Abstract
Thrombocytopenia is common among patients admitted to the intensive care unit (ICU). Multiple pathophysiological mechanisms may contribute, including thrombin-mediated platelet activation, dilution, hemophagocytosis, extracellular histones, ADAMTS13 deficiency, and complement activation. From the clinical perspective, the development of thrombocytopenia in the ICU usually indicates serious organ system derangement and physiologic decompensation rather than a primary hematologic disorder. Thrombocytopenia is associated with bleeding, transfusion, and adverse clinical outcomes including death, though few deaths are directly attributable to bleeding. The assessment of thrombocytopenia begins by looking back to the patient’s medical history and presenting illness. This past information, combined with careful observation of the platelet trajectory in the context of the patient’s clinical course, offers clues to the diagnosis and prognosis. Management is primarily directed at the underlying disorder and transfusion of platelets to prevent or treat clinical bleeding. Optimal platelet transfusion strategies are not defined, and a conservative approach is recommended.
Learning Objectives
Characterize the multiple mechanisms that can contribute to thrombocytopenia in the ICU
Evaluate the trajectory of the patient’s platelet count and apply this information to establish a diagnosis and inform prognosis
Transfuse platelets appropriately to prevent and manage bleeding in thrombocytopenic patients admitted to the ICU
Definition, incidence, and outcomes of thrombocytopenia in the ICU
Thrombocytopenia is a common hematologic abnormality among patients admitted to the intensive care unit (ICU). In adults, the prevalence of thrombocytopenia at the time of ICU admission ranges from 8.3% to 67.6%. The incidence of thrombocytopenia developing during ICU admission ranges from 14% to 44%.1,2 Variability in these estimates reflects differences in patient populations (eg, age, severity of illness, diagnoses), ICU characteristics (eg, medical, surgical, cardiac), and the definition of thrombocytopenia across published observational studies (eg, platelet count of <150 × 109/L, <100 × 109/L, and sometimes <50 × 109/L).1 In the PROTECT trial, a large randomized trial of thromboprophylaxis in patients admitted to medical-surgical ICUs, the incidence of new mild (100-149 × 109/L), moderate (59-99 × 109/L), and severe (<50 × 109/L) thrombocytopenia was 15.3%, 5.1%, and 1.6%, respectively.3 Although thrombocytopenia has many possible causes, risk factors commonly associated with ICU-acquired thrombocytopenia, in multivariate analyses, include severity of illness, organ dysfunction, sepsis, vasopressor use, and renal failure.1,3
In comparison with patients who had a platelet count of >150 × 109/L, the development of thrombocytopenia in critically ill patients is associated with increased bleeding and transfusion risk.1,3,4 In the PROTECT trial, thrombocytopenia of any severity increased the risk of major bleeding and was associated with increased risk of transfusion with red cells, platelets, plasma, or cryoprecipitate.5 In a large observational study, rates of major hemorrhage in patients with thrombocytopenia present at ICU admission (prevalent) or with new-onset thrombocytopenia after ICU admission (incident) were 20% and 19%, respectively, in comparison with 13% in patients without thrombocytopenia.2 Similar to the PROTECT trial, most observational studies have reported thrombocytopenia to be associated with red cell transfusion.1
Among 8 observational studies that used multivariate analysis to determine the association between thrombocytopenia and death, 6 found thrombocytopenia to be an independent predictor of mortality.1 Both the presence and severity of thrombocytopenia have been shown to predict mortality.2,6 In the PROTECT trial, after adjustment for age, severity of illness, baseline use of mechanical ventilation, and inotrope or vasopressor use, both moderate and severe thrombocytopenia were associated with increased ICU and hospital mortality.5 Thrombocytopenia has also been associated with increased ICU and hospital length of stay and need for organ support.2 A blunted platelet recovery appears to further predict increased mortality,7 whereas recovery of platelet count strongly predicts survival.8 As such, thrombocytopenia is a readily accessible marker of clinical status among patients admitted to the ICU.
