Abstract
The US Food and Drug Administration approval of brentuximab vedotin (BV) in 2011 marked an important milestone in the management of classical Hodgkin lymphoma (HL). Although initially approved for use in the relapsed or refractory setting, its high efficacy and favorable toxicity profile led to numerous studies evaluating BV in the front-line, second-line, and posttransplant settings. BV is now approved for use (in combination with chemotherapy) as frontline treatment of advanced-stage patients and as maintenance therapy following autologous stem cell transplant. Additional studies demonstrate its promise as second-line therapy and for elderly patients, as well. Although studies have demonstrated its promise in multiple settings, the ideal timing for use of BV is evolving. Studies evaluating individualized treatment strategies will ultimately define the optimal place for BV in HL treatment.
Learning Objectives
Understand the data supporting the use of brentuximab vedotin in the front-line, second-line, and posttransplant setting for Hodgkin lymphoma
Appreciate the optimal timing for use of brentuximab vedotin in Hodgkin lymphoma treatment based upon currently available data
Introduction
The US Food and Drug Administration FDA (FDA) approval of brentuximab vedotin (BV) in August 2011 was an important advance in the treatment of classical Hodgkin lymphoma (HL). The pivotal BV study in relapsed and refractory (rel/ref) HL demonstrated that it was the most active single agent after failure of autologous stem cell transplant (ASCT); accordingly, there has been an explosion of studies evaluating BV in earlier settings and in combination with other agents.1 Currently, the ideal place for BV in the treatment of HL is evolving. Studies support the use of BV as part of front-line treatment, second-line treatment, post-ASCT maintenance, and post-ASCT failure; however, the optimal timing for BV over the course of HL treatment depends upon patients’ disease characteristics and should be individualized. Discussed here are strategies for incorporating BV into the treatment of HL.
BV
CD30 expression in Hodgkin Reed-Sternberg cells is nearly universal and, thus, has been recognized as a logical target in classical HL. Interestingly, early studies evaluating anti-CD30 monoclonal antibodies failed to demonstrate efficacy in HL2-5 ; however, BV, an anti-CD30 antibody–drug conjugate, established the value of CD30-targeted therapy in HL. BV is conjugated to monomethyl auristatin E, a microtubule-disrupting agent; when BV binds to CD30-expressing cells, it is internalized, leading to release of monomethyl auristatin E and apoptotic cell death. In addition to its mechanism as targeted chemotherapy, recent preclinical studies indicate that BV induces immunogenic cell death.6
The pivotal study that led to initial FDA approval of BV enrolled 102 patients with rel/ref HL following failure of ASCT.1 Patients were treated with BV, 1.8 mg/kg IV every 3 weeks for up to 16 doses. The overall response rate (ORR) was 75%, and the complete response (CR) rate was 34%. Five-year follow-up of this study demonstrated durable benefit for select patients. In particular, 52% of patients who achieved CR to BV were progression free at 5 years. In addition, 9% of patients treated in the study remain in remission following BV, despite never receiving additional therapy, indicating that a small subset of patients with rel/ref HL treated with single-agent BV are likely cured.7 This study established BV as the most effective single agent for rel/ref HL and led to multiple studies aimed to define its role in management of HL.
Although BV is typically well tolerated, its most common side effect is peripheral neuropathy, which occurred in 55% of patients enrolled in the pivotal study.1 Most cases were grade 1 or 2, and only 9% experienced grade 3 or greater. At 5-year follow-up, 15 (14%) patients had ongoing neuropathy, including 11 (10%) with grade 1 and 4 (4%) with grade 2 (limiting instrumental activities of daily living).7 Additional rare, but serious, adverse events reported with BV include pancreatitis and progressive multifocal leukoencephalopathy.8,9
BV in the front-line setting for HL
Studies have evaluated BV in the front-line setting for early-stage disease, advanced-stage disease, as well as for patients older than 60 years of age.
