Abstract
Recent genomic discoveries have improved our understanding of many hematologic diseases and led to novel therapeutic options for many patients. The rapid decrease in the cost of genomic testing has enabled widespread use of clinical genomic testing. However, these advances are accompanied by concomitant challenging ethical concerns. In pediatrics, issues of informed consent for genomic testing, assent, and permission vary significantly by patient age and comprehension. Broader testing strategies, such as whole-exome or whole-genome sequencing, are more likely to yield incidental findings unrelated to the reason for the initial test, and plans to deal with these results when they occur are increasingly important. The lines of clinical care and research are becoming more blurry in the era of precision medicine in which approaches to individual genetic mutations (as opposed to disease phenotypes) occur with increased frequency. Finally, because justice is a fundamental ethical consideration, access to genomic testing and a rigorous approach to utility are critical to individual patients and the field of hematology. In this review, we use 3 cases of genomic testing in pediatric hematology to illustrate core ethical concerns and explore potential solutions.
Learning Objectives
Appreciate that considerations in pediatric cancer genomics include, but are not limited, to issues of informed consent, assent, and permission; incidental findings; ambiguity between research and clinical care; and utility and justice
Learn that approaches to informed consent, assent, and permission should generally be predicated on patient age and comprehension
Understand that developing a plan to deal with incidental findings in the clinical and research setting is crucial to the consent process and for sound ethical practice in the genomic era
Introduction
Genomic science is transforming the practice of benign and malignant hematology at a rapid pace. However, clinicians must carefully consider a set of core ethical concerns to successfully implement these advances.1 In the pediatric setting, many of these ethical concerns are amplified. In this review, we present 3 cases and then discuss 4 sets of ethical issues that demonstrate important themes to consider: (1) issues of informed consent, assent, and permission, (2) incidental findings, (3) research vs clinical care, and (4) utility and access to care.
Cases
Case 1
Jennifer Williams is a previously healthy 5-year-old female who was recently found to have pancytopenia with an absolute neutrophil count of 200/µL. She was referred to a hematologist who performed a bone marrow biopsy that demonstrated hypocellular marrow (5% to 10%), panhypoplasia, and absence of dysplasia. Her hematologist ordered a bone marrow failure gene panel, and testing showed no causative mutations for bone marrow failure. However, results of the gene panel did reveal a heterozygous pathologic mutation in FANCD1 (BRCA2). Jennifer was given a diagnosis of idiopathic aplastic anemia and went on to receive immune-suppressive therapy with equine anti-thymocyte globulin and cyclosporine, with resolution of pancytopenia. Prior to sending the test, Jennifer’s hematologist discussed the gene panel with her parents but did not discuss plans for disclosure of incidental findings or additional implications of detected findings, unrelated to the specific diagnostic question.
Case 2
Michael Smith is a 15-year-old male who underwent a matched unrelated donor hematopoietic cell transplant for hypodiploid acute lymphoblastic leukemia (ALL) 3 years ago. Michael has a 12-year-old brother named Brian who was not a match. Michael’s family has recently moved across the country, and Michael established care with a new pediatric hematologist. Michael has always been known as a free-spirited child. Michael’s new physician recommends targeted testing for germline TP53 mutation given the association between this mutation and hypodiploid ALL.2,3 Michael’s physician explains that TP53 mutations cause the cancer predisposition condition, Li-Fraumeni syndrome (LFS), and are associated with high rates of second malignancies.4 If the testing were to be positive, the hematologist will recommend a surveillance program to provide early detection and possible intervention (eg, excision of melanoma in situ found on dermatologic examination). Both of Michael’s parents agree to targeted TP53 testing; however, Michael refuses to provide assent. He understands that, if he has LFS, a surveillance program would be beneficial but states that he wants to get on with his life and does not want to know if he is likely to have another cancer. Michael states that knowing the results would be detrimental for his plans for the future, causing him to fixate on things that are not under his control. Both of Michael’s parents and the physician believe that genetic testing and a malignancy-screening program (if a TP53 mutation is identified) are in Michael’s best interest. Michael is not legally empowered to make his own decisions. Should he be tested against his wishes, persuaded to accede to parental and physician recommendations, or should testing be deferred because he does not assent?
