Abstract
Adolescents and young adults (AYAs) with cancer, defined by the National Cancer Institute as having been diagnosed between the ages of 15 and 39 years old, have not benefited from the same improvements in quality of outcomes and survival that have been seen for individuals diagnosed in childhood or as older adults. Although is leukemia composed of a diverse group of diagnoses, leukemia AYA survivors share unique vulnerabilities with other AYA diagnostic groups. They will spend the majority of their lives as survivors, with clear evidence of adverse medical conditions, health care requirements, and social and psychological needs that differ not only from their peers but also, from the needs of other cancer survivor populations. Furthermore, they share a developmental stage of life in which careers, finances, and family concerns are uniquely impacted by the cancer diagnosis and treatment. Leukemia in AYAs typically presents with higher-risk biologic features, and treatment requires multiagent chemotherapy, including alkylating agents, anthracyclines, high-dose steroids, frequently intrathecal chemotherapy, and sometimes, cranial radiation. Thus, AYAs have significant risks for long-term complications, subsequent malignancies, and accelerated development of usual age-related comorbid conditions, such as cardiovascular disease and dyslipidemias. AYAs require specialized health care monitoring, surveillance for late effects, and periodic evaluation of psychosocial, health behavior, and life goal outcomes.
Learning Objectives
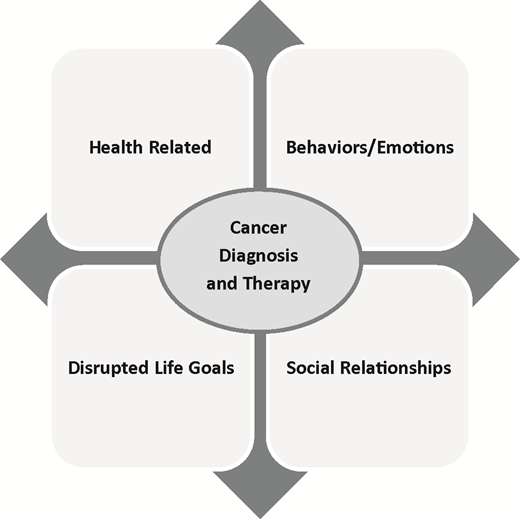
Describe the major challenges/concerns facing adolescents and young adults (AYAs) survivors from the 4 domains presented, including health related, behaviors/emotions, disrupted life goals, and social relationships
Identify how late effects in AYA survivors differ from those of survivors diagnosed in childhood or as older adults
Introduction
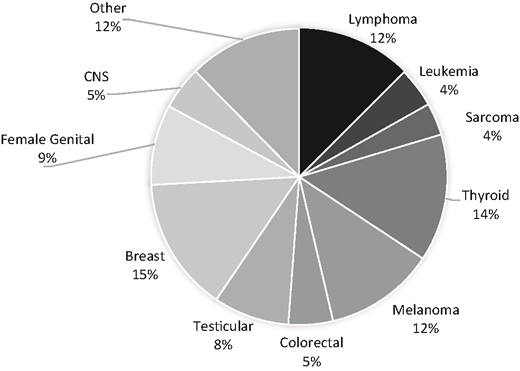
The adverse medical, physical, developmental, and psychosocial outcomes experienced by adolescents and young adults (AYAs; ages 15-39 years old as defined by the National Cancer Institute [NCI]) who are diagnosed with cancer constitute a significant understudied public health priority, with meaningful health disparities as highlighted in recent reports of the NCI and the Health and Medicine Division of the National Academies (previously the Institute of Medicine).1-3 Although composed of a diverse group of diagnoses, AYA survivors share unique vulnerabilities compared with older adults or younger children. AYAs are transitioning from dependence to independence and adult responsibilities. They are graduating from school, getting their first jobs, finding life partners, having children, and setting their goals for the future. A cancer diagnosis is not only an interruption, but it is also life changing. AYAs will spend the majority of their lives as cancer survivors with physical, social, and emotional needs that differ not only from their peers but also, from the needs of younger or older cancer survivors. They have significant risks for long-term complications, second cancers, and accelerated development of usual age-related comorbid conditions.4,5 Existing survivorship guidelines developed for pediatric or adult survivors are only beginning to recognize the impact of this unique developmental life stage and subsequent issues that AYA survivors face. As shown in Figure 1, AYA survivors are defined by distinctive types of cancer.3,6 The 5-year relative survival rate for AYA cancers overall is 81.1% (76.7% males, 84.1% females).1
Cancer incidence rates per 100 000 in AYAs age 15-39 years old. Source: SEER, 2007-2011. CNS, central nervous system
Cancer incidence rates per 100 000 in AYAs age 15-39 years old. Source: SEER, 2007-2011. CNS, central nervous system
AYA cancer survivors: a diverse group that experiences disparities in outcomes