Patients aged 60 years and older
Compared with younger patients, patients older than 60 years of age with HL have significantly inferior outcomes. Older HL patients are not only more likely to have risk factors associated with poor prognosis, such as B symptoms, mixed cellularity type, or poor performance status, a major reason for their poor outcomes is reduced tolerability of treatment.10,11 In an effort to improve tolerability and efficacy of therapy, BV has been evaluated in the front-line setting for older patients as monotherapy, combined with chemotherapy, and given sequentially with chemotherapy. In a phase 2 study, BV was evaluated as a single agent in previously untreated patients aged ≥60 years who were determined to be ineligible for conventional front-line chemotherapy.12 Among 27 patients treated in this study, the median age was 78 years, and 63% had advanced-stage disease. The ORR was 92%, with 73% of patients achieving CR. Unfortunately, responses were not durable, because the median progression-free survival (PFS) was only 10.5 months. As expected with BV, peripheral neuropathy was the most commonly observed adverse event; however, the frequency was higher than typically observed in the younger population. Overall, 78% experienced peripheral sensory neuropathy, and these were grade-3 events in 26%. We learned from this study that, although the response rate to BV is very high, most patients ultimately develop disease progression. In addition, despite its favorable toxicity profile, older patients need to be observed closely for adverse events, such as neuropathy, which occurs more commonly and with greater severity in this patient population.
BV was subsequently evaluated in combination with bendamustine, as well as dacarbazine.13 The BV plus bendamustine cohort aimed to enroll 30 patients; however, it closed early after only 20 patients because of a significant rate (65%) of serious adverse events, including 2 deaths (1 disease related, 1 cause unknown). BV plus dacarbazine was better tolerated; ORR and CR rate were 100% and 62%, respectively, for the 22 patients enrolled. Responses were more durable than observed with BV alone, because the median PFS was 17.9 months; therefore, BV plus dacarbazine may be a reasonable option for frail patients who are not candidates for standard-combination chemotherapy.
Perhaps the most compelling results for patients aged 60 years and older were observed in a multicenter phase 2 study evaluating BV sequentially with adriamycin, vinblastine, dacarbazine (AVD).14 In this study, patients with stage IIB, III, or IV disease were treated with 2 cycles of BV followed by 6 cycles of AVD and then another 4 cycles of BV. Among 48 patients enrolled, 42 completed 6 cycles of AVD; among these patients, ORR and CR rate were 95% and 90%, respectively. By intent-to-treat, the 2-year PFS and overall survival were 84% and 93%. The rate of grade 3 or 4 peripheral neuropathy was only 4%, whereas grade 2 peripheral neuropathy occurred in 33% of patients. These results are among the most favorable ever reported in this patient population; although it is a prolonged treatment (10 months), it represents a promising option for patients aged 60 years and older.
Early-stage disease
Studies evaluating BV for early-stage HL have generally aimed to reduce or eliminate the need for radiotherapy (RT). In a pilot study led by Kumar and colleagues, patients with unfavorable early-stage disease received BV plus AVD for 4 cycles, followed by 30 Gy involved site radiation therapy.15 Among 30 patients enrolled, 77% had disease bulk by Memorial Sloan Kettering Cancer Center criteria (>7 cm)16 ; CR rates after 2 and 4 cycles of BV + AVD (BV-AVD) were 90% and 93%, and 1-year PFS was 93%. Given the promising results in this cohort, a subsequent cohort received reduced-dose involved site radiation therapy (20 Gy).17 A total of 29 patients was enrolled in this cohort, including 69% with disease bulk (>7 cm); similarly favorable results were seen, with 93% achieving CR after 4 cycles of BV-AVD and 1-year PFS of 93%. Additional cohorts are evaluating reduced-field radiation (termed “consolidation volume radiation therapy”) as well as complete elimination of RT for patients who achieve CR. Similar studies for nonbulky early-stage disease have evaluated replacement of RT consolidation with BV or simply using BV-AVD for 4 to 6 cycles and eliminating RT for patients who achieve CR.18,19 All of these studies show promising results; however, the optimal treatment of early-stage disease will ultimately need to be determined in a randomized study.