Case 3
Sarah Davis is a 25-year-old female with a history of alopecia and insulin-dependent diabetes mellitus. She had idiopathic thrombocytopenic purpura as a young child that remitted after a short course of oral corticosteroids. She now is referred to a hematologist with a recurrence of idiopathic thrombocytopenic purpura, as well as autoimmune hemolytic anemia (Evans syndrome). Sarah’s hematologist attempted to treat her using a combination of steroids and immunoglobulin, but her blood counts did not significantly improve. Other immunosuppressive options are discussed, but Sarah is not interested because of their side-effect profiles. Her hematologist notes that multiple genetic mutations have recently been described in association with autoimmune disease, including cytopenias, and these mutations may be amenable to targeted therapy.5 The hematologist recommends genetic testing using an autoimmunity panel of 80 genes, to which Sarah agrees. Results demonstrate a STAT3 gain-of-function mutation. Based on a single case report in the literature, Sarah’s hematologist recommends “off-label” use of the anti–IL-6 receptor monoclonal antibody tocilizumab.6 Sarah has a favorable response; given the paucity of data on this approach in the literature, the hematologist asks her permission to submit a case report for publication.
Sarah happily provides verbal consent, hoping that the publication may help others. Before agreeing to send the case report out for peer review, the journal editors ask Sarah’s hematologist if he had Institutional Review Board (IRB) approval for the use of the medication in this “experiment” and request that he include a statement to that effect in the manuscript. Is IRB approval really needed for this case report, and what changes to this case might make approval strictly necessary?
Good ethics start with good scientific facts
The major advances in genomic science in the early 21st century include somatic and germline discoveries. Germline mutations are more ethically fraught than somatic mutations because they carry implications for family members of the index case, whereas somatic mutations generally do not. Additionally, in pediatric hematology, the patient’s age, by definition, implies that germline variants will be relatively more common than in adult hematology, which are more likely to involve somatic lesions.
Multiple mutations, including biallelic mutations in BRCA2 (FANCD1) are known to cause the bone marrow failure syndrome Fanconi anemia.7 In contrast, a single pathogenic mutation, BRCA2, will lead to hereditary breast and ovarian cancer (HBOC) syndrome.8 In HBOC, affected females have a 50% to 80% lifetime risk for breast cancer and a 30% to 50% lifetime risk for ovarian cancer. Additionally, those with BRCA2 mutation have a 20-fold increased risk for prostate cancer and a 10-fold increased risk for pancreatic cancer.8 Accordingly, not only would an affected female (eg, Jennifer Williams in case 1) be at an extremely high risk for breast and ovarian cancer in adulthood, the parent who is the carrier of the gene also has a significantly increased risk for malignancy.
Several germline mutations associated with pediatric ALL have been newly discovered. For example, variants in ETV6 have been associated with familial clusters of ALL and have been identified in a minority of pediatric ALL patients.9 Alterations in IKZF1, which encodes the lymphoid transcription factor IKAROS, are a hallmark of Philadelphia-positive ALL and Philadelphia-like ALL.10,11 More recently, germline variants in IKZF1 have been observed in familial ALL and a small proportion of sporadic ALL and are associated with drug resistance.12 The increasing identification of affected individuals and families with leukemia-predisposing conditions has led the American Association of Cancer Research to develop consensus recommendation on disease surveillance.13 In contrast to many germline variants that are only associated with an increased risk for a single malignancy, LFS is associated with a high risk for a multitude of neoplasms. An important scientific fact related to case 2 is that early malignancy detection using screening guidelines has been demonstrated to be associated with improved survival.14
Evans syndrome has historically been treated with immunomodulatory agents, such as cyclosporine or mycophenolate mofetil, when refractory to steroids and/or intravenous immunoglobulin. In case 3, the hematologist offers genetic testing, followed by targeted therapy using tocilizumab. More generally accepted agents include cyclosporine and mycophenolate mofetil, each of which has been used for many more years than tocilizumab, although neither has been evaluated in a randomized controlled trial for Evans syndrome.15 The risk of developing malignancy or serious infection from the use of tocilizumab is less well defined than in older immunosuppressive agents, and the long-term effects are not well known.16
Ethical issues
Informed consent, assent, and permission
Informed consent is a foundation of the ethical practice of medicine and a cornerstone of efforts to protect human subjects from research risk. Despite the firmly established role of informed consent in practice and research, many questions persist regarding its application in the context of pediatrics,17 and many of these issues are amplified in the setting of genetic testing.18 For pediatric ethics, informed consent is more properly understood as a combination of informed parental permission and (when appropriate) the assent of the child.17 The age of each patient in our case examples (5, 15, and 25 years) corresponds with the need for a nuanced approach that is distinctive to pediatric ethics and decision making. For the 5-year-old in case 1, parental permission is the relevant construct, because patients this young do not have decision-making capacity. By contrast, most 15-year-old patients (as in case 2) are able to participate in decision making and do so when clinicians and/or investigators solicit their assent. Finally, the 25-year-old patient in case 3 is capable of independently autonomous informed consent.
