Abstract
The remarkable clinical activity of tyrosine kinase inhibitors (TKIs) in chronic myeloid leukemia (CML) has transformed patient outcome. Consequently, allogeneic stem cell transplantation (allo-SCT) is no longer the only treatment modality with the ability to deliver long-term survival. In contrast to the central position it held in the treatment algorithm 20 years ago, allografting is now largely reserved for patients with either chronic-phase disease resistant to TKI therapy or advanced-phase disease. Over the same period, progress in transplant technology, principally the introduction of reduced intensity conditioning regimens coupled with increased donor availability, has extended transplant options in patients with CML whose outcome can be predicted to be poor if they are treated with TKIs alone. Consequently, transplantation is still a vitally important, potentially curative therapeutic modality in selected patients with either chronic- or advanced-phase CML. The major causes of transplant failure in patients allografted for CML are transplant toxicity and disease relapse. A greater understanding of the distinct contributions made by various factors such as patient fitness, patient-donor HLA disparity, conditioning regimen intensity, and transplant toxicity increasingly permits personalized transplant decision making. At the same time, advances in the design of conditioning regimens coupled with the use of adjunctive posttransplant cellular and pharmacologic therapies provide opportunities for reducing the risk of disease relapse. The role of SCT in the management of CML will grow in the future because of an increase in disease prevalence and because of continued improvements in transplant outcome.
Learning Objectives
Understand the criteria for identifying patients with CML who should be considered for allogeneic SCT
Acquire the ability to characterize disease and transplant-related factors that determine transplantation outcome
Understand manipulable factors that can improve the outcome of SCT in CML
Introduction
The outcome of patients with chronic myeloid leukemia (CML) has been transformed by the remarkable clinical activity of BCR-ABL tyrosine kinase inhibitors (TKIs).1 As a result, many newly diagnosed patients with chronic-phase (CP) CML now routinely achieve survival rates approaching those of age-matched controls.2 Allogeneic stem cell transplantation (allo-SCT), which until the turn of the century represented the only treatment with the capacity to deliver long-term disease-free survival to patients with CML, is now indicated in only a minority of patients in CP, although it remains an important curative modality for many patients with advanced-phase (AP) CML. Over the same period, improvements in transplantation technologies, notably the introduction of reduced intensity conditioning (RIC) regimens, has dramatically increased the number of patients who could potentially benefit from an allograft.3,4 As a consequence, the role of SCT in the management of patients whose outcome can be predicted to be poor if treated with TKIs alone continues to evolve.5 Consistent with best practice in other hematologic malignancies, transplantation decision making in CML is dynamic and is determined by considering the predicted patient outcome with either standard non-transplant therapy or allo-SCT.6 Optimal management of patients who are or can be predicted to be resistant to TKI monotherapy therefore requires regular dialogue with an experienced transplantation center.
General principles of allo-SCT in CML
The toxicity of allo-SCT has fallen substantially over the last 3 decades.7 This has resulted not only in reductions in transplant-related mortality (TRM) but also in lower morbidity, notably a reduced risk of chronic graft-versus-host disease (cGVHD).8 This is particularly relevant when considering treatment options for patients with TKI-resistant CML for whom the possibility of late transplant complications often represents a major disincentive to proceeding with an allograft. Disease recurrence, as in other hematologic malignancies, is now the most common cause of transplant failure in patients allografted for CML. Although there has been very little progress in improving the outcome of patients who relapse after an allograft for acute myeloid leukemia (AML), there are now several effective pharmacologic and cellular salvage strategies for patients who relapse after an allograft for CML.