The age-adjusted incidence rate (per 100 000) for all of cancer in AYAs (age 15-39) is 67.7 (52.6 for males and 82.9 for females). Although AYA cancer remains relatively uncommon, almost 70 000 new AYA cancer diagnoses were reported in 2011, which is about 6 times higher than the number of cases diagnosed in children <15 years old,1 and this rate has steadily increased over the past 25 years. Cancer remains the leading cause of nonaccidental death in this age group. Despite advances in cancer prevention, early detection, and treatment over the past several decades, survival rates for AYAs have not improved to the extent that they have for other cancer populations.3,6-8 AYAs are more likely than older or younger groups to be diagnosed with Hodgkin lymphoma, testicular cancer, and sarcomas. However, incidence varies with age groups among AYAs. Although leukemia, lymphoma, testicular cancer, and thyroid cancer are the most common cancers among 15 to 24 year olds, among 25 to 39 year olds, breast cancer and melanoma are most common. Compared with pediatric populations, evidence indicates poorer outcomes in AYAs with colon cancer, breast cancer, acute leukemias, and other cancer types, which may be related to their biology as well as other factors, with evidence for different genomic risks, tumor histopathology, oncogenic pathway deregulation, chemotherapy sensitivities, and disparities in access to health care generally and clinical trials participation specifically.6,9 AYAs are also diagnosed in later stages than their older counterparts, and there are suggestions of different side effect profiles, such as more nausea, neuropathy, glucose intolerance, pancreatitis, and osteonecrosis (ON) in AYA vs older survivors.6,9,10
Significance of patient-reported outcomes in AYA cancer survivors
Much of what is known about AYA needs and gaps in care has been derived from patient-reported outcomes (PROs) in AYA survivors of childhood cancer rather than directly from those diagnosed and treated as AYAs, with the exception of one cross-sectional study, the Adolescent & Young Adult Health Outcomes & Patient Experience Study (AYA HOPE) study, that included 524 AYA survivors assessed between 6 and 14 months after diagnosis, which will be discussed later.11 In developmental milestones, lifestyle, and psychosocial priorities, AYAs do not fit into either the pediatric setting or the adult oncology setting. Among AYA survivors, 41% are uncertain of their health care needs as a result of their cancers, and >50% do not receive needed cancer follow-up care.12,13 AYA survivors have greater emotional distress and fewer positive health beliefs compared with age-matched controls.14 Their survival is often accompanied by challenges, such as physical impairment, infertility, uncertainty, fears about recurrence, interruption of life plans, and discrimination in employment/insurance (Figure 2). Although adherence and health behavior problems have been identified, the actual scale, nature, and reasons for nonadherence or high-risk health behaviors have been only minimally examined.15
Major patient-centered domains of concerns facing AYA survivors impacted by a cancer diagnosis and subsequent therapy.
Major patient-centered domains of concerns facing AYA survivors impacted by a cancer diagnosis and subsequent therapy.
AYA leukemia and health-related long-term outcomes
Approximately 60% of leukemia in AYAs is acute lymphoblastic leukemia (ALL), and 40% is acute myeloid leukemia (AML). ALL occurs more frequently in males (65%), with a peak incidence between 15 and 20 years old. AYAs present with higher-risk biologic features (T cell, unfavorable cytogenetics, including Philadelphia chromosome positive [BCR-ABL fusion protein]) or an aggressive “Ph-like” ALL characterized by aberrations in cytokine receptor and tyrosine kinase (TK) pathways, rendering them responsive to TK or other signaling pathway inhibitors.16,17 ALL treatment requires multiagent chemotherapy, including alkylating agents, anthracyclines, high-dose steroids, and frequently, intrathecal chemotherapy and cranial radiation.18 Survival rates of ALL in AYAs are ∼75%.17,18 For AML, the 5-year survival in AYAs remains inferior compared with children (54% vs 64%).19 Treatment of AML in the AYA population is not significantly different than in children, and all receive anthracycline-based induction chemotherapy, which contributes to their overall late effect risk profile. Long-term toxicities of AML or ALL therapy in AYAs include obesity, insulin resistance, hyperlipidemia, venous thrombosis, and ON.20-22 Both AML and ALL survivors receive significant cumulative doses of anthracyclines, placing them at risk for cardiomyopathy and cardiometabolic abnormalities.23-25 Importantly, in this age group, TK inhibitors (eg, imatinib, dasatinib) are routinely used in therapy for Philadelphia chromosome–positive or Ph-like ALL; minimal data are available on the long-term toxicities of these targeted agents, raising concerns related to long-term adherence in the AYA population.
