Abstract
Thrombotic microangiopathies (TMAs), specifically, thrombotic thrombocytopenic purpura (TTP) and complement-mediated hemolytic uremic syndrome (CM-HUS) are acute life-threatening disorders that require prompt consideration, diagnosis, and treatment to improve the high inherent mortality and morbidity. Presentation is with microangiopathic hemolytic anemia and thrombocytopenia (MAHAT) and variable organ symptoms resulting from microvascular thrombi. Neurological and cardiac involvement is most common in TTP and associated with poorer prognosis and primarily renal involvement in CM-HUS. TTP is confirmed by severe ADAMTS13 deficiency (which can be undertaken in real time) and CM-HUS by an abnormality in complement regulators, confirmed by mutational analysis (in 60% to 70% of cases) or the presence of Factor H antibodies (which may not be available for weeks or months). Plasma exchange (PEX) should be started as soon as possible following consideration of these TMAs. Differentiation of the diagnosis requires specific treatment pathways thereafter (immunosuppression primarily for TTP and complement inhibitor therapy for CM-HUS). As the diagnosis is based on MAHAT, there are a number of other medical situations that need to be excluded and these are discussed within the article. Other differentials presenting as TMAs may also be associated with micro- or macrovascular thrombosis, yet are more likely to be due to direct endothelial damage, many of which do not have a clear therapeutic benefit with PEX.
Learning Objectives
Understand that the presentation of thrombocytopenia, with anemia, requires exclusion of TTP or CM-HUS, by reviewing a blood film and undertaking hemolytic parameters
Understand other causes of TMAs are more likely the result of direct endothelial damage, but may need to be excluded following presentation with MAHAT, for example, DIC, autoimmune disease, infection, or drugs
Thrombotic microangiopathy (TMA) can be defined at a histopathological level or as a clinical syndrome. Histologically, there is thrombus formation affecting small or larger vessels. Thrombi vary in their constituents depending on the underlying cause of the TMA. For example, the thrombi may contain fibrin-platelets (in disseminated intravascular coagulation [DIC] or heparin-induced thrombocytopenia [HIT]), endothelia cells with inflammatory components (as in autoimmune conditions such as lupus or scleroderma), and cancer cells (in malignancy-associated TMA). Von Willebrand factor (VWF)-platelet thrombi are specific in thrombotic thrombocytopenic purpura (TTP) and endothelia damage with fibrinoid necrosis in hemolytic uremic syndrome (HUS). Both HUS and TTP are not typically associated with an inflammatory histological component.
Clinically, TMA is defined by microangiopathic hemolytic anemia and thrombocytopenia (MAHAT). Specifically, there is anemia and a reduced platelet count, with fragmentation and often polychromasia on the blood film. Confirmation of an underlying hemolytic process includes an elevated lactate dehydrogenase (LDH), reticulocytosis, and a low/absent haptoglobin. A direct antiglobulin test should be negative.1
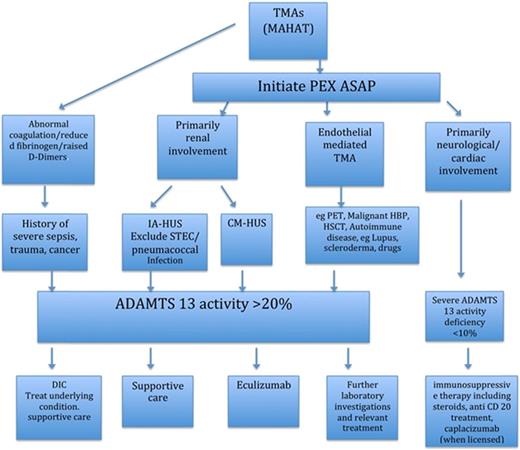
It is important to differentiate TTP and HUS in patients presenting with MAHAT as quickly as possible, as initiation of disease-specific treatment has a critical impact on outcome (Figure 1).2
Summary of diagnosis and treatment of TMAs. ASAP, as soon as possible; PET, pre-eclampsia toaxemia.
Summary of diagnosis and treatment of TMAs. ASAP, as soon as possible; PET, pre-eclampsia toaxemia.