The evolution of transplantation modalities in CML
The principle that myeloablative chemoradiotherapy followed by stem cell rescue is an effective treatment strategy in CML was first established in the early 1970s when it was observed that CP disease could be reestablished in patients with blastic transformation after transplantation of cryopreserved autologous cells.9 This pioneering observation was followed by the demonstration that durable cytogenetic remissions could be achieved in patients transplanted with bone marrow harvested from syngeneic donors.10 In 1982, myeloablative sibling allografts were shown to have the capacity to deliver long-term survival to a proportion of patients with CP CML.11,12 Subsequently, after recognizing that only one-third of patients possess an HLA-identical sibling adult donor, the role of transplantation in the management of CML was dramatically extended by the demonstration that long-term survival could be achieved in patients allografted using a matched unrelated donor (MUD). Although they were initially associated with substantially increased risks of graft failure and GVHD, MUD transplants matched using molecular typing of HLA class I and class II genes now deliver outcomes to patients with CML that are nearly equivalent to those achievable with a sibling donor.4 In 2018, thanks to the altruism of the more than 30 million volunteers recruited to worldwide unrelated donor registries, the likelihood of identifying a well-matched volunteer unrelated donor for a white patient with CML who lacks a matched sibling donor now approaches 90%. However, there are still major barriers to identifying a donor for nonwhite patients.
Two important developments promise to increase donor availability for allo-mandatory patients with CML who lack a well-matched donor. First, transplantation of 1 or 2 units of cryopreserved umbilical cord blood (UCB) with an adequate nucleated cell dose now delivers predictable if delayed engraftment with an acceptable TRM.13 Although outcomes after UCB transplantation were historically compromised by a high risk of graft failure, transplantation of two 4/6 HLA-matched UCBs has been shown to result in faster neutrophil and platelet engraftment and a substantially reduced risk of graft failure. There is a possibility that UCB transplantation may result in a lower risk of disease relapse in patients with high-risk AML, consequent upon enhanced alloreactivity, and this may be relevant in patients with AP CML.14,15 Recent reports of the ability of ex vivo expansion techniques to accelerate neutrophil engraftment make it likely that in the future, UCB will represent a graft source increasingly used in patients who lack a well-matched unrelated donor.16 Consequently, a search for suitably matched UCB units with an acceptable nucleated cell dose should be initiated in allo-mandatory patients with CML who lack a suitable MUD. Second, although transplantation using haplo-identical donors was historically associated with unacceptably high rates of transplant toxicity (specifically graft failure and severe GVHD), infusing cyclophosphamide shortly after infusing stem cells has been shown to result in predictable donor stem cell engraftment and acceptable risks of acute GVHD (aGVHD) and cGVHD, dramatically reducing the toxicity associated with using this source of stem cells.17 It remains unclear whether haplo-identical transplants in myeloid diseases are associated with an increased risk of disease relapse compared with alternative sources of stem cells, and much remains to be done in terms of identifying an optimal conditioning regimen and stem cell source. Nonetheless, haplo-identical donors represent an increasingly important potential source of stem cells for patients with CML for whom no other suitable donor can be identified.
A potent graft-versus-leukemia (GVL) effect is exerted in patients allografted for CML in first CP. Evidence supporting the existence of a GVL effect in CML initially came from registry studies that showed a decreased risk of relapse in patients who developed cGVHD and increased relapse rates in patients receiving in vivo T-cell depletion.18,19 The importance of the GVL effect was unequivocally demonstrated by the observation that donor lymphocyte infusion (DLI) could serve as salvage therapy and restore durable molecular remissions in patients who relapsed after an allograft for CML.20 Increasing awareness of the potency of the GVL effect in patients allografted for a range of hematologic malignancies, coupled with the excessive toxicity of myeloablative conditioning (MAC) regimens in older patients, led to the development of RIC regimens in the late 1990s.21 In the subsequent 2 decades, the ability of RIC regimens to substantially reduce transplant toxicity and permit the delivery of a potentially curative GVL effect to patients as old as age 75 years has transformed transplant practice internationally. Specifically, it has significantly extended the role of allo-SCT in older patients with TKI-resistant or intolerant CML in whom an allograft might previously have been deemed to be associated with unacceptable toxicity.3
Current indications for allo-SCT in CML
CP CML
Which patients with CP CML should be considered for transplantation?