What are the specific implications of this for our cases? In case 1, an incidental finding is discovered, and the hematologist is unsure how to proceed with disclosing the results. Given the patient’s young age when the test was ordered, assent from the patient was not appropriate. However, because the implications of the findings are not likely to affect the patient until adulthood, should the physician and family wait until the patient is 18 years of age to determine whether she wants to know the results?19 Case 3 is clearer because the patient is over 18 years of age, and her opinion regarding the testing is determinative. Case 2 is likely the most complex and illustrates conflicting attitudes toward cancer surveillance in a known cancer-predisposition syndrome.20 In case 2 the patient is not of legal age, but his opinion should certainly be taken into consideration.
Germline mutations in cancer-predisposing genes have recently been identified in 8.5% of children and adolescents with cancer.21 Most cases of childhood leukemia occur in the absence of known germline mutations; however, some mutations (such as TP53 and hypodiploid ALL as discussed in case 2) significantly increase the risk of developing hematopoietic malignancies in childhood.22 LFS is an autosomal-dominant cancer-predisposition syndrome caused by a mutation in the tumor suppressor gene TP53, and ethical challenges associated with this mutation were noted almost 25 years ago.23 Approximately 50% of patients will develop a malignancy in the first 3 decades of life, and ∼50% of patients surviving a first cancer will develop another cancer at a median of 10 years.24 Patients with LFS in formal screening programs noted that benefits include early detection, peace of mind, knowledge providing power, and screening making LFS seem more livable, whereas perceived drawbacks included logistical issues, challenges navigating the system, screening being draining, and significant negative emotions, including anxiety, fear, and skepticism.25 When parents are offered TP53 testing for children, most families decide in favor of testing.26 However, a minority of families decline testing, often citing psychosocial risks and privacy/discrimination/insurance issues.
Siblings of childhood cancer survivors have higher rates of malignancy than the general population.27 For example in case 2, a diagnosis of TP53 mutation in the patient suggests a 50% likelihood of the same mutation in a sibling or parent. Michael’s refusal to be tested has implications for his younger brother Brian, but it is not clear whether this information should be used to attempt to persuade Michael to be tested. Another possibility would be to respect Michael’s decision and test both parents. If both parents were negative, this would suggest a de novo mutation in Michael or the possibility of nonpaternity. This latter possibility raises a well-known ethical dilemma associated with genetic testing, which is beyond the scope of discussion of this article.28 Assuming that both parents tested negative and Michael has a true de novo mutation, there would be no indication to test Brian. By contrast, if parental testing were to show 1 of Michael’s parents to be positive for a TP53 mutation, there would be strong ethical and clinical justification for soliciting assent from Brian to have testing for TP53.