Mechanisms of thrombocytopenia in the ICU
The mechanisms contributing to thrombocytopenia in critically ill patients remain poorly understood. Moreover, thrombocytopenia arising in an individual patient may have multiple possible causes, and our tools to distinguish between them are limited. In the discussion that follows, we focus on the causes that are most pertinent to critically ill patients (Box 1).
Decreased production
The extent to which decreased platelet production contributes to thrombocytopenia in multiorgan failure is unknown and has never been critically assessed. Whereas inflammatory cytokines (eg, interleukin-6 [IL-6]) are known to blunt erythropoiesis, they stimulate thrombopoiesis. As such, decreased production is unlikely to be the dominant factor unless there was preexisting marrow disease or the patient has undergone cytotoxic chemotherapy. Thrombocytopenia associated with acute alcohol toxicity is a notable exception.
Sequestration
Splenic sequestration is usually a factor only if it was a preexisting problem (most often in liver disease with portal hypertension), but it may contribute to the severity of thrombocytopenia and reduce posttransfusion platelet increments. An exception is the acute splenic sequestration crisis that can occur in infants with hemoglobin (Hb) SS and adults with sickle variants such as Hb SC.
Destruction, consumption, or both
The destruction or consumption of platelets likely explains the bulk of thrombocytopenia in the ICU. Mechanisms may include the following.
Thrombin.
Thrombin-mediated platelet activation may be physiologically appropriate but extensive, as in major surgery or trauma, or uncontrolled, as in disseminated intravascular coagulation (DIC). In either case, platelets may be activated and consumed. In thrombocytopenic patients, serial measurement of international normalized ratio and fibrinogen may allow recognition of nonovert DIC.9 Regrettably, high-quality evidence to inform optimal management of DIC (apart from identification and correction of the underlying cause) is lacking.9
Antibodies.
The antibodies that most often lead to thrombocytopenia in ICU patients are those against the heparin-PF4 complex mediating heparin-induced thrombocytopenia (HIT). Even so, as was discussed by L. Rice in the 2017 American Society of Hematology Education Program, HIT is confirmed in fewer than 5% of thrombocytopenic ICU patients.10 Screening tests for HIT should be performed in any patient who has a rapid drop in platelet count occurring between 5 and 10 days after first heparin exposure regardless of baseline count.10,11 Other drug-immune thrombocytopenias involving agents such as ranitidine, β-lactam antibiotics, vancomycin, and linezolid can occur but are infrequent. For example, although vancomycin is a recognized cause of thrombocytopenia, in a major American referral laboratory, only 34 cases of vancomycin antibody-dependent thrombocytopenia were confirmed over a 5-year period.12 Comprehensive and unbiased estimates of the incidence of drug-immune thrombocytopenia are lacking. Glycoprotein IIb/IIIa receptor antagonists are known to cause thrombocytopenia; however, use of these agents has declined considerably. Autoantibodies (ie, immune thrombocytopenia [ITP]) are unlikely to be the cause of thrombocytopenia in an ICU patient unless there is a previous history of the disorder. Alloantibodies (posttransfusion purpura) are even less common.
Hemophagocytosis.
Hemophagocytic syndrome (HPS) in adults (also known as hemophagocytic lymphohistiocytosis or macrophage activation syndrome) is a poorly understood process of uncontrolled cytokine production and unregulated macrophage activation triggered by a variety of infections, malignancies, or autoimmune processes. It is characterized by cytopenias, fevers, splenomegaly, extreme hyperferritinemia, and usually (but not always) hemophagocytosis on bone marrow biopsy.13 Although uncommon, it is an increasingly recognized clinical entity with evolving diagnostic criteria.14 Bone marrow findings of hemophagocytic activity are neither highly sensitive nor specific. Histological evidence of hemophagocytic activity has been reported to be common in patients with thrombocytopenia and sepsis.15 However, it is unknown whether similar findings would be present in nonthrombocytopenic critically ill patients, and thus the contribution of hemophagocytosis to the peripheral cytopenias is uncertain.