Although the majority of patients with newly diagnosed CML achieve a complete cytogenetic response (CCR) or major molecular response with first-line TKI therapy, 25% to 40% will demonstrate either drug intolerance or disease resistance and will require treatment with second-line TKI therapy.22,23 Approximately 50% of such patients will achieve a durable CCR or deeper response if they are switched to dasatinib, nilotinib, bosutinib, or ponatinib.24-28 Patients for whom second-line TKI therapy fails can still achieve a CCR after treatment with a third-line TKI, especially if their reason for switching is resistance rather than intolerance to prior TKI therapy, but response rates are variable and the durability of response is less certain.29 Consequently, allo-SCT should be considered as a potential treatment option in all fit patients who are unable to achieve a durable CCR after treatment with 2 TKIs (Table 1). Factors that predict the likelihood of achieving CCR after second-line treatment with dasatinib or nilotinib include Sokal score at presentation, cytogenetic response to first-line therapy, and degree of neutropenia during first-line therapy.30 A recent analysis also identified failure to achieve a reduction in the BCR-ABL ratio to <10% 3 months after starting second-line therapy as a predictor of failure to achieve a subsequent CCR; this helps to quickly identify patients whose outcome with a second-line TKI can be predicted to be poor.31
Indications for allo-SCT in CML in 2018
| Chronic phase |
| Failure of first-line TKI and predicted poor response to second-line TKI |
| Failure to respond to first- and second-line TKIs |
| Presence of T315I mutation and/or failure to respond to ponatinib |
| Presence of repeated grade 4 cytopenias in response to treatment with different TKIs despite appropriate dose reduction and cytokine support |
| Advanced phase |
| TKI naïve |
| TKI naïve with suboptimal response to TKI |
| TKI resistant |
| Blast phase |
| Acquisition of second CP after TKI or chemotherapy salvage |
| Chronic phase |
| Failure of first-line TKI and predicted poor response to second-line TKI |
| Failure to respond to first- and second-line TKIs |
| Presence of T315I mutation and/or failure to respond to ponatinib |
| Presence of repeated grade 4 cytopenias in response to treatment with different TKIs despite appropriate dose reduction and cytokine support |
| Advanced phase |
| TKI naïve |
| TKI naïve with suboptimal response to TKI |
| TKI resistant |
| Blast phase |
| Acquisition of second CP after TKI or chemotherapy salvage |
In approximately 20% of patients who are unable to achieve a durable CCR after first-line TKI therapy, mutational analysis demonstrates the presence of the gatekeeper T315I mutation, which predicts resistance to imatinib, nilotinib, dasatinib, and bosutinib and confers a poor prognosis. Until recently, such patients were deemed allo-mandatory because transplantation represented the only realistic prospect of long-term survival. The demonstration that ponatinib possesses significant activity in patients with CML who have the T315I mutation represents a significant advance.26 Although longer follow-up is required, it seems that ponatinib has the potential to deliver durable responses in a proportion of patients with CP CML, and it may be reasonable to defer allo-SCT in a proportion of such patients.32 However, it is still the case that patients with a T315I mutation who demonstrate primary or secondary resistance to ponatinib should proceed swiftly to an allograft if possible (Table 1).33
A small number of patients develop serious (grade 4) cytopenias after exposure to a range of TKIs despite appropriate dose reduction and cytokine support. This results in frequent interruption of treatment and can prevent effective sustained delivery of TKI therapy (Table 1). In these patients, who likely have insufficient residual normal hematopoiesis to repopulate the bone marrow, allo-SCT can deliver excellent long-term survival and represents the only effective form of therapy.
Transplantation outcomes in first CP CML.