Cardiomyopathy
Anthracyclines have well-known cardiotoxic potential and are used in almost all regimens for treatment of both AML and ALL. The risk of cardiomyopathy and subsequently, congestive heart failure (CHF) is dose dependent, and regimens for high-risk disease (including most in the AYA age range) typically use higher cumulative dose exposures. In a large cohort of 607 childhood cancer survivors, the relative risk of anthracycline-induced clinical heart failure in patients treated with anthracyclines and followed for a median of 6.3 years was found to be 11.8 times higher for patients treated with doses <300 mg/m2 compared with cumulative doses >300 mg/m2.23 In the total cohort that had a median exposure of 301 mg/m2, the cumulative risk of CHF was 5% after 15 years of follow-up. Lipshultz et al26 examined echocardiograms from 120 children and adults with ALL (n = 87) or osteogenic sarcoma (OS; n = 33) who had received doxorubicin at cumulative doses of 244 to 550 mg/m2 in childhood and at the time of the study, were a median of 8.1 years (range, 2-14 years) since completion of therapy and had a current median age of 14.2 years old for ALL and 23.4 years old for OS. Results were analyzed in comparison with a cohort of 296 normal subjects and found that female sex was associated with depressed contractility (P ≤ .001) and that higher cumulative doses of doxorubicin were associated with depressed contractility and depressed left ventricular (LV) function (P ≤ .001); younger age at diagnosis was associated with reduced LV wall thickness and mass and increased afterload. With a longer time from completion of therapy there was also an associated reduction in LV wall thickness and increased afterload (P ≤ .001). There was no difference based on diagnosis, and the age range at diagnosis of the OS cohort in this study was very similar to that of a typical AYA cohort. Although there is not a clear cutoff for a “safe dose” of anthracyclines, additional recent data from the Children’s Oncology Group as well as a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group have provided strong evidence in support of higher risk of cardiomyopathy in survivors of childhood cancer who receive cumulative anthracycline doses of 250 mg/m2 or more.27,28 ASCO clinical practice guidelines published in 2017 also define adult survivors who have received doses of 250 mg/m2 or higher to be at risk for developing cardiac dysfunction.29
Screening for at-risk survivors has been controversial; however, the Children’s Oncology Group and Harmonization Group’s recommendation is to obtain an echocardiogram ≤2 years after completion of cardiotoxic therapy, repeat at 5 years after diagnosis, and continue every 5 years thereafter. The National Comprehensive Cancer Network (NCCN) recommendation is that an echocardiogram be performed between 6 and 12 months after completion of cancer-directed therapy in asymptomatic patients considered to be at increased risk of cardiac dysfunction, but in the absence of evidence, no recommendations can be made regarding the frequency and duration of surveillance in patients at increased risk who are asymptomatic and have no evidence of cardiac dysfunction on their 6- to 12-month posttreatment echocardiogram.
In addition to anthracyclines, additional therapeutic exposures, such as mediastinal radiation, cyclophosphamide, and uncontrolled hypertension, can further add to overall cardiovascular (CV) risks. Other relevant considerations for AYA females who may become pregnant include the fact that, during the third trimester of pregnancy, cardiac volume increases, leading to increases in cardiac workload, which can precipitate overt CHF in pregnant women with LV dysfunction (even if subclinical).
Cardiometabolic abnormalities
Leukemia survivors are also at risk for the development of other CV risk factors, including obesity, dyslipidemia, hypertension, and insulin resistance, all of which are known to be potent risks for premature CV disease in adults. Although there have not been studies specifically performed in the AYA population, a large study of childhood cancer survivors >5 years after diagnosis who were a mean age of 14.5 years old and carefully evaluated for CV risk and insulin resistance found that, relative to a sibling comparison group, the survivors had greater adiposity, a higher percentage of body fat, and lower lean body mass.25 These survivors were found to have higher levels of total cholesterol, low-density lipoprotein cholesterol, and triglycerides, and they were more insulin resistant as measured by euglycemic insulin clamp study than controls. These findings are significant, because it has been shown that CV risk factors track from childhood into adulthood, and when already present at a younger age, these very likely contribute to the risk of early CV disease and potentially, mortality. AYA survivors should have baseline fasting lipid profile and blood glucose obtained at their entry into survivorship. Blood pressure should be monitored on a yearly basis. Counseling regarding the importance of exercise, a nutritionally balanced diet, and maintaining a healthy body weight should be encouraged at each follow-up visit along with referral to resources to maintain healthy behaviors.