Histones.
Extracellular histones are released during cellular injury and as part of neutrophil extracellular traps. Histones induce platelet aggregation, and infusion of histones in animal models causes a rapid drop in platelet count and accompanying tissue toxicity.16 In ICU patients, plasma histone levels have been shown to correlate with thrombocytopenia,17 but whether the association is causal or reflects illness severity needs to be further explored.
ADAMTS13 depletion.
Nguyen et al have shown that ADAMTS13 levels are reduced in sepsis, although not typically as severely as is seen in thrombotic thrombocytopenic purpura (TTP).18 It is unknown whether this degree of deficiency contributes to microvascular occlusion, thrombocytopenia, or multiorgan failure in septic patients.
Complement activation.
Immune complexes may activate the classical pathway, and bacterial cell walls may activate the alternative pathway of the complement cascade. Complement can bind and activate platelets, contributing to platelet destruction in sepsis.19 Complement activation and completion of the membrane attack complex is a powerful platelet activator and contributor of platelet destruction.6
Thrombocytopenia in sepsis
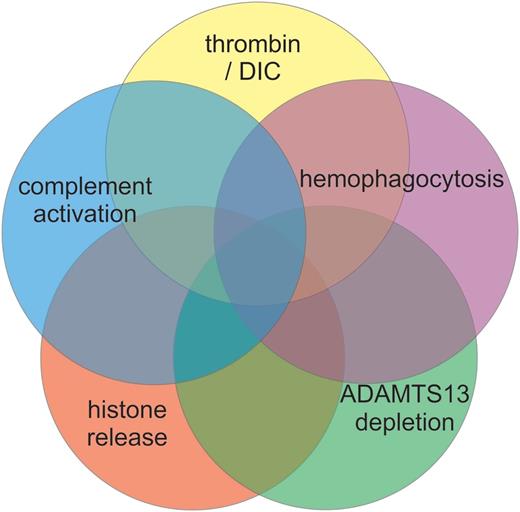
Sepsis is a common clinical problem, accounting for 10% of ICU admissions and roughly 50% of all thrombocytopenias in critically ill patients, yet the mechanisms underlying thrombocytopenia in sepsis are poorly understood.20,21 Several pathological processes likely contribute to thrombocytopenia in sepsis (Figure 1). Thrombocytopenia may also independently modify the host immune response to infection.22 Consideration of the contributing mechanisms suggests directions for possible therapeutic trials.
Mechanisms of thrombocytopenia in sepsis. Multiple mechanisms have been proposed to contribute to the thrombocytopenia of sepsis. The relative contribution of each potential mechanism may vary among patients and within a given patient over time. DIC, disseminated intravascular coagulation.
Mechanisms of thrombocytopenia in sepsis. Multiple mechanisms have been proposed to contribute to the thrombocytopenia of sepsis. The relative contribution of each potential mechanism may vary among patients and within a given patient over time. DIC, disseminated intravascular coagulation.