The outcomes of patients with first CP CML after a MAC allograft from a matched sibling or volunteer unrelated donor have steadily improved over the last 30 years.4 Retrospective registry and single-center series report 3-year survival rates ranging from 70% to 90% in patients who received a transplant using a matched sibling donor and a myeloablative preparative regimen.5,34 Even though the transplantation outcomes achieved using an unrelated donor now approximate those achieved using a matched sibling donor, predicting outcome after a MUD transplantation is more complicated. Factors that determine outcome after MUD transplantation include patient age, patient cytomegalovirus status, and the degree of donor-patient HLA disparity.35-37 There is no consensus on the optimal source of stem cells for patients who receive a transplant using a matched sibling or unrelated donor. Although the use of peripheral blood stem cells, as opposed to bone marrow, is associated with a higher risk of cGVHD, this does not seem to have an impact on survival.38 Nonetheless, in a disease such as first CP CML in which relapse is eminently salvageable, optimal GVHD prophylaxis with in vivo T-cell depletion can be justified, despite the associated increase in relapse rates.39 It is worth noting that, although up-front TKI therapy has indisputably supplanted allo-SCT as the therapy of choice in newly diagnosed patients, several randomized trials have demonstrated that first-line transplantation results in outcomes broadly equivalent to those achieved with TKI therapy, particularly in patients with high-risk features, which emphasizes the substantial progress that has been made in improving transplant outcome in recent years.34 In contrast to previous assumptions, evidence is emerging that delayed transplantation is not necessarily associated with an inferior outcome.40,41
Transplant toxicity is substantially increased in older patients who receive a transplant using a MAC regimen, and the advent of preparative RIC regimens has been highly effective in reducing transplant morbidity and mortality. Several studies have reported encouraging survival rates in patients who received a transplant using a RIC regimen with either a matched sibling or an unrelated donor.42,43 The increased risk of disease relapse after a RIC allograft mandates both meticulous monitoring of minimal residual disease (MRD) after the transplant and development of novel transplant strategies that have the potential to reduce the risk of disease recurrence.5
In patients with TKI-resistant CML who lack a well-matched unrelated donor, emerging data support the use of either cryopreserved UCB units or a haplo-identical donor. Promising results have been reported in patients with CP CML who receive a transplant that uses 1 or 2 units of UCB with a high nucleated cell dose; survival rates are ∼40%.44 Favorable long-term survival is also reported in patients with high-risk CML after transplantation of haplo-identical grafts with and without posttransplant cyclophosphamide.45
AP CML
The outcomes of patients with AP CML treated with TKIs alone compares unfavorably with those achieved in first CP, and although ∼20% to 40% of patients in AP CML will achieve a CCR after treatment with a TKI, such responses are often not durable.41,46 In contrast, allo-SCT represents a valuable, potentially curative treatment modality in patients with AP CML, and 5-year progression-free survival rates of 50% to 80% have been reported in patients allografted using a sibling or unrelated donor (Table 1). One randomized study has suggested that allo-SCT may be deferred in patients with low-risk AP disease defined by the absence of the following characteristics: disease duration >12 months, hemoglobin <100 g/L, and peripheral blood blasts >5%.41 A superior outcome was observed after allograft for patients with any 1 of these features, and patients with 2 or more features had an especially poor outcome if treated with TKIs alone.
In patients with blastic transformation, the immediate focus of therapy should be restoration of second CP because transplantation outcomes for patients with untreated blastic phase remain very poor. At the same time, an urgent search for donors should be started so that patients achieving second CP can proceed as swiftly as possible to transplantation.47,48 In patients with a myeloid blast crisis, salvage options include TKI therapy guided by the results of mutational profiling or intensive chemotherapy using an anthracycline-cytosine-arabinoside backbone or a combination of TKIs and intensive chemotherapy. Although there are no prospective comparisons, a recent analysis identified improved outcomes in patients treated with induction chemotherapy in combination with a TKI.49 Several small studies highlight encouraging results when using fludarabine, cytarabine, granulocyte-colony stimulating factor, and idarubicin (FLAG-IDA) as induction chemotherapy.50 In patients with a lymphoid blast crisis, combination TKI and induction chemotherapy with vincristine-prednisolone is commonly used.51 More recently, the cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) regimen has been combined with dasatinib with promising results.52,53 Long-term survival rates for patients who achieve a second CP range from 30% to 40% for those who received a transplant that used a MAC regimen (Table 1). Inferior outcomes are reported in patients who received a transplant using a RIC regimen, presumably because the GVL response is attenuated in AP CML,42 and such patients should receive a transplant using a MAC regimen when possible.