Advanced-stage disease
The safety of BV combined with adriamycin, bleomycin, vinblastine, dacarbazine (ABVD) or AVD was originally assessed in a phase 1 study.20 Initially, 22 patients received BV plus ABVD; although the CR rate was high (96%), there was an unacceptable rate of pulmonary toxicity (44%) with this combination. The next cohort of patients was treated with BV plus AVD (no bleomycin); among 25 patients enrolled, the CR was again high (96%), and no cases of pulmonary toxicity were observed. Furthermore, the combination of BV plus AVD was determined to be safe and associated with primarily grade 1 or 2 adverse events; therefore, the BV-AVD regimen was chosen to be further assessed in the ECHELON-1 study. ECHELON-1 was the first randomized study to compare a BV-based regimen (BV-AVD) with a standard HL regimen (ABVD).21 The primary end point for the study was 2-year modified PFS, defined as disease progression, death, or modified progression (ie, incomplete response after completion of frontline therapy, followed by subsequent anticancer therapy). For the purpose of this study, an incomplete response was defined as a Deauville score ≥ 3. The study enrolled 1334 patients with stage III or IV disease and demonstrated a statistically significant improvement in the primary end point. The results led to FDA approval of BV combined with chemotherapy for front-line treatment of advanced-stage HL; however, this study does not necessarily support widespread use of BV-AVD, because the observed improvement in modified PFS was small (82.1% vs 77.2%), and, compared with ABVD, BV-AVD is associated with increased toxicity and cost. The particular toxicities observed with BV-AVD included higher rates of neuropathy (all grades, 67% vs 13%; grade 2, 20% vs 9%; ≥grade 3, 11% vs 2%), as well as higher rates of febrile neutropenia necessitating the use of growth factor. However, once the study began mandating the regular use of growth factor support with BV-AVD, the rate of neutropenic fever decreased from 21% to 11% (in comparison, the rate with ABVD was 7%-8%). In addition, although ABVD is known to have only limited impact on fertility, the impact of BV on fertility is not known. As expected, the most notable toxicity in the ABVD arm was pulmonary toxicity, which occurred in 7% (3% grade 3 or higher) compared with 2% (<1% grade 3 or higher) with BV-AVD. Subgroups that appeared to benefit most from BV-AVD included higher-risk groups, such as those with stage IV disease and males. Therefore, it may be reasonable to consider BV-AVD for the highest-risk patients (such as those with international prognostic scores ≥ 4); however, for the majority of patients with advanced-stage HL, PET-adapted therapy with ABVD (as was done in the RATHL study)22 is a good option, because it is highly curative and associated with lower toxicity (although perhaps not an optimal strategy for patients older than age 60 years because of the requirement for escalated BEACOPP for PET-2+ patients).
Patients aged ≥60 years represented only ∼14% of the patients enrolled in the ECHELON-1 study. Interestingly, although BV-AVD lacks bleomycin, this group attained no benefit from BV-AVD, potentially as a result of increased toxicity. Thus, rather than using BV-AVD, sequential therapy with BV and AVD (discussed above) appears to be a better option for these patients.14
BV as a bridge to ASCT
Although the standard approach for a patient who fails front-line therapy is to administer salvage therapy, followed by consolidation with ASCT, the choice of salvage therapy for HL is not standard and, instead, is center or investigator dependent.23,24 The traditional salvage regimens are considered equal with respect to efficacy and include platinum-based regimens, such as ICE (ifosfamide, carboplatin, etoposide), DHAP (dexamethasone, cytarabine, cisplatin), and ESHAP (etoposide, cytarabine, cisplatin, methylprednisolone), and gemcitabine-based regimens, such as ifosfamide, gemcitabine, and etoposide; gemcitabine, dexamethasone, and cisplatin; and gemcitabine, vinorelbine, and doxil.25-29 PET− rates up to 60% are observed with these regimens.30
Given the favorable toxicity profile of BV, as well as its efficacy, there has been significant interest in evaluating it in the second-line setting. The primary outcome for these studies has been the rate of PET normalization, based upon multiple studies demonstrating pre-ASCT PET normalization as 1 of the strongest predictors of outcome following ASCT.31-37 BV has been studied in combination with chemotherapy, such as bendamustine, ICE, DHAP, and ESHAP.38-42 In addition, BV has been studied as a single agent given sequentially with combination chemotherapy for patients who remain PET+ after BV.43,44 Although some of these studies are still ongoing, combination and sequential approaches with BV produce CR rates ranging from 69% to 90% (Table 1). More recently, BV was evaluated in combination with nivolumab as second-line therapy before ASCT, and interim results showed a PET− rate of 61% among 62 patients. Although the CR rate is not as high as the other BV-based salvage regimens, it is considerably higher than observed with either drug alone, suggesting synergy between the 2 drugs.45