Endocrinopathies and gonadal dysfunction
The endocrine system is quite susceptible to the impact of cancer therapies, although particular risks vary depending on the age of exposure. In particular, for patients with leukemia, the greatest risk is seen in patients who have received cranial radiation therapy for prophylaxis or especially, treatment of central nervous involvement of the disease. Fortunately, with current risk stratification and regimens used, the use of radiation has significantly declined. However, those patients in whom cranial radiation therapy has been delivered are at risk for deficiencies in one or more anterior pituitary hormones, including growth hormone (GH), follicle-stimulating hormone, luteinizing hormone, adrenocorticotrophic hormone, and thyroid-stimulating hormone. The risk of pituitary dysfunction increases with time from radiation and with increasing radiation dose. Although the majority of AYA patients will have completed most of their growth and development, GH plays an important role throughout life. GH deficiency in adulthood can leady to fatigue, abnormal body composition (increased fat mass, decreased muscle mass), osteopenia, and it can contribute to an abnormal CV risk profile (hyperlipidemia and insulin resistance).30 GH therapy in adulthood can improve quality of life, increase lean mass, decrease fat mass, increase bone mineral density, and improve the CV risk profile.31 GH is vital for development of bone mass, which peaks in the third decade. AYAs with GH deficiency may not reach peak bone mass and thus, have an increased risk of osteoporosis in the future. For patients with ALL, this risk may be further potentiated by the use of steroids in their treatment. Despite these critical functions of GH, there are no specific guidelines or recommendations for GH testing or replacement in adult-aged survivors.
Gonadal function can certainly be affected in both men and women after exposure to chemotherapy and/or radiation. Standard treatment protocols for ALL or AML are not particularly toxic to gonadal tissues in the AYA population. However, for patients with higher-risk or relapsed disease who receive more intensive therapies, the risk of developing premature ovarian failure or impaired testicular function exists. Exposure to high-dose alkylating agents and total body irradiation in those ALL or AML patients who require hematopoietic stem cell transplantation will lead to gonadal failure in the majority of females.32 For males, alkylating agent exposure can cause failure of the germinal epithelium, leading to oligospermia or azoospermia, although testosterone deficiency is rare. However, radiation to the testis can lead to germinal epithelium damage and azospermia as well as Leydig cell dysfunction and testosterone deficiency.
ON
Although ON typically develops while on therapy, the latency period has been as long as 13 years after treatment. The primary risk factors for development of ON are systemic corticosteroids and radiation therapy. Thus, this is primarily a complication seen in ALL survivors or those who have undergone hematopoietic cell transplantation and received steroids for treatment of graft-versus-host disease. The overall incidence in patients with ALL is ∼5%, but there is a higher risk in AYAs.33 Dexamethasone has a higher incidence of ON than equivalent doses of prednisone, particularly in the AYA age range.
Subsequent neoplasms
Available data on the risk of subsequent neoplasms (SNs) are primarily from long-term survivors of ALL in childhood. A large study from St. Jude reported on a cohort of 2169 ALL patients who were followed for over 30 years.34 In total, 123 SNs were found, and the majority of these were low-grade tumors (meningiomas and basal cell carcinomas), likely secondary to the use of cranial radiation. The cumulative incidence of SN increased steadily over the 30 years of follow-up: 4% at 15 years and up to nearly 11% at 30 years. This was a 13.5-fold increase in overall risk compared with the general population. Other less common SN diagnoses included myeloid malignancy (n = 46), lymphoma (n = 3), other brain tumors (n = 22), other carcinomas (n = 16), and sarcomas (n = 6). Given the infrequent use of radiation in modern leukemia therapy, it is likely that the overall risks of SN are significantly lower in current survivors. Given the variety of SN seen, no specific screening guidelines can be provided over those that are recommended for the general population. Preventative measures, such as vigilant use of sunscreen, maintaining a healthy weight and diet, physical activity, and avoiding smoking, should be recommended at each touch point with health care providers.
Late mortality
The increased risk of late mortality in childhood cancer survivors has been well described and is ∼10-fold higher than in the general population. Although there is not as much data on AYA survivors, a study from the Finland Cancer Registry identified 16 769 5-year survivors ages 0 to 34 years old at diagnosis (5352 ages 0-19 years old and 11 417 ages 20-34 years old) and examined late mortality in these two age groups.35 The overall standardized mortality ratio (SMR) for the entire cohort was 4.6 (95% confidence interval [95% CI], 4.4-4.8), with the highest SMRs for cancer-related deaths including primary and secondary malignancies (SMR = 12.8) followed by infectious diseases (SMR = 4.8), all CV causes (SMR = 1.9), cardiac ischemia (SMR = 1.9), and respiratory diseases (SMR = 1.7). Most SMRs were higher in the childhood cancer survivors than in the young adult survivors, with overall SMRs of 7.6 and 4.2, respectively. The highest mortality from nonmalignant causes was due to CV disease, and there was an ongoing increase noted throughout the follow-up period. An additional population-based study from the British Columbia Cancer Registry included 1248 5-year survivors who had been diagnosed between the ages of 20 and 24 years old.36 The overall mortality rate was almost 6-fold higher compared with the general population, with the leading cause of mortality from the primary cancer diagnosis. Importantly, however, the SMR for a second malignancy was 5.2, and for noncancer-related deaths, it was 1.7. These data all highlight the importance of ongoing long-term follow-up for AYA survivors, with particular attention focused on modifiable risk factors, particularly those related to CV disease and subsequent malignancies.