Optimizing transplant outcome in patients allografted for CML
Although allo-SCT is now indicated in only a minority of patients with CP CML, it remains a highly effective treatment modality that reliably delivers long-term survival without a requirement for ongoing TKI therapy. Although the risk of transplant-related complications has decreased substantially, the small but distinct possibility of either early death or long-term complications (principally cGVHD) remains daunting for many patients compared with the relative short-term safety of continued TKI therapy. Consequently, decision making is highly nuanced in patients who are eligible for a transplant but for whom TKI therapy has failed. For some younger patients, the prospect of long-term molecular remission without the requirement for ongoing therapy and regular monitoring represents an attractive option. Many other patients find the complexity and unpredictability of transplantation unacceptable, even if their predicted outcome with TKI therapy is unsatisfactory. Therefore, it is vital that discussions with patients and their families contain balanced information about the benefits and risks of both allo-SCT and long-term treatment with TKIs.
Several scoring systems that predict transplant outcome in CML have been developed. Gratwohl and Apperley were the first to develop a model that predicted the outcome of patients after allo-SCT choosing CML as the index disease.54 The European Society for Blood and Marrow Transplantation (EBMT) score identified 5 factors predictive of survival: donor type (sibling vs unrelated donor), pretransplant disease stage, patient age, donor and recipient sex, and time from diagnosis to transplant. In addition to predicting survival, the EBMT score was also strongly predictive of TRM, which varied from 20% in the lowest-risk cohort to more than 70% in high-risk patients.55 Although it was formulated before the advent of TKI therapy and RIC regimens, the EBMT score embodies the principle that disease-specific factors coupled with patient and donor characteristics can inform transplant decision making, a principle that is just as important in 2018 as it was 20 years ago. The more recent demonstration that meticulous assessment of pretransplant comorbidities (expressed as the hematopoietic cell transplant-comorbidity index [HCT-CI])56 permits accurate prediction of both survival and TRM in patients allografted for a hematologic malignancy and has been confirmed in patients with CML transplanted using a MAC regimen.4 The adverse impact of a high HCT-CI also applies in patients older than age 60 years allografted using a RIC regimen, in which even an HCT-CI >1 seems to be associated with a very substantial increase in TRM.57
Reducing the risk of GVHD and long-term complications in patients allografted for CML
The major causes of treatment failure in patients allografted for CML are transplant toxicity, GVHD, and disease relapse. The advent of RIC regimens, coupled with advances in supportive care has substantially reduced TRM. Significant progress has also been made in reducing the risk of cGVHD which can be as high as 66% in patients undergoing a T-replete allograft from an unrelated donor.35 Retrospective studies demonstrate the ability of both antithymocyte globulin and alemtuzumab to reduce the risk of aGVHD and cGVHD, although use of these agents seems to increase the risk of disease relapse.19,58 Recent randomized trials have confirmed that pretransplant antithymocyte globulin reduces the risk of aGVHD and cGVHD without compromising survival in patients allografted for a range of hematologic malignancies, although this has not been confirmed in all studies.39,59 Given the salvageability of patients who relapse after an allograft for CML, there is therefore a compelling case for the use of in vivo T-cell depletion in patients allografted for CML, particularly those who receive a transplant from an alternative donor.