TTP and HUS
This includes congenital and immune-mediated TTP (cTTP and iTTP) and infection-associated or complement-mediated HUS (IA-HUS and CM-HUS). Without treatment, mortality in TTP is 90%. The majority of cases are acquired and immune mediated, and presenting symptoms associated with MAHAT include neurology (such as stroke, transient ischemic attacks, migraines, and seizures), renal impairment, abdominal pain, and cardiac involvement. Cardiac TTP is often less obvious clinically and defined by a raised troponin at presentation.3 A diagnosis of TTP is confirmed by a severe deficiency in ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), the metalloprotease specifically associated with a diagnosis of TTP.4
IA-HUS has typically been associated with Shiga toxin-producing Escherichia coli (STEC), typically serotype O157 but also other non-O157 serotypes, as well as Shiga toxin-secreting strains, including Shigella and Campylobacter. It results from ingestion of infected water or food, in animals or person to person. The median incubation period is 3 to 4 days. The toxin acts on vascular endothelium, specifically binding to glycolipid glotriaosylceramide (Gb3) receptors, particularly in the glomerulus of the kidney and, therefore, associated with acute kidney injury. Cases may present with bloody diarrhea, but the TMA usually presents 4 to 7 days after this has resolved. Children are more commonly affected with a mortality of 5% to 10%.5 A small percentage of cases of IA-HUS are caused by Streptococcus pneumoniae, which are more likely to be associated with pneumonia and may lead to multi-organ failure. CM-HUS presents with similar laboratory features as IA-HUS and may have diarrhea, but not necessarily bloody. Clinically, an infectious precipitant may need excluding. A viral or bacterial trigger or vaccination may be associated with presentation. However, dysregulation of complement is the underlying defect, although identification of a genetic or acquired abnormality can only be confirmed in 60% to 70% of cases. Mortality has been up to 25% with high rates of end-stage renal failure within 1 year.6
Differential diagnosis of TMA
Despite the urgency of diagnosis and treatment of TTP and HUS (Table 1), there are a number of medical conditions that may present with TMA. The most common is DIC.7 This can be precipitated by severe sepsis or trauma, for example. Monitoring the laboratory parameters within scoring systems for DIC correlates with clinical features and outcomes. In particular, thrombocytopenia, which is often the first and most sensitive sign of DIC, is present in >90% of cases with 50% of cases having a platelet count <50 × 109/L. A reduced platelet count is associated with increased thrombin formation and amplified fibrinlytic activity, associated with raised D-Dimers. There is variability in derangement of the coagulation screen in 60% to 70% of cases (PT [prothrombin time] and/or APTT [activated partial thromboplastin time] may be increased, but they may be normal or indeed shortened). Fibrinogen may be reduced, but it is less commonly below the normal laboratory range unless there is very severe DIC. The sequential changes in these parameters may be more helpful in confirming a diagnosis of DIC.8 The International Society of Thrombosis and Haemostasis scoring system for DIC is >90% sensitive and specific and predictive of mortality. This was confirmed comparing other scoring systems.9 A normal coagulation screen, thrombocytopenia, raised D-Dimers, and fragmentation on a blood film require exclusion of DIC from other TMAs.
Summary of the differential diagnosis and treatment of thrombotic microangiopathies
| Condition . | Comments . | Summary of treatment . |
|---|---|---|
| TTP | Diagnosis confirmed by severe ADAMTS13 deficiency | PEX, immune suppression including rituximab (or CD20 monoclonal antibody therapy) |
| Immune mediated | Presence of antibodies to ADAMTS13 | Newer therapies under investigation include nanobodies and recombinant protein |
| Secondary to an underlying precipitant | Antibody mediated, but precipitating event identified on investigation | Congenital TTP requires replacement of missing enzyme, eg, with plasma infusion |
| Congenital TTP | Typically in pregnancy, but infection/neonatal period may be associated with younger diagnoses | In secondary TTP, requires treatment of underlying condition as well as TTP therapy |
| HUS | Diagnosis of exclusion Genetic analysis may confirm complement mutations | STEC/IA-HUS: confirm by serology/PCR |
| Complement mediated | Exclude STEC- and non-STEC-causing HUS presenting up to 7 days following hemorrhagic colitis, confirm by serology/PCR but also pneumococcus, HIV and viral associated | Pneumococcus: supportive treatment including antibiotics and washed blood products |