Activity of second-line BV-based regimens
| Regimen . | N . | Relapsed (n) . | Refractory (n) . | PET− rate (%) . | ASCT (%) . | PFS . |
|---|---|---|---|---|---|---|
| Sequential PET-adapted therapy with single-agent BV, followed by combination chemotherapy | ||||||
| BV → augmented ICE43 | 65 | 31 | 34 | 83† | 98 | 82% at 3 y |
| BV → salvage therapy44 | 37 | 13 | 24 | 73† | 89 | 72% at 18 mo |
| BV combinations | ||||||
| BV-bendamustine41 | 54 | 27 | 27 | 74 | 74 | 63% at 2 y |
| BV-ICE38 * | 16 | 5 | 11 | 69 | 75 | Too soon |
| BV-DHAP39 * | 12 | 10 | 2 | 90 | 100 | Too soon |
| BV-ESHAP40 * | 66 | 26 | 40 | 70 | 92 | Too soon |
| BV-nivolumab45 | 62 | 34 | 28 | 61 | 87 (68% directly after BV-nivo) | 89% at 6 mo |
| Regimen . | N . | Relapsed (n) . | Refractory (n) . | PET− rate (%) . | ASCT (%) . | PFS . |
|---|---|---|---|---|---|---|
| Sequential PET-adapted therapy with single-agent BV, followed by combination chemotherapy | ||||||
| BV → augmented ICE43 | 65 | 31 | 34 | 83† | 98 | 82% at 3 y |
| BV → salvage therapy44 | 37 | 13 | 24 | 73† | 89 | 72% at 18 mo |
| BV combinations | ||||||
| BV-bendamustine41 | 54 | 27 | 27 | 74 | 74 | 63% at 2 y |
| BV-ICE38 * | 16 | 5 | 11 | 69 | 75 | Too soon |
| BV-DHAP39 * | 12 | 10 | 2 | 90 | 100 | Too soon |
| BV-ESHAP40 * | 66 | 26 | 40 | 70 | 92 | Too soon |
| BV-nivolumab45 | 62 | 34 | 28 | 61 | 87 (68% directly after BV-nivo) | 89% at 6 mo |
BV-nivo, BV-nivolumab.
Published only in abstract form thus far.
PET− rate for entire PET-adapted approach (only patients who were PET+ after single-agent BV received combination chemotherapy).
With the multitude of studies demonstrating high efficacy of BV-based therapy in the pretransplant setting, there are many reasonable approaches. Although BV is not FDA approved as second-line therapy, for a patient who has not received BV as part of their front-line treatment, I prefer to start with single-agent BV. Two different dosing schedules could be considered: 1.8 mg/kg IV every 3 weeks for 2 to 4 cycles (which was evaluated by Chen et al44 ) or 1.2 mg/kg IV weekly, 3 weeks on and 1 week off, for 2 cycles (evaluated at Memorial Sloan Kettering Cancer Center43 ). Patients who achieve normalization of PET after 2 cycles of BV proceed to ASCT, whereas those with residual abnormalities on PET after single-agent BV proceed to additional salvage chemotherapy, such as ICE, before consideration for ASCT. At my institution, using this PET-adapted approach with weekly BV, followed by augmented ICE for PET+ patients, 28% achieved CR (Deauville score ≤ 2) after BV alone, and 75% achieved CR following the entire PET-adapted program.46 PET-adapted therapy with single-agent BV allows a small portion of patients to avoid platinum-based salvage therapy before ASCT and also establishes whether individual patients are sensitive to single-agent BV, which is helpful in deciding whether to use BV maintenance following ASCT.
BV as maintenance following ASCT
The role of BV maintenance following ASCT was established in the AETHERA trial, a randomized double-blind phase 3 study evaluating post-ASCT BV maintenance for higher-risk patients who had undergone ASCT (higher risk defined as the presence of extranodal disease at relapse, relapse within 1 year of initial treatment, or primary refractory disease). A total of 329 patients was randomized to receive BV or placebo for up to 16 cycles following ASCT.47 Median PFS in the BV group was significantly better (42.9 months vs 24.1 months, P = .0013). After 2 years of follow-up, no difference in overall survival was observed; however, this is not surprising given that the median survival for patients following ASCT failure in the BV era is far longer than 2 years.48 Based upon the AETHERA study, the FDA extended the label for BV to include post-ASCT maintenance for up to 16 doses. Patients who attained the most benefit from BV maintenance were those with ≥2 adverse risk factors, where the risk factors included primary refractory disease, relapse within 1 year of initial treatment, less than CR to salvage therapy, extranodal disease at relapse, B symptoms at relapse, or requiring ≥2 salvage therapies before proceeding to ASCT. It is important to note that all patients enrolled in the AETHERA study were BV naive, and, therefore, the role of BV maintenance for patients previously treated with BV is unclear; however, patients who previously achieve PR or CR to BV typically respond again upon retreatment.49 Therefore, BV maintenance should be considered for higher-risk patients (as defined in the AETHERA study) and is reasonable to consider for patients who previously achieved response to BV.