Thrombin
Sepsis is a well-recognized trigger of DIC, largely driven by the upregulation of tissue factor expression on monocytes and other cells in response to inflammatory mediators such as endotoxin. Even in the absence of overt DIC, markers of coagulation activation (eg, thrombin-antithrombin complexes, prothrombin fragment 1.2) are increased. Several anticoagulants, including tissue factor pathway inhibitor, antithrombin, and activated protein C have been evaluated in the treatment of sepsis, with negative or mixed results.23 Compared with prophylactic-dose unfractionated heparin or no thromboprophylaxis, the use of low-molecular- weight heparin for venous thromboprophylaxis has been shown to be associated with lower risk of moderate thrombocytopenia (hazard ratio, 0.62; 95% confidence interval, 0.47-0.84).3 Data from published randomized trials indicate the potential for heparin to improve clinical outcomes in sepsis.24 To better define the role of heparin in sepsis, the HALO (Heparin AnticoaguLation to improve Outcomes in septic shock) research program investigators have recently completed a feasibility pilot randomized trial of unfractionated heparin in septic shock and are preparing to commence an international trial of heparin in septic shock.25
Hemophagocytosis
Unfortunately, high-quality evidence to inform the optimal treatment of sepsis-associated HPS in adult patients is lacking. Recommended therapies target T cells (steroids, cyclosporine) and macrophages (etoposide).26 Intravenous immunoglobulin (IVIG) to support defective humoral immunity and reduce systematic inflammation has also been suggested.26 Data from randomized trials suggest a potential survival benefit of IVIG in patients with sepsis.27 Whether the potential beneficial effects of IVIG correlate with the presence of HPS is unknown.
ADAMTS13 deficiency
The possible contribution of ADAMTS13 deficiency to thrombocytopenia and microvascular injury in sepsis should lead to consideration of future trials evaluating plasma exchange28 or infusion of recombinant ADAMTS13.
Complement
A coming wave of targeted complement inhibitors, of which eculizumab is a prototype, should permit elucidation of the complement’s contribution to the thrombocytopenia and other pathologies of sepsis.
How we approach thrombocytopenia in the critically ill patient
In assessing a critically ill patient with thrombocytopenia, there are 3 time frames to consider. First, the past: what is the context of the ICU admission, and what was the platelet count profile prior to the current illness? Second, the present: what is the trajectory of the platelet count, and how does it relate to the patient’s clinical course; how severe is the thrombocytopenia; and is there evidence of thrombosis? Third, the future: what trajectory or changes in the platelet count should we expect on the basis of what we have deduced to be the cause of the thrombocytopenia? (Box 2 provides questions for evaluating thrombocytopenia in the ICU patient.)
Past
To understand the likely causes of a patient’s thrombocytopenia, it is essential to have a full picture of the prior medical history, comorbidities, and medications (eg, cytotoxic chemotherapy, antiproliferative agents, and relevant antibiotics), as well as a clear framing of the illness that precipitated the ICU admission. It is especially important to obtain previous complete blood counts, because they may show whether the patient has a preexisting disorder causing the thrombocytopenia. Preexisting thrombocytopenia may signify an unrecognized marrow disorder, such as myelodysplasia or a lymphoproliferative disorder, in which case concomitant anemia would be likely. Thrombocytopenia may represent splenic sequestration due to portal hypertension, usually in the setting of cirrhosis, in which case mild leukopenia may also be present. Alternatively, the patient may have chronic ITP, in which case the other counts should be normal. These conditions will persist during the critical illness, although transient pseudocorrection of the platelet count may occur later with the reactive rise in platelet count when the acute phase response sets in.
The illness that precipitated ICU admission must also be fully characterized. Although the critical illness is usually obvious, patients may become acutely ill and require transfer to the ICU before a diagnosis has been established. One must consider whether the critical illness is one of those catastrophic multisystem disorders in which platelets are directly involved, such as an aggressive myeloid or lymphoid neoplasm, HPS, or TTP. Significant platelet consumption may be expected when the admission was precipitated by polytrauma or major surgery. Massive transfusion or large-volume fluid resuscitation resulting in hemodilution may also contribute to a transient fall in platelet count. An underlying preexisting condition such as cancer or a complicated pregnancy may predispose to DIC and consequent thrombocytopenia.
Present
The trajectory of a patient’s platelet count during the ICU stay (and pre-ICU hospitalization) allows inferences about likely causes and may inform prognosis. The platelet trajectory can only be interpreted, however, with reference to the course of the patient’s critical illness. The earliest blood test results (eg, from the emergency room or hospital from which the patient was transferred) should be obtained to capture the complete platelet profile.