In case 2, Michael makes a reasonable argument that knowing his TP53 status could adversely affect his well-being, and other families have chosen this approach. Many survivors of pediatric malignancy suffer from anxiety and posttraumatic stress,29 and further testing might lead to or worsen these issues for Michael. If Michael was instead 18 years old, his parents’ opinion should not be considered by the new physician, and Michael’s beliefs about the importance of various aspects of his health would be determinative. (If Michael was 18 years old, his parents’ opinions might reasonably still influence Michael’s decision, but they should not influence the physician.) From an ethical standpoint, Michael likely has the capacity to make his own decision at age 15 years. In a pilot study, the feasibility of a tool to assess children’s competence to consent for genetic testing was investigated.30 With 90% specificity, children older than 11.8 years were judged competent. A similar situation was recently described by Bester et al; a parent with LFS asked that her child be tested for TP53 mutation without her knowledge and the results only be reported if they demonstrated LFS.31 When physicians encounter decisional discord between parents and older children, they may use 1 of 3 approaches: the advocative, deferential, or arbitrative model.32 However, clinicians should remember that to test against the wishes of the patient will, in a sense, foreclose the patient’s “right to an open future.”33
Incidental findings
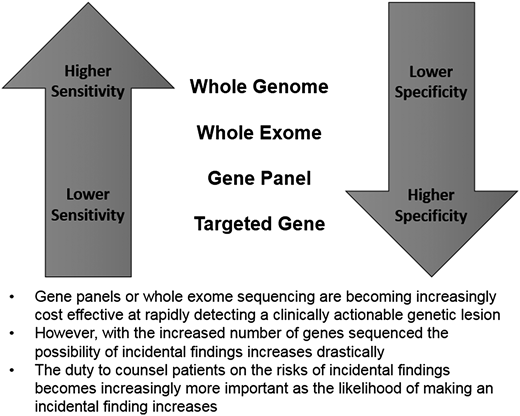
As the cost of gene sequencing becomes less expensive, the use of gene panels and whole-exome sequencing has dramatically increased. Although targeted testing of a specific gene is often the most appropriate test, in the setting of nonspecific unexplained symptoms (as in case 3) a broader approach is often warranted. Targeted gene testing offers the least sensitive, but most specific, results when a mutation is identified, whereas findings on whole-exome sequencing are much more likely to yield false-positive findings (Figure 1). Algorithms have been developed to help physicians determine the appropriate indications for single-gene, gene panel, and exome/genome sequencing and may be helpful in some situations.34 In general, the consensus recommendation is to test in the narrowest way feasible to answer the clinical question at hand.
In 2013, the American College of Medical Genetics and Genomics issued recommendations for reporting incidental findings from clinical whole-genome sequencing and whole-exome sequencing.35 The recommendations call for evaluating a specific set of 57 genes associated with malignancy (eg, BRCA1, TP53, MEN1), cardiovascular disease, and malignant hyperthermia, as part of all whole-genome sequencing/whole-exome sequencing (even when the clinical question is not relevant to these genes) and reporting all probable or known pathogenic variants, irrespective of patient age.35,36 Although many of these genes are actionable, these recommendations have been met with significant controversy because they are often unrelated to the reason for clinical testing and do not allow patients to decline this specific testing.36,37 Further, many of the conditions are adult onset in nature, and testing for them in children does not afford the patient an opportunity to make a choice about a finding that may not affect them until after they reach the age of majority. Potential proposed solutions to this include allowing patients and families to opt out or contacting pediatric patients at age 18 years to determine whether they would like to know the results of incidental findings.