| Infection associated | Cobalamin C (Cbl-C) deficiency is a rare cause in neonates/childhood associated with | B12 and folic acid for Cbl-C deficiency |
| Secondary HUS | Eculizumab for CM-HUS | |
| DIC | Thrombocytopenia is often the initial feature Coagulation abnormalities are variable, but associated low fibrinogen suggests severity | Treat underlying precipitant Supportive blood products for active bleeding (anticoagulation if thrombosis) |
| Sepsis; malignancy; trauma; hematological disorders, eg, acute myeloid leukemia; obstetric complications | ||
| B12 deficiency | Anemia, reticulocytosis, and thrombocytopenia, but typical MAHA features not present | Careful review of blood film |
| Vitamin B12 replacement | ||
| Cancer | TMA maybe the initial feature of a cancer presentation or in relation to drugs required to treat active cancer | No role for PEX |
| May represent bone involvement | Treat underlying disease | |
| Usually adenocarcinomas or hematological malignancies | No contraindication to platelet transfusions in thrombocytopenia | |
| TA-TMA | Results from endothelia cell damage, from underlying conditioning therapies, immunosuppressives, or complications relating to the transplant, eg, graft-versus-host disease | No role for PEX |
| Symptomatic therapy | ||
| Reduce or stop offending drugs | ||
| Question role for complement inhibitor therapy | ||
| DA-TMA | A number of drugs very rarely associated with antibody-mediated TTP | No proven role for PEX, unless antibody-mediated ADAMTS13 deficiency confirmed |
| Chemotherapy and newer specific treatments, eg, vascular endothelial growth factor inhibitors, associated with an HUS-type picture | Question role for complement inhibitor therapy | |
| Underlying pathophysiology may be varied | ||
| Autoimmune disease/vasculitis | A variety of autoimmune/vasculitic conditions may present as a trigger to TTP/HUS or may have MAHAT features, but with normal ADAMTS13 levels | PEX if associated with low (<10%) ADAMTS13 activity levels |
| Relevant immunology investigations +/− biopsy will confirm | Immunosuppressive therapy depending on results of further investigations | |
| Question role of complement inhibitor therapy, eg, in anti-phospholipid syndrome | ||
| Infections | May precipitate TTP or HUS | Supportive care |
| Viral-, bacterial-, or atypical/fungal-associated damage to endothelia cells | Treat underlying infection following confirmation by serology, culture, or PCR | |
| Malignant hypertension | May be primary or in association with a specific disorder, eg, IgA nephropathy | Symptomatic treatment |
| Cannot reliably differentiate from CM-HUS, especially if normal renal size on radiological examination and no chronic features of high blood pressure, eg, on ophthalmic examination | If pathogenesis is unclear, consider a trial of complement inhibitor therapy | |
| Renal biopsy if possible to help differentiate | ||
| Pregnancy | ||
| TMA precipitated by pregnancy | TTP or CM-HUS | PEX and complement inhibitor therapy if CM-HUS |
| Pregnancy-associated TMA | PET/HELLP: delivery usually helps resolution, but with progressive symptoms/worsening laboratory parameters, consider TTP or HUS | Control blood pressure, deliver baby if severe |
| Condition . | Comments . | Summary of treatment . |
|---|---|---|
| TTP | Diagnosis confirmed by severe ADAMTS13 deficiency | PEX, immune suppression including rituximab (or CD20 monoclonal antibody therapy) |
| Immune mediated | Presence of antibodies to ADAMTS13 | Newer therapies under investigation include nanobodies and recombinant protein |
| Secondary to an underlying precipitant | Antibody mediated, but precipitating event identified on investigation | Congenital TTP requires replacement of missing enzyme, eg, with plasma infusion |
| Congenital TTP | Typically in pregnancy, but infection/neonatal period may be associated with younger diagnoses | In secondary TTP, requires treatment of underlying condition as well as TTP therapy |
| HUS | Diagnosis of exclusion Genetic analysis may confirm complement mutations | STEC/IA-HUS: confirm by serology/PCR |
| Complement mediated | Exclude STEC- and non-STEC-causing HUS presenting up to 7 days following hemorrhagic colitis, confirm by serology/PCR but also pneumococcus, HIV and viral associated | Pneumococcus: supportive treatment including antibiotics and washed blood products |
| Infection associated | Cobalamin C (Cbl-C) deficiency is a rare cause in neonates/childhood associated with | B12 and folic acid for Cbl-C deficiency |
| Secondary HUS | Eculizumab for CM-HUS | |
| DIC | Thrombocytopenia is often the initial feature Coagulation abnormalities are variable, but associated low fibrinogen suggests severity | Treat underlying precipitant Supportive blood products for active bleeding (anticoagulation if thrombosis) |