Though neither rigid nor comprehensive, we believe several distinct trajectories may be recognized:
Platelets are low at presentation and stay low, without apparent relation to the course of critical illness: This pattern suggests an independent cause of thrombocytopenia, such as marrow failure or hypersplenism. Investigation directed at those possibilities could include assessment of spleen size, review of the peripheral blood film, and bone marrow examination.
Platelets fall immediately and recover quickly: In the absence of a major operative complication, this pattern is expected after major surgery, especially those procedures using cardiopulmonary bypass, and can be observed in patients requiring a massive transfusion.
Platelets fall within the first few days and recover after the critical illness improves: This pattern is typical of sepsis and similar non-infection-related multisystem inflammatory illness (eg, pancreatitis, burns). In septic shock, the median time from ICU admission to the development of thrombocytopenia (<100 × 109/L) is 2 days (interquartile range [IQR], 1-3). The median duration of thrombocytopenia in survivors is 6 days (IQR, 4-8).29
Platelets fall and stay low in a patient whose clinical course is otherwise recovering: In these patients, an iatrogenic cause of thrombocytopenia should be considered. In HIT, the signature drop in platelet count evolves over a day or 2 and occurs between 5 and 10 days after initial heparin exposure. Testing for HIT antibodies, cessation of all heparin exposure, and the use of alternative anticoagulants are recommended.11 Posttransfusion purpura and other drug-related immune thrombocytopenias would also follow this pattern but are far less common.
Platelets fall within the first few days and stay low in a patient with persistent multiorgan failure: This is the pattern observed in the sickest of patients who have the worst prognosis. Sepsis, DIC, HPS, and shock are possible. It is not clear to what extent the thrombocytopenia contributes to their poor outcomes, and in such patients the thrombocytopenia is probably significant mainly as an indicator of the severity of their illness.1
Apart from the temporal pattern of platelet counts, the severity of thrombocytopenia and the presence or absence of thrombosis are readily available clues that may help to determine the mechanism of thrombocytopenia and the outlook for the patient. Severe thrombocytopenia (<20 × 109/L) has a much more limited differential diagnosis than do milder degrees of thrombocytopenia. In the critical care patient, severe thrombocytopenia is most likely to reflect one of the following:
Profound bone marrow failure, due to either cytotoxic chemotherapy or an aggressive hematologic neoplasm. The absolute neutrophil and reticulocyte counts should also be severely depressed.
Overt DIC. Fibrinogen and other coagulation factors should be consumed and tests for d-dimer and fibrin degradation products should be strongly positive.
Antibody mediated, whether autoimmune (ITP), alloimmune (posttransfusion purpura), or drug-related immune. HIT is a notable exception, in which the typical platelet nadir is >20 × 109/L.11
Hemophagocytic syndrome.
The occurrence of thrombosis in the thrombocytopenic patient is a paradox, which should trigger consideration of HIT, antiphospholipid syndrome, and prothrombotic DIC (usually associated with cancer, and occasionally with sepsis) as possible diagnoses. Coagulation activation in the setting of depleted natural anticoagulants (eg, warfarin initiation, which depletes protein C faster than procoagulant factors, or after ischemic liver injury) may trigger a process of peripheral small-vessel thrombosis and gangrene despite thrombocytopenia and despite adequate arterial inflow.30
Future
By scrutinizing the clinical and laboratory features of the case, including the trajectory of the platelet count, one aims to arrive at a working diagnosis of the cause of thrombocytopenia. In most instances, treatment of the thrombocytopenia is not required, but establishing a diagnosis permits one to predict the future trajectory of the platelet count. If the count follows the projected course of recovery, unnecessary investigation can be avoided; conversely, timely investigation can be pursued if the platelet count deviates from the anticipated course.