In the broadest sense, case 1 can be viewed as a problem with adequate informed consent. Informed consent should include a review and discussion about results of testing, intended and unintended (ie, incidental findings), and agreement should be reached on whether and how results will be disclosed, including implications of the results of the specific test (in this case HBOC syndrome), psychosocial and privacy concerns, the implications of the results for other relatives (in this case the parents and possibly other children or even second-degree relatives), and the option of deferring or declining testing.38,39
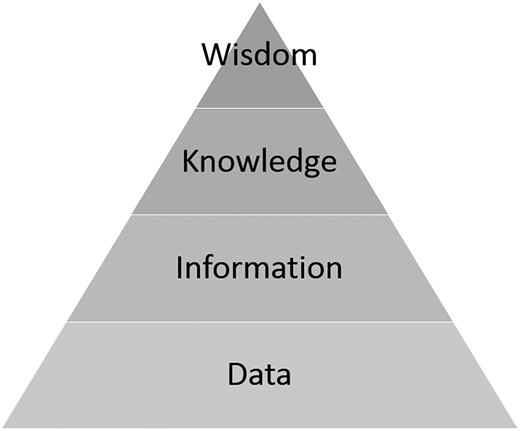
In case 3, an autoimmune gene panel was sent, resulting in the finding of a STAT3 gain of function. Alternatively, the panel could have reported an incidental finding of STAT3 loss of function, which is associated with large granular lymphocytic leukemia, myelodysplastic syndrome, and aplastic anemia.40 Although these findings may be important clinical knowledge for Sarah Davis, perhaps resulting in a screening plan with her hematologist, she may not want to know these results. Likewise, the autoimmune panel may have included testing for XIAP, a gene known to cause X-linked lymphoproliferative disease, and demonstrated that Sarah was a carrier for this disease with a 50% likelihood of passing it on to future male offspring. Sarah’s opinion regarding this, and perhaps implications for disclosure to a future partner, would add to the ethical complexity. With the increased use of these panels, clinicians may not even contemplate all genes being tested and potential incidental findings. Oftentimes data may not be actionable, or the field, as a whole, may not possess enough knowledge of the condition to provide a clear recommendation (Figure 2). Future research must focus on optimal ways to convey the range of possibilities of incidental findings and help patients navigate their preferences for disclosure. Pretest consultation with a genetic counselor is extremely desirable in helping patients and doctors grapple with these challenges.
Wisdom, Knowledge, Information, Data pyramid in clinical cancer genomics. In the era of big data, genomic sequencing, and precision medicine, ethically sensitive physicians must attend to the differences between data and information, moving up the ladder to knowledge. Seasoned physicians having spent decades in practice may still strive for wisdom, but we would assert that knowledge will suffice. Data are often accompanied by background noise, and the transition to information may mark an actionable point. For other patients, however, the bar may be higher, and they would take action only at the point of knowledge. Physicians in the 21st century must be able to distinguish among these categories, apply them in the clinical setting, and develop communication skills that will allow them to share the uncertainty.
Wisdom, Knowledge, Information, Data pyramid in clinical cancer genomics. In the era of big data, genomic sequencing, and precision medicine, ethically sensitive physicians must attend to the differences between data and information, moving up the ladder to knowledge. Seasoned physicians having spent decades in practice may still strive for wisdom, but we would assert that knowledge will suffice. Data are often accompanied by background noise, and the transition to information may mark an actionable point. For other patients, however, the bar may be higher, and they would take action only at the point of knowledge. Physicians in the 21st century must be able to distinguish among these categories, apply them in the clinical setting, and develop communication skills that will allow them to share the uncertainty.
Incidental findings of genomic testing in the research setting is somewhat different from the clinical setting because of the protocol-driven nature of research and oversight by the IRB. This regulatory difference will often provide a “road map” for informed consent and disclosure of incidental findings that is more likely to be absent in the clinical context. On occasion, the very nature of research, with a goal of producing generalizable knowledge, may justify the collection of information of indeterminate meaning without an obligation to disclose results to research participants. Researchers are obliged to address the possibility of discovering incidental findings in their protocol and communications with the IRB, as well as in their consent forms and communications with research participants.41 Pathways should be established for handling incidental findings. Results may be categorized into those that must be disclosed to research participants, those that may be disclosed, and those that should not be disclosed.41 This categorization aligns with the concepts of actions that are ethically obligatory, ethically permissible, and ethically impermissible.
Research vs clinical care
Case 3 demonstrates the successful off-label use of a US Food and Drug Administration– approved drug to treat a condition diagnosed using a gene panel test. The physician subsequently submitted a case report on this experience with the patient’s consent, which is an ethically permissible and common practice; IRB approval is generally not required in these situations. However, one could imagine several modifications to the scenario that would constitute an ethically ambiguous area between clinical care and research. For example, if the patient’s gene sequencing occurred in a non–Clinical Laboratory Improvement Amendments–certified laboratory as part of a research study in which the physician was the principal investigator, some may construe this as ethically concerning, whereas others may see it as cutting edge research results that benefit the patient. Alternatively, if the drug being considered had not been used previously in a similar situation and the treatment rationale was based on a hypothesis derived from basic science research in the physician’s laboratory, it may have been unnecessarily risky to give the agent. What if, in case 3, the hematologist empirically performed gene panel testing on all Evans syndrome patients (with appropriate consent for clinical testing) but then planned to retrospectively publish a case series of results? One important question raised in these situations is economic: who is responsible for the costs of genomic testing or downstream medical care? Clearly, the traditional bifurcation of research vs clinical care has become more blurry in the setting of translational genomic research.42 Each of these variations on case 3 may be scientifically reasonable, but that does not make them inherently justified from an ethical perspective. As we move more toward precision medicine approaches in the practice of hematology, the ethical framework for delineating research from “pure” clinical care will need to adapt.
