dasatinib is FDA approved for chronic myeloid leukemia but has off-target activity against KIT.32 KIT mutations and KIT overexpression occur in AML and are particularly common in patients with core binding factor (CBF) cytogenetic abnormalities33 ; clinical strategies to target KIT with dasatinib have shown some promise,34-37 and there may be a role for dasatinib in this setting either during induction and consolidation or as a postremission maintenance therapy. With the recent approval of gemtuzumab ozogamicin (GO) for CD33+ AML patients and meta-analysis data showing a particularly significant OS benefit for CBF patients,38 use of induction with GO for these patients has become widely adopted. Whether the addition of GO will mitigate the worse prognosis for KIT+ patients and whether patients treated with GO would still benefit from dasatinib are unclear. However, given the lack of experience with combining these agents, it may be best to withhold dasatinib for KIT+ CBF patients treated with GO-based induction and consolidation regimens until data are available describing the toxicity and efficacy of such a regimen.
As more targeted therapies are approved for other non-AML indications, it is possible that we will be able to repurpose more therapies for AML given the extensive knowledge that clinical profiling affords for each patient’s disease.
Potential new drugs inspired by genomics
The literature is replete with hypotheses for targeting genomically defined subsets of AML patients with particular therapies. Nearly all results from clinical trials now include breakdowns of efficacy based on genomic signatures, no matter how small the study. Despite some promising leads, there are few examples as logical and promising as targeting FLT3 and IDH.
It is now well known that a significant subset of AML patients has recurrent mutations affecting genes that encode RNA splicing factors.5 These mutations result in missplicing, which may be therapeutically exploited.39 H3B-8800 is a splicesome inhibitor that may directly target cells with these abnormalities,40 and clinical trials investigating this are ongoing (NCT02841540). Partial tandem duplications involving KMT2A occur in as many as 10% of AML patients, and they are associated with a worse prognosis.41 Targeting these patients via inhibition of DOT1L histone methyltransfase activity with pinometostat has a clear rationale,42 and clinical experiences show some efficacy with this approach; however, relatively few patients have been treated.43,44 Mutations in genes responsible for the cohesion complex, like STAG2, are recurrently mutated and represent a distinct subgroup of AML patients.5 Extrapolating from work done on glioblastoma cells that harbor these mutations, the use of poly ADP ribose polymerase inhibitors may be a potential strategy for targeting cells with mutant copies of these genes.45 Mutations in the RAS pathway are relatively frequent in AML4 ; although RAS is not conventionally thought to be druggable, MEK, in the same pathway, is the target of the drug trametinib. When used as a single agent in patients with AML and myelodysplastic syndrome, only 1 of 30 patients without an RAS mutation responded compared with 10 of 50 (20%) with an RAS mutation.46 Finally, an RNA interference screen to identify genes that would be synthetically lethal with IDH mutations suggested BCL-2 as a candidate gene.47 In the clinic, patients with IDH mutations treated with the BCL-2 inhibitor venetoclax may have responded better to this strategy than patients without IDH mutations, suggesting that this strategy may be prioritized based on genomic information.48-50
When to use them
One common theme with nearly all of the above genomically defined therapies is a relatively short duration of response when response is even achievable. Given that all of these examples are of therapies that target a single gene or pathway, this should not necessarily come as a surprise when dealing with a disease that is driven, on average, by multiple mutations or genetic lesions simultaneously.4 The ability to target one of those mutations, simply because it is druggable, should not lead to the conclusion that a significant impact on the disease will follow. One obvious place to use these therapies would be as an attempt to force a response, although it may be cosmetic, that would allow a patient to proceed to a potentially curative allogeneic stem cell transplantation based on longstanding data showing better outcomes when patients enter a transplant in remission.51 The durability of posttransplantation remissions with this strategy is unclear, but if responses that arise from a single-gene targeting strategy are not very deep, leaving behind measurable residual disease (MRD), one would not expect transplantations that occur after remissions from targeted therapies to have a high rate of success.52 This topic needs to be comprehensively studied, and both the percentage of patients who proceed to a transplantation after receiving salvage therapy with a genomically targeted agent and their post transplantation outcomes must be reported.
There is an important exception to observations that single-target therapeutic strategies do not allow for deep responses. Using a sensitive assay for IDH1 allele frequency, 7 of 34 patients who achieved a CR on the ivosidenib study did achieve clearance of the detectable IDH1 clone; these patients had longer remissions and survival.25 Therefore, the ability to achieve MRD negativity with single gene–targeted therapies may allow for these treatments to be used in the pretransplantation MRD-positive setting as a tool to achieve MRD negativity and hopefully, a better posttransplantation outcome, which is an FDA-approved indication for blinatumomab in acute lymphoblastic leukemia.53 When this is not feasible, superior outcomes with the deeper responses are intriguing, and work should be done to attempt to predict who these patients may be and how to expand the pool of patients able to achieve such responses.
Perhaps genomically defined therapies could be combined with each other, and this may increase their efficacy. Obvious limitations to this strategy include the potential for cumulative toxicity, a limited number of patients whose disease has more than 1 druggable mutation, and of course, the evolutionary nature of the disease, which adapts to its environment and selects clones for expansion that are not targeted.54
Introducing targeted therapies into the treatment paradigm earlier is one way to potentially render them more potent along with combining them with conventional chemotherapy. It is this scenario that resulted in the survival benefit for midostaurin,12 and the framework to rapidly test other therapies in this way is being piloted with the Beat AML Master trial.55
Conclusions
After the first AML genome was sequenced,6 it was declared that the era of discovery in AML was over.56 Because FLT3 was a well-known target long before whole-genome/exome sequencing, this means that, in the years since the end of discovery, we have found a total of 2 gene mutations (IDH1 and IDH2) that seem to be druggable and whose targeting may provide clinical benefit (Figure 1). Active research into this area is ongoing, but surely, this reality falls short of the expectations that many once had for the era of genomically driven personalized therapies in AML. It is instructive to consider the reasons for this and how improvements may be made.
Clearly, management of expectations is necessary with strategies that target single-gene mutations or pathways,57 and targeting IDH provides an excellent case study. It is an early and stable mutation with a unique biomarker (2HG); enasidenib and ivosidenib are highly specific, orally bioavailable, and well-tolerated targeted therapies against IDH2 and IDH1, respectively. However, in this best of circumstances, only a minority of patients respond to this treatment in the relapsed setting.23,25
Genomic characterization of AML was a huge leap forward, and the high-level sequencing that is performed now as standard of care in newly diagnosed patients provides an incredibly sophisticated understanding of each individual’s disease. It may have seemed logical that genomically defined therapies would, therefore, revolutionize the treatment of AML. However, it is clear that these treatments are not a panacea; they have a role, but currently, that role is limited. The proper setting and/or combinations to fully realize the potential of genomically derived targeted therapies have not yet been determined.
Correspondence
Daniel A. Pollyea, Division of Hematology, University of Colorado School of Medicine, 1665 Aurora Court, Aurora CO 80045; e-mail: daniel.pollyea@ucdenver.edu.
References
Competing Interests
Conflict-of-interest disclosure: D.A.P. is on an advisory committee for Celgene, Pfizer, Argenx, Agios, Abbvie, and Celyad and has received research funding from Agios, Pfizer, and Abbvie.
Author notes
Off-label drug use: This chapter discusses off-label drug use of quizartinib, crenolanib, gilteritinib, enasidenib, ivosidenib, sorafenib, ponatanib, and dasatinib.