Managing disease relapse after transplantation
Thirty percent to 70% of patients allografted for CML will relapse.42 Several factors determine the risk of disease recurrence after a transplant, including transplantation for AP disease, use of a RIC as opposed to a MAC regimen, and the incorporation of either in vivo or in vitro T-cell depletion.49 Three monthly BCR-ABL quantitation posttransplant permits identification of patients with isolated molecular relapse who may benefit from early intervention with DLI or a TKI and is mandatory for the first 3 years posttransplant. Late disease relapse in patients allografted for CML is also well documented and serves as a justification for longer-term BCR-ABL transcript monitoring.60
DLI remains the most effective salvage therapy in patients relapsing after allograft, which results in restoration of molecular remission in 60% to 90% of patients allografted in first CP.20,61 Response rates to DLI are determined by the disease stage at transplant (CP CML vs AP CML), the presence of molecular or cytogenetic vs morphologic relapse, and time to relapse posttransplant.62 The major complication of DLI is the development of GVHD, which typically occurs between 4 and 8 weeks after infusion. Administration of DLI more than 12 to 18 months posttransplant as well as the use of escalating doses of DLI seems to reduce the risk of severe GVHD.63 A large EBMT study reported a 69% 5-year survival rate in patients with relapsed CML who were treated with DLI. Thirty-eight percent of patients developed GVHD, which typically presented as aGVHD, although one-third of patients developed features of cGVHD without evidence of preceding aGVHD.63 The GVHD-related mortality was 11%.
TKIs represent an alternative salvage strategy in patients who relapse after allograft, those with evidence of active GVHD, or those in whom DLI cannot otherwise be delivered, even in patients who received a transplant for TKI resistance. Although the majority of patients who relapse after allo-SCT for CML demonstrate persistence of the ABL kinase mutation documented before a transplant, a proportion of patients who relapse after allograft have evidence of novel BCR-ABL clones and seem to respond particularly well to salvage therapy with TKIs.64 This mirrors recent reports of clonal evolution in patients who relapse after an allograft for AML.65 In patients who are predicted to have a reduced likelihood of responding to DLI, such as those who relapse after an allograft for AP CML, there may be a case for combined treatment with a TKI and DLI guided by the results of ABL kinase mutational analysis.66
Reducing the risk of disease relapse after allo-SCT in CML
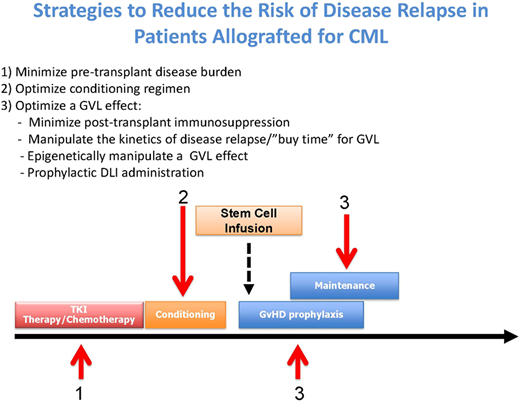
Broadly, there are 3 approaches for reducing disease relapse posttransplant: minimizing the disease load pretransplant, increasing the antitumor activity of the conditioning regimen without higher toxicity, and optimizing a GVL effect posttransplant (Figure 1).
Strategies to reduce the risk of disease relapse after allo-SCT in CML.
Strategies to reduce the risk of disease relapse after allo-SCT in CML.
Does pretransplant cytoreduction have an impact on outcome in patients allografted for CML?
In patients allografted for AML, the pretransplant MRD status is an important predictor of disease relapse posttransplant.67 Similarly, in patients who have received a transplant for CML in blast crisis, the risk of disease relapse risk is very high for those who did not achieve a second CP before transplantation. In patients allografted for CML in first CP, there was no compelling evidence that disease load predicted relapse risk before the introduction of imatinib. Indeed, there were initial concerns that routine use of TKIs as first-line therapy in CML might increase TRM. However, retrospective studies seem to demonstrate that prior TKI exposure does not increase transplant toxicity, but instead may reduce relapse risk.68 However, therapeutic interventions to reduce the pretransplant MRD burden may be of particular benefit in patients allografted for AP CML.47
Defining the optimal conditioning regimen in patients allografted for CML
Retrospective studies have demonstrated broad equivalence of the busulphan-cyclophosphamide and cyclophosphamide-total body irradiation (TBI) myeloablative preparative regimens in patients with CML in first CP. The busulphan-cyclophosphamide (Bu/Cy) regimen has reportedly delivered encouraging results in patients allografted for first CP CML.69 More recent registry studies in patients allografted for AML have shown reduced toxicity and a trend toward improved survival using intravenous (IV) preparations of busulphan, and parenteral preparations have now almost entirely supplanted oral busulphan.70 Building on these observations, a recent large randomized trial in patients allografted for AML demonstrated reduced toxicity and preserved antileukemic activity of a myeloablative combination of IV busulphan and fludarabine (busulphan-fludarabine for 4 days [FB4]) compared with IV busulphan-cyclophosphamide.71 As a result, FB4 is now arguably the standard MAC regimen for fit patients with AML or CML who are younger than age 50 years.