Poorer outcomes in the AYA population may be related to multiple factors beyond biology and treatment
Barriers to health care in the AYA population include lack of access, which historically has included lack of health insurance.37 Even now, the Affordable Care Act does not cover the full age spectrum of AYA survivors, and those without health insurance report worse psychosocial outcomes. In addition, AYAs lack adequate information about the cancer treatments that they received and follow-up needs based on their diagnosis and treatments.12,13 AYAs’ multiple competing roles also can be a barrier to focusing on health care needs. Balancing nonhealth priorities, such as career and family, along with limited practical and economic resources and lack of perception of needs as well as avoidance behaviors with regard to health care may all be issues.5,38
Psychosocial and health behavior outcomes
Cancer-related concerns may impact AYA transitions, choices, and developmental milestones related to education, employment, identity, relationships, and family. In qualitative interviews and systematic reviews, AYAs have identified needs in three areas: social wellbeing, information access, and health care services.39,40 Small or disease-specific studies have documented poorer emotional wellbeing in AYAs compared with age-matched controls or other survivor populations and unmet needs for psychosocial care.5,14,41,42 Recent data suggest higher rates of depression, posttraumatic stress symptoms, fatigue, poor attention, and sexual dysfunction in AYA cancer survivors relative to controls or older survivors but also, a greater sense of personal growth for the AYA survivors, although studies have not been done specific to leukemia AYA survivors.41-44
Health behaviors
A study utilizing data from the 2009 Behavioral Risk Factor Surveillance System (BRFSS) general population cohort of 350 000 found that AYA survivors across diagnoses, compared with the age-matched general population, had a higher prevalence of smoking (26% vs 18%), obesity (31% vs 27%), chronic health conditions (14% vs 7%), and poor mental health (20% vs 10%).5 These results have been confirmed with several national population-based studies. In addition, AYA’s report more fatigue than controls, and their health-related quality of life is worse than those without a cancer history.45 These health problems may stem from multiple sources, including barriers to access to care, such as lack of insurance; knowledge barriers; competing priorities of families and careers; developmental differences with older adults; psychosocial experience of feeling isolated or otherwise different from peers; and differences in communication style and interests, such that traditional health messages are not connecting with AYA survivors.44
Sexual dysfunction, poor body image, and infertility
These issues are well-known consequences and concerns of many AYAs after cancer treatment.46-48 These are particular concerns of AYA survivors who are unmarried, may be prematurely postmenopausal, and are without children yet.46,47 Treatment can lead to not only estrogen or testosterone deficiencies but also, hypothalamic and pituitary hormone deficits. At a time of life when many AYAs feel vulnerable about their body image, cancer can exacerbate this self-consciousness, with 60% reporting a negative body image, which may, in turn, impact sexual function.49 Some AYA survivors may be challenged by physical deformities, particularly during this phase of life when body image is critical to their sense of self. In interviews with AYA survivors, they reported thinning hair, scars, and stretch marks as negatively affecting their body image.50 AYA survivors consistently report poorer sexual function and satisfaction than older survivors.42 Lack of information about treatments for vaginal dryness, dyspareunia, lack of desire, and erectile dysfunction can have major impact on their relationships, but they can be reluctant to ask questions, whereas health care providers may not be anticipating these concerns in AYAs. When health care providers routinely address these issues along with safe sexual behaviors, use of contraception, sexually transmitted infections, and vaccination for human papilloma virus, it normalizes these problems and assures the survivors that they do not need to worry unnecessarily or try to figure out what to ask, whom to ask, and when to ask about these concerns.