BV as a bridge to allogeneic stem cell transplant
The availability of checkpoint inhibitors for rel/ref HL has led to less frequent use of reduced-intensity allogeneic stem cell transplant (alloSCT) for patients who relapse after ASCT. However, it is not known whether checkpoint inhibitors can be curative for HL; therefore, alloSCT, which is the only known curative therapy after ASCT failure, needs to be considered for some patients. The use of BV as a bridge to alloSCT has been shown to be quite successful, likely as a result of its effectiveness in achieving disease control. A retrospective analysis of 21 consecutive patients treated with BV before alloSCT showed a 2-year PFS of 59.2%.50 In comparison, the 2-year PFS for 23 consecutive patients treated with alloSCT in the pre-BV era (2003-2008) was only 23.8%. Reduced rates of peritransplant toxicity were noted for patients in the BV group, as well. Patients who received BV before alloSCT were more commonly in remission before alloSCT (28.6% vs 4.3%) and less commonly received prior treatment with combination chemotherapy or radiation. Similarly favorable outcomes were observed for the 7 patients treated in the BV pivotal trial who went on to receive alloSCT consolidation.51
Should every patient diagnosed with HL receive BV?
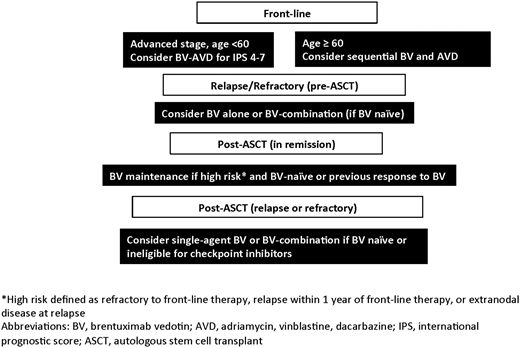
The optimal place for BV in the treatment of HL is likely to evolve over the next few years as studies evaluate how to best individualize therapy. The settings in which to consider incorporating BV into the treatment of HL, based upon currently available data, are shown in Figure 1. The ECHELON-1 study showed that BV-AVD is minimally more effective than ABVD at the cost of higher rates of (manageable) toxicity.21 Therefore, until we have more data from randomized studies, front-line BV-AVD is best reserved for the higher-risk advanced-stage patients who appeared to glean more benefit from BV-AVD in the ECHELON-1 study. Studies aimed at identifying baseline biomarkers to help guide which patients should receive novel agents, such as BV or checkpoint inhibitors, are needed. Baseline metabolic tumor volume has been shown to be prognostic in untreated early-stage disease, as well as in rel/ref disease, and studies evaluating its role in stratifying patients for more- or less-intense therapy are warranted.46,52 The utility of cell-free DNA in identifying high-risk patients is emerging and may also aid in risk stratification.53 Baseline programmed death ligand-1 expression or 9p24.1 amplification is predictive of response to checkpoint inhibitors and may aid in identifying patients appropriate for front-line incorporation of checkpoint inhibition, either instead of or in addition to BV.54 Finally, interim PET is consistently prognostic in early- and advanced-stage disease and should potentially be used to select patients in need of novel agents. For patients who do not receive BV with front-line treatment, second-line therapy is the logical place for BV. In this setting, high CR rates are observed with sequential BV strategies, as well as BV combinations; therefore, use in this setting appears to increase rates of pretransplant PET normalization and ultimately improves outcomes for rel/ref patients. After transplant, BV maintenance should be considered for patients with higher-risk relapse, and previous BV use should not necessarily dissuade use in the maintenance setting, unless refractoriness to BV was already observed. Finally, for patients who relapse following ASCT, BV, or BV combination, studies should be considered for BV-naive patients or for those with a prior response to BV. For a select few, it could serve as a bridge to alloSCT. Overall, BV has undoubtedly prolonged survival and improved the lives of countless patients with HL, although the optimal place for BV in HL treatment is not yet entirely clear. The most appropriate timing for BV should ultimately be defined through studies that aim to individualize therapy for patients with HL by evaluating risk-adapted and response-adapted treatment approaches.
Correspondence
Alison J. Moskowitz, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: moskowia@mskcc.org.
References
Competing Interests
Conflict-of-interest disclosure: A.J.M. has received research funding and honoraria from Seattle Genetics, Merck, and Bristol-Myers Squibb.
Author notes
Off-label drug use: Off-label use of brentuximab vedotin is discussed.