Surgery or trauma: with major surgery, there is typically a modest, immediate drop in the platelet count due to consumption, hemodilution, or both. The count normally begins to rise 3 to 4 days after surgery and should continue to rise over the next 10 days to levels above baseline because of the thrombopoietic activities of IL-6 and other acute-phase cytokines; the same pattern is expected with polytrauma.31
Cytotoxic chemotherapy: with most cyclic chemotherapy regimens, the nadir platelet count typically occurs on days 12 to 18, or a few days longer with platinum drugs, with recovery proceeding briskly thereafter.32
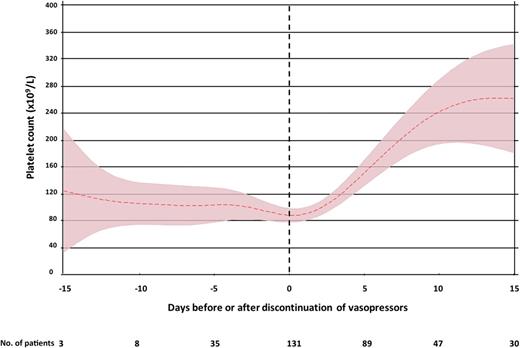
Septic shock: in patients with septic shock who develop thrombocytopenia, despite signs of clinical improvement, on average, platelet recovery is not anticipated until several days after vasopressor independence. The median time from discontinuation of vasopressors to recovery of platelet counts above 100 × 109 per liter is 2 days (IQR, 0-4) (Figure 2).29
Time course of thrombocytopenia in septic shock. Mean platelet count (±95% confidence interval) in patients with septic shock who developed thrombocytopenia after ICU admission. Time axis is anchored to the day that vasopressors were discontinued (day 0). Only data for survivors are included.29
Time course of thrombocytopenia in septic shock. Mean platelet count (±95% confidence interval) in patients with septic shock who developed thrombocytopenia after ICU admission. Time axis is anchored to the day that vasopressors were discontinued (day 0). Only data for survivors are included.29
Management of thrombocytopenia in critical illness
Given that incident (new) thrombocytopenia in the ICU is generally due to physiologic perturbation associated with increased severity of illness, treating the underlying cause of the critical illness is paramount and likely to result in improved platelet counts. If thrombocytopenia is due to sepsis, prompt and appropriate antimicrobial therapy, source control, and organ support are required.33 If the thrombocytopenia is due to severe bleeding (eg, trauma), hemostatic control is the priority. In the presence of mechanical circulatory support devices, such as intra-aortic balloon pumps, device-related thrombocytopenia may persist and may be further influenced by underlying cardiogenic shock and multiorgan failure. Although uncertainty exists whether extracorporeal membrane oxygenation (ECMO) is independently associated with thrombocytopenia, the conditions associated with the need for ECMO certainly are.34 Until these underlying conditions improve, resolution of platelet counts is not anticipated.
Thrombocytopenia is consistently associated with increased risks of bleeding, transfusion, and death; however, the effectiveness of platelet transfusion to stop bleeding, reduce transfusion of other blood components, or improve clinical outcomes is uncertain.1,3,35 The need for evidence addressing this uncertainty is especially relevant in the ICU. Past practices of liberally correcting abnormal laboratory values (eg, hemoglobin, albumin) or aggressively supporting deranged physiologic parameters that are recognized to be associated with poor prognosis (eg, supranormal oxygen therapy or high tidal volume mechanical ventilation) have either failed to benefit or harmed patients when evaluated in randomized trials. Platelet transfusion may be associated with harm, including increased incidence of nosocomial infection and transfusion-associated acute lung injury.36-38 Randomized trials of transfusion strategies to treat or prevent bleeding in thrombocytopenic ICU patients have not been conducted, and a conservative approach seems warranted.