| Sepsis; malignancy; trauma; hematological disorders, eg, acute myeloid leukemia; obstetric complications | ||
| B12 deficiency | Anemia, reticulocytosis, and thrombocytopenia, but typical MAHA features not present | Careful review of blood film |
| Vitamin B12 replacement | ||
| Cancer | TMA maybe the initial feature of a cancer presentation or in relation to drugs required to treat active cancer | No role for PEX |
| May represent bone involvement | Treat underlying disease | |
| Usually adenocarcinomas or hematological malignancies | No contraindication to platelet transfusions in thrombocytopenia | |
| TA-TMA | Results from endothelia cell damage, from underlying conditioning therapies, immunosuppressives, or complications relating to the transplant, eg, graft-versus-host disease | No role for PEX |
| Symptomatic therapy | ||
| Reduce or stop offending drugs | ||
| Question role for complement inhibitor therapy | ||
| DA-TMA | A number of drugs very rarely associated with antibody-mediated TTP | No proven role for PEX, unless antibody-mediated ADAMTS13 deficiency confirmed |
| Chemotherapy and newer specific treatments, eg, vascular endothelial growth factor inhibitors, associated with an HUS-type picture | Question role for complement inhibitor therapy | |
| Underlying pathophysiology may be varied | ||
| Autoimmune disease/vasculitis | A variety of autoimmune/vasculitic conditions may present as a trigger to TTP/HUS or may have MAHAT features, but with normal ADAMTS13 levels | PEX if associated with low (<10%) ADAMTS13 activity levels |
| Relevant immunology investigations +/− biopsy will confirm | Immunosuppressive therapy depending on results of further investigations | |
| Question role of complement inhibitor therapy, eg, in anti-phospholipid syndrome | ||
| Infections | May precipitate TTP or HUS | Supportive care |
| Viral-, bacterial-, or atypical/fungal-associated damage to endothelia cells | Treat underlying infection following confirmation by serology, culture, or PCR | |
| Malignant hypertension | May be primary or in association with a specific disorder, eg, IgA nephropathy | Symptomatic treatment |
| Cannot reliably differentiate from CM-HUS, especially if normal renal size on radiological examination and no chronic features of high blood pressure, eg, on ophthalmic examination | If pathogenesis is unclear, consider a trial of complement inhibitor therapy | |
| Renal biopsy if possible to help differentiate | ||
| Pregnancy | ||
| TMA precipitated by pregnancy | TTP or CM-HUS | PEX and complement inhibitor therapy if CM-HUS |
| Pregnancy-associated TMA | PET/HELLP: delivery usually helps resolution, but with progressive symptoms/worsening laboratory parameters, consider TTP or HUS | Control blood pressure, deliver baby if severe |
An uncommon but eminently treatable cause of TMA is vitamin deficiency, in particular severe B12 deficiency. While there is anemia, raised reticulocytes, and thrombocytopenia, the blood film findings are not specifically in keeping with MAHA. The laboratory parameters at presentation may be identical to TTP, and plasma exchange (PEX) has been initiated in some cases.10 PEX can be stopped and patients respond promptly to B12 injections. Further follow-up to exclude pernicious anemia with intrinsic factor antibodies or celiac disease is suggested. Therefore, it is proposed that B12 and folate levels are undertaken as part of routine screening samples for acute TMAs.
TMA may be the presenting feature of an underlying cancer, either in undiagnosed disease or in association with metastases. Furthermore, chemotherapeutic medications have been associated with TMA, characterizing a drug-associated HUS (DA-HUS). One of the largest reviews of cancer-associated microangiopathy confirms the majority of cases are solid tumor malignancies, but hematological cancers such as lymphoma make up approximately 8% of all cases. Gastric, lung, breast, and prostate, primarily adenocarcinoma, are the most likely diagnoses. Respiratory symptoms were more commonly featured (not normally seen in TTP or HUS), with an increase in DIC7 noted. Treatment with antitumor therapy was associated with improved survival, and there was no role for plasma therapy.11 Distinguishing cancer-associated TMA from TTP may be difficult initially, but the former is more likely to have respiratory symptoms (70% of cases) and bone pain at presentation and have an inadequate response to PEX.12 The TMA picture is the result of microthrombi, which may include tumor-associated thrombi or bone marrow involvement by the underlying cancer. Review of the peripheral blood smear and an early bone marrow aspirate and trephine may help expedite the underlying cancer diagnosis.