Infertility can add long-term stress to cancer survivors who have not yet completed their families.51 In ongoing work in AYA survivors, we have examined the impact of fertility concern in 872 young adult survivors (72.3% female) between ages 18 and 39 years old who were 1 to 5 years from diagnosis and ≥1 year from therapy completion, across all diagnoses.52 Participants were randomly selected from tumor registries of 7 participating sites and asked to complete an online PRO survey. With median ages at diagnosis and the time of survey of 32 and 36 years old, respectively, and with most married (61%), 20% had a high or intermediate risk of infertility based on their treatment exposures. Only 13% of women and 36% of men reported fertility preservation (FP) attempts before treatment. Of the majority who did not pursue FP, 38% were not interested, and 19% reported lack of information on risks or options as the reason. FP in men was associated with age under 30 years old at diagnosis, higher educational attainment, and treatment with chemotherapy. For women, FP was associated with age 31 to 35 years old, and it was marginally associated with age under 30 years old vs over age 35 years old at diagnosis, being married, and treatment with chemotherapy. For both males and females, having no children at diagnosis was a strong predictor of FP. As other research has found, among both genders, survivors who had children before diagnosis were less likely to pursue FP. Interestingly, in this cohort of broad diagnoses, including leukemia, of the men who tried to father a pregnancy, 70% were successful, and most conceived naturally. Of the women who attempted pregnancy, 73% were successful, and nearly all conceived naturally. A substantial minority of survivors in our study reported that they were not aware of the potential risks of treatment or FP options, indicating that fertility may be underaddressed for some AYA survivors. Our results also suggest that those who need assisted reproduction infrequently are having children, showing a possible gap that needs additional exploration. Other research confirms that AYA survivors who have the highest level of long-term fertility concern are female, are younger, are a member of an ethnic minority group, did not have children at the time of diagnosis, and received chemotherapy.51
Emotional health, fear of recurrence, and posttraumatic stress symptoms
Compared with age-matched controls or other survivor populations, AYAs report poorer emotional wellbeing.41 Many endorse a heightened awareness of the uncertainties in life and a sense of being out of sync with their peers. They worry about recurrence and can be hypervigilant about symptoms. The stress of managing health needs as well as changes in self-perceptions, body image, and feelings of vulnerability can all add to their emotional distress. These symptoms may not meet full clinical criteria for anxiety or depressive disorders, although they often include somatic symptoms at rates above those in the general population, such as difficulty with concentration, fatigue, and sleep.53 AYAs report higher rates of fear of recurrence than older cancer survivors.42 As with any other type of anxiety, fear of recurrence can lead to avoidance of health care, interfering with potentially lifesaving surveillance behaviors, or it can lead to hypervigilance to all body or sensation changes, potentially resulting in overtesting and overtreatment. Greater distress in AYAs is associated with younger age (adolescence) at the time of treatment and higher intensity of treatment.14 These cancer-specific concerns require brief but thoughtful evaluation at follow-up beyond the standard assessment of anxiety and depression.
Clinical anxiety and depression
Data on rates of clinical anxiety or depression in AYA cancer survivors are limited, and they are generally extrapolated from survivors of childhood cancer who are in the AYA age range when evaluated. Those AYAs at risk for depression include females, survivors with reproductive concerns, those at older age at diagnosis, and those with more visible disfigurement, particularly of head and neck.42,54 Suicidal ideation is more common in AYA survivors than their siblings, and one Norwegian study found that they were more likely to commit suicide than their noncancer peers (hazard ratio, 2.6; 95% CI, 1.5-4.2).55 In contrast to anxiety and depression, posttraumatic stress symptoms are better described in AYA survivors, with 8% to 29% meeting criteria for posttraumatic stress disorder.43 Risk factors include female sex, poorer family functioning, presence of late effects of treatment, unemployment, lower education, cranial irradiation, lower social support, and poorer self-image. Because effective treatments are available for all of these emotional complications of treatment, regular evaluation with brief standardized measures assures that symptoms are detected and treated before they become chronic or impact function for AYAs, particularly because these survivors have less reference for what would be “normal” emotional responses after life-threatening illness.
Functional limitations in AYA cancer survivors
Most available data on AYA cancer survivors come from national-level surveillance data or single-institution/single-disease cohorts. These available data indicate that AYA survivors have more functional limitations than noncancer controls and that those with reduced physiological capacity report that it interferes with their quality of life. In a study from the National Health Interview Survey that included 100 female breast/gynecological cancer survivors diagnosed when 15 to 29 years old, survivors scored lower on all questions about functional abilities and participation in social or leisure activities than noncancer controls.56 Data from the BRFSS cohort indicated that survivors of AYA cancers were more likely than population-based controls to report disability (36% vs 18%) and poor physical health (24% vs 10%).5 Finally, the AYA HOPE study reported lower scores on the physical component summary of the Short Form-12 among those age 18 to 34 years old compared with population controls (49.7 vs 53.1).53 Additional research on tested actual physical function capability is required for this population rather than depending solely on PRO or extrapolation from childhood survivors.