Therapeutic platelet transfusion
In the context of massive transfusion, treatment of bleeding with a 1:1:1 or a 1:2:1 ratio of red cells, plasma, and platelets is supported by clinical studies and widely recommended in clinical guidelines to prevent severe thrombocytopenia or hemodilution of coagulation factors. The optimal ratio of platelet concentrates in this setting remains unknown.39 Caring for patients requiring massive transfusion is complex; comprehensive transfusion protocols can help achieve transfusion goals and improve outcomes.40 In general medical-surgical ICU patients with severe bleeding and, in the absence of informative clinical trials, current guidelines recommend that platelet transfusion be considered if the platelet count is <50 × 109/L or if significant platelet dysfunction is suspected or confirmed (Table 1).38 Platelet dysfunction is relatively common in the ICU and may be aggravated by factors including uremia, liver failure, antiplatelet agents, and extracorporeal circuits. The extent to which platelet transfusion can overcome these effects is unclear.
| Indication . | Platelet threshold* . | Strength of recommendation . | Quality of evidence . |
|---|---|---|---|
| Severe bleeding | Maintain PLT > 50 × 109/L; consider using an MTP | Strong | Low |
| Prophylaxis in adults | 10 × 109/L | Moderate | Low |
| Prior to elective central venous catheter | 20 × 109/L† | Weak | Low |
| Prior to chest tube insertion or thoracentesis | 50 × 109/L | Weak | Low |
| Prior to bronchoscopy with lavage | 20 × 109/L | Weak | Low |
| Prior to paracentesis | Not routinely required | Weak | Low |
| Prior to bone marrow biopsy | Not routinely required | Weak | Low |
| Prior to elective diagnostic lumbar puncture | 50 × 109/L | Weak | Very low |
| Prior to urgent diagnostic lumbar puncture | 20 × 109/L | Weak | Very low |
| Prior to major elective surgery (excluding neurosurgery) | 50 × 109/L | Weak | Very low |
| Prior to neurosurgery | 100 × 109/L | Weak | Very low |
| Traumatic brain injury, intracranial hemorrhage | 100 × 109/L | Weak | Low |
| Prior to insertion of an intraventricular drain | 100 × 109/L | Weak | Very low |
| Indication . | Platelet threshold* . | Strength of recommendation . | Quality of evidence . |
|---|---|---|---|
| Severe bleeding | Maintain PLT > 50 × 109/L; consider using an MTP | Strong | Low |
| Prophylaxis in adults | 10 × 109/L | Moderate | Low |
| Prior to elective central venous catheter | 20 × 109/L† | Weak | Low |
| Prior to chest tube insertion or thoracentesis | 50 × 109/L | Weak | Low |
| Prior to bronchoscopy with lavage | 20 × 109/L | Weak | Low |
| Prior to paracentesis | Not routinely required | Weak | Low |
| Prior to bone marrow biopsy | Not routinely required | Weak | Low |
| Prior to elective diagnostic lumbar puncture | 50 × 109/L | Weak | Very low |
| Prior to urgent diagnostic lumbar puncture | 20 × 109/L | Weak | Very low |
| Prior to major elective surgery (excluding neurosurgery) | 50 × 109/L | Weak | Very low |
| Prior to neurosurgery | 100 × 109/L | Weak | Very low |
| Traumatic brain injury, intracranial hemorrhage | 100 × 109/L | Weak | Low |
| Prior to insertion of an intraventricular drain | 100 × 109/L | Weak | Very low |
MTP, massive transfusion protocol; PLT, platelet count.
May be modified by several factors including platelet dysfunction or other risk factors for bleeding, indication for the procedure, urgency, and medical comorbidities.
Inserted with bedside ultrasound and by experienced personnel. Avoid noncompressible vessels if platelets are <50 × 109 per liter. Choosing a compressible site is preferable to platelet transfusion.