The optimal RIC regimen in patients allografted for CML has yet to be defined, but many centers use a combination of busulphan and fludarabine delivered over 2 days (FB2). Of interest, promising results have been reported using the 8-Gy TBI RIC regimen pioneered by the German AML Cooperative Group.72 It is likely that nonmyeloablative regimens using low-dose (2 Gy) TBI deliver inferior outcomes, and RIC regimens should therefore be preferred.43 There are opportunities for developing regimens with augmented antileukemic properties and reduced toxicity in older patients, particularly those with AP CML, by building on emerging data in patients allografted for AML.73
Posttransplant maintenance strategies
Posttransplant administration of a well-tolerated agent with antitumor activity has the potential to reduce disease relapse through several mechanisms: direct targeting of residual leukemic stem and progenitor cells, manipulation of the kinetics of disease relapse thereby either buying time for the genesis of a GVL effect or postponing the requirement for DLI, or direct pharmacologic manipulation of the allo-reactive response (Table 2). Proof of principle of such an approach was highlighted by the demonstration that elective administration of imatinib for the first 12 months posttransplant in patients allografted for CML abolished disease recurrence during this period.74 As a result, administration of DLI for those patients who relapse could be postponed to a time when the risk of severe GVHD is substantially reduced. Consequently, although posttransplant pharmacologic maintenance therapies have not yet been evaluated in prospective randomized trials, there is increasing interest in their role in patients allografted for high-risk myeloid malignancies including CML.
Mechanisms by which posttransplant maintenance therapy can reduce the risk of disease relapse
| Antitumor activity against residual leukemic stem and progenitor cells |
| Postponement of disease relapse thereby buying time for the genesis of a GVL effect |
| Manipulation of the kinetics of disease relapse permitting postponement of DLI to a later time posttransplant when the risk of severe GVHD is reduced |
| Pharmacologic manipulation of a GVL response using agents such as azacitidine or sorafenib |
| Antitumor activity against residual leukemic stem and progenitor cells |
| Postponement of disease relapse thereby buying time for the genesis of a GVL effect |
| Manipulation of the kinetics of disease relapse permitting postponement of DLI to a later time posttransplant when the risk of severe GVHD is reduced |
| Pharmacologic manipulation of a GVL response using agents such as azacitidine or sorafenib |
Conclusion
The success of TKI therapy in CML has dramatically reduced the number of patients requiring an allograft. However, allo-SCT remains a highly effective therapeutic modality in patients with first CP CML who are resistant or intolerant to TKIs as well as patients with AP CML. Advances in transplant technology and donor availability have simultaneously increased transplant availability and improved patient outcome. We anticipate that increased precision in our ability to identify patients whose outcome if treated with TKIs will be poor coupled with continued improvements in transplant outcome will increase the role of allo-SCT in managing CML. It will also be important to ensure that transplant advances in other hematologic malignancies that have the potential to reduce transplant toxicity and disease relapse are rapidly applied to patients allografted for CML, the disease in which many of the fundamental principles of allo-SCT were first established.
Acknowledgment
C.F.C. acknowledges support from the Birmingham ECMC programme.
Correspondence
Charles F. Craddock, Centre for Clinical Haematology, Queen Elizabeth Hospital, 6 Mindelsohn Way, Birmingham B15 2SY, United Kingdom; e-mail: charles.craddock@uhb.nhs.uk.
References
Competing Interests
Conflict-of-interest disclosure: C.F.C. has received honoraria from Novartis, BMS, and Pfizer and research support and honoraria from Celgene.
Author notes
Off-label drug use: None disclosed.