Social relationships: family dynamics, peers, dating, intimacy, social wellbeing or isolation, and health care relationships
Social functioning is worse in AYA survivors even 2 years after treatment compared with population norms.45 Many AYA survivors are accompanied to health care appointments by their mothers beyond the age that would be expected for other adults. They also report feeling closer to their families at the end of treatment.57 The need to depend on family at an age when most young adults are becoming increasingly independent can result in AYA survivors being vulnerable to family dysfunction, either with parents or spouses. A commonly expressed concern in qualitative studies is that spouses are ready to move beyond the cancer experience well before the survivor or that the spouse is unaware of the long-term effects of cancer and treatment that the survivor experiences. This is frequently expressed by female survivors. Similarly, AYA survivors may feel different from their peers, and at the same time, when they go to their cancer centers, they feel quite different from the older patients who fill reception areas. This sense of being different can result in a perception of not belonging anywhere and contribute to isolation. For AYAs with long-term complications, this isolation can be exacerbated by persisting symptoms of fatigue or cognitive difficulties. Another area to recognize when addressing the needs of AYA survivors is the difficulty with dating and intimacy after cancer, particularly for those with scars, hormonal changes that impact sexual function, or infertility. Although these issues may also arise with couples in stable relationships, for those who are single, questions of when to discuss the impacts of cancer and how to bring up the topic of a cancer history and its consequences can add a whole new dimension to developing new relationships and intimacy. Referral to numerous online groups and resources as well as in-person community groups in larger cities can reduce this isolation, normalize an AYA survivor’s experience, and facilitate social connections, while providing solutions to some of these common AYA concerns.
Disrupted life goals: school, work, finances, and insurance
Financial concerns and return to work or school can be challenging for many AYA survivors, and they may interact with physical and psychosocial limitations and other disparities.39 Work provides not just financial benefits but also, meaning and social support. Approximately one-third of AYAs report a negative impact of cancer on their work, including increased likelihood of stopping work or working part time.49,58,59 AYAs are less likely to be in managerial occupations than their siblings, and they are likely to have lower income in any job category.60
We have also examined the impact of cancer on employment and finances.61 Survivors provided a baseline PRO survey, and diagnostic and treatment data were abstracted from medical records. Survivors were grouped into 4 diagnostic categories: leukemia and lymphoma (n = 163), breast (n = 241), thyroid (n = 126), and all other diagnoses (n = 342). Most survivors (736; 84.4%) reported they were working for pay at some time between diagnosis and survey completion. Of those, 70% reported having a physical component to their job, and over one-half of those reported that their cancer and its treatment interfered with their ability to perform the physical or mental tasks that their jobs required. Males were more likely than females to be working for pay at any time after diagnosis (odds ratio [OR], 1.6; 95% CI, 1.0-2.5; P = .05). Survivors treated with surgery alone were less likely to have impairments that limited their ability to perform physical (OR, 0.5; 95% CI, 0.3-0.7; P < .005) or mental tasks (OR, 0.4; 95% CI, 0.3-0.5; P < .005) at work compared with those treated with chemotherapy with or without radiation. Over one-half of these survivors reported that they needed to take extended paid or unpaid time off from work or made a change in their hours, duties, or employment status. Unpaid time off from work was taken by 39% of survivors, with 37% of those taking >6 months unpaid time off. When survivors with leukemia and lymphoma were examined separately, there was no association found between cancer-directed treatment exposure categories (surgery, chemotherapy, or radiation) and interference with physical or mental tasks required by their job or with paid or unpaid time off work. Additional analyses that focus on specific chemotherapy agent exposures may provide information on the impact of specific agents. Nearly one-third of all survivors reported that they or their family borrowed money or went into debt because of cancer and treatment; 47% borrowed >$10 000, and 5% reported bankruptcy. These findings highlight the significant impact that cancer and its treatment have on work and the associated financial toxicity for AYA survivors.
Conclusions
Progress in advancing health and psychosocial outcomes for AYAs has been hindered by several factors, including suboptimal knowledge of patient-centered needs both during and after the cancer experience.3,12 There has been a critical lack of evidence-based standards of posttreatment survivorship care specific for AYAs with cancer. However, guidelines from the Children’s Oncology Group (http://www.survivorshipguidelines.org) or the NCCN (www.nccn.org/professionals/physician_gls/default.aspx#survivorship) do provide recommendations that can be adapted to the AYA population. In addition, the NCCN has developed AYA-specific guidelines (www.nccn.org/professionals/physician_gls/pdf/aya.pdf) that include topics relevant to the care of AYA patients diagnosed with cancer from the time of diagnosis through survivorship and/or palliative care. These also include information on psychosocial/behavioral considerations relevant to this age group. Table 1 provides a list of organ system or patient-centered components that should be evaluated as part of the comprehensive long-term follow-up of AYA leukemia survivors. Epidemiologic longitudinal research with AYA survivors will allow for better understanding of causes of disparities in outcomes in this population and will further support the development of evidence-based and age-appropriate survivorship care.