Prophylactic platelet transfusion
Most platelet units in an ICU are transfused to prevent clinical bleeding. Clinical trials to guide best practice are needed, and considerable practice variability exists. Extrapolating from randomized trials evaluating prophylactic platelet transfusion in hospitalized adult patients with chemotherapy-induced hypoproliferative thrombocytopenia, the American Association of Blood Banks (AABB) and the Society for Critical Care Medicine (SCCM) 2016 Surviving Sepsis Guideline recommends prophylactic platelet transfusion below a threshold of 10 × 109/L.33,38 This extrapolation, however, may not be valid; in patients admitted to medical-surgical ICUs, thrombocytopenia is frequently multifactorial and may be accompanied by acquired platelet dysfunction but also increased platelet turnover. Furthermore, posttransfusion platelet increments and the durability of platelet increases are known to be attenuated in ICU patients. In observational studies, the median increase in platelet count per platelet transfusion was 15 × 109/L.41 It should also be recognized that major hemorrhage is a recognized consequence of critical illness with multiple organ failure and can occur at any platelet count.
Periprocedural platelet transfusion
Platelet transfusion is recommended to minimize bleeding during placement of an elective central venous catheter if the platelet is <20 × 10/L38,42 (Table 1). Data to support this recommendation come from observational studies, of which a subset of patients were admitted to an ICU. In 2 large observational studies, only patients with a preprocedural platelet count of <20 or 25 × 109/L were at risk for bleeding.43,44 Central venous catheters should ideally be placed under ultrasound guidance and by expert operators to reduce the risk of bleeding. With the use of bedside ultrasound and clear vascular windows, the incremental benefit of platelet transfusion is uncertain. In absence of informative trials, practice variability exists. Recommended platelet thresholds for lumbar puncture and surgical procedures are itemized in Table 1.
Conclusion
Thrombocytopenia in the ICU is common, especially among those with septic shock, and correlates with an adverse prognosis. Multiple mechanisms may contribute to thrombocytopenia, and differentiating the pertinent cause (or causes) in individual patients is challenging. Looking back at the patient’s medical history and presenting illness, while observing the platelet trajectory and the clinical course, offers clues to the diagnosis and prognosis. Pragmatic guidelines are presented; however, optimal platelet transfusion strategies to prevent or treat bleeding in thrombocytopenic ICU patients remain to be defined.
Causes of thrombocytopenia in the ICU
Common causes of thrombocytopenia in ICU patients:
Sepsis
Disseminated intravascular coagulation
Consumption (eg, major trauma, cardiopulmonary bypass)
Dilution (with massive transfusion)
Myelosuppressive chemotherapy
Mechanical circulatory support devices (eg, intra-aortic balloon pump)
Less common but important causes of thrombocytopenia that should not be missed:
Heparin-induced thrombocytopenia
Hemophagocytic syndrome
Uncommon causes of thrombocytopenia that develop during ICU admission:
Drug-induced thrombocytopenia (other than heparin or cytotoxic chemotherapy)
Leukemia, myelodysplasia, aplastic anemia, etc, unless abnormalities were already present before ICU admission
Thrombotic thrombocytopenic purpura
Immune/idiopathic thrombocytopenia
Posttransfusion purpura
Questions to ask when evaluating thrombocytopenia in the ICU patient
Past:
What is the context of the patient’s ICU admission?
Is there evidence of a preexisting illness or the use of a drug known to cause thrombocytopenia?
Could the ICU admission have been precipitated by a catastrophic illness associated with thrombocytopenia, such as thrombotic thrombocytopenic purpura, hemophagocytic syndrome, or acute leukemia?
Was there major trauma or surgery that would consume platelets, or transfusion and fluid resuscitation that would cause dilution?
Present:
What is the trajectory of the platelet count, and how does it relate to the patient’s clinical course?
How low is the platelet count?
Is there thrombosis?
Future:
Is the platelet count following the expected trajectory, given your analysis of the cause?
Correspondence
Ryan Zarychanski, Department of Medical Oncology and Haematology, Cancercare Manitoba, ON2051, 675 McDermot Ave, Winnipeg, MB R3E 0V9, Canada; e-mail: rzarychanski@cancercare.mb.ca.
References
Competing Interests
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.