Clinicians and scientists must also be vigilant in their awareness of any upcoming regulatory changes. For example, changes to the US Federal Common Rule will establish new categories of exempt or excluded research based on subject risk, and they allow investigators to obtain broad consent for biospecimens use for future undefined studies.43 Regardless of specific regulation, continued good faith efforts to disclose risk, benefits, and alternatives during the informed consent process and in the informed consent document are critical.
Utility and access to testing
Justice is a fundamental ethical consideration and lens through which to view conundrums in genomics and hematology. Health care disparities exist, and access to genomic testing is no exception. Data published in 2009 demonstrated significant differences by racial and ethnic groups with regard to concerns about the possible misuse of genomic testing.44 Groups differed by types of health insurance coverage and level of mistrust in physicians and the medical system.44 A more recent study of focus group participants demonstrated a decreased willingness to participate in whole-exome studies and receive results of sequencing analysis among African Americans.45 Racial and ethnic differences may play a role in the heterogeneity of cancer biology.46,47 Differences in research participation may lead to future disparities in our fundamental understanding of malignancy and our ability to offer precision treatment approaches. For example, as part of the National Institutes of Health–sponsored Cancer Genome Atlas project, white patients were overrepresented, and Asian and Hispanic patients were underrepresented, compared with the entire United States population.47 Due to decreased research participation with less background data, individuals from minority groups are at a higher risk for false-positive and false-negative genomic testing results.1 Increasing the availability of genomic tests to historically underserved populations is critical to the amelioration of health disparities and ensuring distributive justice.
In addition to racial and ethnic disparities, geographic variation may lead to different uptake of genomic testing. For example, genomic counseling services may be more available in urban medical centers than in the rural setting. Genomic testing without genetic counseling is associated with a lack of informed decision making, misinterpretation of results and inappropriate clinical management, potential breaches of ethical standards, and adverse psychosocial outcomes.39 However, consultation with a genetic counselor is not strictly required prior to testing, and the American Society of Clinical Oncology opposes any activities or requirements limiting the ordering of genomic testing when appropriate by current guidelines.48 Those who receive care in academic medical centers may also have increased access to genomic testing, although data on this are lacking. Physicians at a National Cancer Institute–Designated Cancer Center vary considerably in how they incorporated somatic genomic tests into practice.49 Patients in specific age groups or geographic locations may be more likely to be referred to an academic medical center when diagnosed with a malignancy,50 and this could conceivably affect utilization of genomic tests. As genomic testing is used more frequently and clinicians become more comfortable interpreting results, it is hoped that disparities based on geographic location and socio-economic factors will dissipate.
Conclusions
As genomic testing becomes more cost effective and readily available, it will continue to be incorporated into clinical and research practice. The hematologist must be thoughtful about the core ethical tenets of informed consent, parental permission and assent, approaches to unintended consequences (incidental findings), the blurring boundaries between research and clinical care, and advocating for equitable access to testing. In general, involvement of genetic counselors, when available, is preferred. At a time when much more data and information than knowledge or wisdom are available (Figure 2), testing as narrowly as clinically feasible to prevent incidental findings and having clear plans in a clinical and research setting on how different categories of incidental findings will be disclosed (or not disclosed) is crucial. Finally, if testing is part of research, clear guidance to patients should be given on the risks and benefits of testing and what is the standard of care vs research. Further, care should be taken to clearly explain a potential link between diagnostic information and potential downstream therapeutic implications (or present lack thereof).
Correspondence
Eric Kodish, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: kodishe@ccf.org.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: Use of tocilizumab, mycophenolate mofetil, and cyclosporine for treatment of Evans syndrome is considered off-label use.