Components of comprehensive long-term follow-up of AYA leukemia survivors
| Organ system or patient-centered care category . | Specific toxicities or outcomes that impact survival, health, or quality of life . |
|---|---|
| Overall survival | Premature mortality |
| Cancer recurrence or SN | |
| Cardiac | Cardiomyopathy |
| Cardiometabolic | Obesity |
| Dyslipidemia | |
| Hypertension | |
| Insulin resistance, diabetes | |
| Endocrine/gonadal | Anterior pituitary hormone deficiencies in GH, FSH, LH, ACTH, TSH |
| Ovarian dysfunction, estrogen and progesterone deficiency | |
| Testicular dysfunction, oligospermia or azoospermia, testosterone deficiency, germinal epithelium damage, Leydig cell dysfunction | |
| Reproductive | Infertility |
| Sexual dysfunction | |
| Musculoskeletal | ON |
| Osteopenia/osteoporosis | |
| Sarcopenia with increased adiposity | |
| Health behaviors | Risky behaviors (smoking, alcohol, sexual behavior) |
| Diet and exercise | |
| Health care needs | Chronic health conditions |
| Demands of routine surveillance | |
| Nonadherence to medication and screening | |
| Lack of information | |
| Access to care | |
| Health fears leading to over- or undertreatment | |
| Emotional adjustment | Living with uncertainty, fear of recurrence |
| Infertility impacts | |
| Sexual dysfunction impacting relationships and self-perception | |
| Body image with self-consciousness | |
| Hypervigilance | |
| Posttraumatic stress | |
| Distress, anxiety, depression | |
| Suicidal ideation | |
| Sense of personal growth | |
| Social relationships | Family difficulties: parents, spouse, children |
| Peers comparison or separation | |
| Dating, intimacy | |
| Decreased social and leisure activities | |
| Isolation with decreased social support | |
| Need for multiple health care provider relationships | |
| Functional needs/symptoms | Managing physical limitations |
| Fatigue | |
| Cognitive dysfunction | |
| Sexual function symptoms | |
| Insomnia | |
| Employment/finances | Education completion |
| Career advancement | |
| Debt and financial stress | |
| Insurance coverage |
| Organ system or patient-centered care category . | Specific toxicities or outcomes that impact survival, health, or quality of life . |
|---|---|
| Overall survival | Premature mortality |
| Cancer recurrence or SN | |
| Cardiac | Cardiomyopathy |
| Cardiometabolic | Obesity |
| Dyslipidemia | |
| Hypertension | |
| Insulin resistance, diabetes | |
| Endocrine/gonadal | Anterior pituitary hormone deficiencies in GH, FSH, LH, ACTH, TSH |
| Ovarian dysfunction, estrogen and progesterone deficiency | |
| Testicular dysfunction, oligospermia or azoospermia, testosterone deficiency, germinal epithelium damage, Leydig cell dysfunction | |
| Reproductive | Infertility |
| Sexual dysfunction | |
| Musculoskeletal | ON |
| Osteopenia/osteoporosis | |
| Sarcopenia with increased adiposity | |
| Health behaviors | Risky behaviors (smoking, alcohol, sexual behavior) |
| Diet and exercise | |
| Health care needs | Chronic health conditions |
| Demands of routine surveillance | |
| Nonadherence to medication and screening | |
| Lack of information | |
| Access to care | |
| Health fears leading to over- or undertreatment | |
| Emotional adjustment | Living with uncertainty, fear of recurrence |
| Infertility impacts | |
| Sexual dysfunction impacting relationships and self-perception | |
| Body image with self-consciousness | |
| Hypervigilance | |
| Posttraumatic stress | |
| Distress, anxiety, depression | |
| Suicidal ideation | |
| Sense of personal growth | |
| Social relationships | Family difficulties: parents, spouse, children |
| Peers comparison or separation | |
| Dating, intimacy | |
| Decreased social and leisure activities | |
| Isolation with decreased social support | |
| Need for multiple health care provider relationships | |
| Functional needs/symptoms | Managing physical limitations |
| Fatigue | |
| Cognitive dysfunction | |
| Sexual function symptoms | |
| Insomnia | |
| Employment/finances | Education completion |
| Career advancement | |
| Debt and financial stress | |
| Insurance coverage |
ACTH, adrenocorticotrophic hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; TSH, thyroid-stimulating hormone.
Acknowledgments
This work was funded by grants from the National Cancer Institute (CA215134, 5P30 CA015704, CA 204378, and CA201179).
Correspondence
K. Scott Baker, Pediatric Blood and Marrow Transplantation and Survivorship Programs, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: ksbaker@fredhutch.org.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Author notes
Off-label drug use: None disclosed.