Diagnosis is important, as there is no beneficial role for PEX, steroids, or other immunosuppressive used in TTP; but, the use of platelet transfusions for severe thrombocytopenia, normally withheld in TTP, would be appropriate. An ADAMTS13 activity level not demonstrating severe deficiency should point to consideration of another TMA, including cancer.13 A difficult scenario is the differential among cancer/chemotherapy/CM-HUS, none of which is associated with severe ADAMTS13 deficiency. Furthermore, chemotherapeutic medications have been associated with TMA, typically a DA-HUS picture. There is no current evidence for the use of eculizumab in malignancy-associated TMA. However, there have been case reports/small series demonstrating a possible beneficial effect of complement inhibitor therapy for chemotherapeutic agents causing a TMA.14
Transplant-associated TMA (TA-TMA) may affect either solid organ or hematopoietic stem cell transplant (HSCT) patients. The underlying pathology is endothelial damage,15 and it may be the result of the prior conditioning therapy, HLA-mismatched transplants, and calceneurin inhibitors used to prevent rejection. It is important to consider an additional underlying infection such as adenovirus; indeed, nearly 50% of patients at post mortem relating to HSCT had evidence of viremia, with some cases only diagnosed at post mortem.16 The effect of systemic viral infections on already damaged endothelia, from treatment or complications of the transplant, may lead to laboratory features of a TMA.
PEX is not of benefit and treatment is based on symptomatic support. Mortality is raised in such scenarios, and parameters associated with increased mortality include proteinuria, raised LDH, and hypertension.17 More recently, inherited or acquired defect in complement have been identified and thought to compound the endothelial damage.18 The use of complement inhibitor therapy has been associated with positive results in some cases/small series.14,19,20
Drugs are a rare but important cause of TMA (Table 2).21 The most definitive cause of DA-TTP was with ticlopodine.22 DA-TMAs maybe idiosyncratic (eg, quinine) or dose dependent (eg, IFN).23 In many cases, the clinical presentation primarily involves severe hypertension with principally a renal element in conjunction with MAHAT. Intravenous abuse of Opana ER (oxymorphone hydrochloride) presents with TMA associated with acute renal injury as well as cardiac and retinal ischemia.24 Other drug-related causes for TMA include chemotherapies21,25 and therapies used in nonmalignant conditions.26,27
Drugs associated with thrombotic microangiopathy
| Drug . | Possible pathogenesis and treatment . |
|---|---|
| Ticlodopine | Specific to ticlodopine and not other thienopyridine derivatives Association with ADAMTS13 antibodies and respond to PEX |
| Estrogen-containing drugs, eg, COCP | Precipitation of congenital TTP Association with immune TTP cases |
| Quinine | Antibodies against platelets, leukocytes, erythrocytes, and endothelial cells, leading to damage to endothelial cells Question role of PEX |
| Gemcitabine | Dose-dependent endothelial damage, affecting primarily the glomerular arterioles and capillaries Response recorded to eculizumab |
| Mitomycin C | Delayed onset, dose-dependent toxicity, and cumulative Microthrombi affect glomerular capillaries and arterioles Cumulative dose results in irreversible renal damage and no response to PEX |
| VEGF inhibitors, eg, bevacizumab and aflibercept | TMA features limited to the glomerular structures differentiate anti-VEGF-induced thrombotic microangiopathy from other drug causes Supportive care and drug discontinuation |
| Proteosome inhibitors, eg, carfilzomib and bortezomib | Occurs at a median of 3 weeks following medication initiation Favorable response to stopping therapy |
| Thyrosine kinase inhibitors, eg, carfilzomib and bortezomib | Small molecule inhibition of the VEGF receptor Supportive care and drug discontinuation |
| Interferon-β | Direct dose-dependent effect causing endothelial hyperplasia, luminal occlusion, and microaneurysm formation Very delayed (years after initiation) Variable recovery |
| Calcineurin inhibitors, eg, ciclosporin and tacrolimus | Primarily affect glomerular arterioles Reducing the dose/stopping the drug can improve/reverse the TMA |
| Platinum-based drugs, eg, oxaliplatin | Anemia and thrombocytopenia could result from oxaliplatin-dependent antibodies against erythrocytes and platelets |
| Emicizumab (in conjunction with bypassing agents) | Unknown, question excess thrombin resulting in endothelial damage Stop drug, symptomatic symptom control PEX used Drug has been reintroduced on resolution |
| Drug . | Possible pathogenesis and treatment . |
|---|---|
| Ticlodopine | Specific to ticlodopine and not other thienopyridine derivatives Association with ADAMTS13 antibodies and respond to PEX |
| Estrogen-containing drugs, eg, COCP | Precipitation of congenital TTP Association with immune TTP cases |
| Quinine | Antibodies against platelets, leukocytes, erythrocytes, and endothelial cells, leading to damage to endothelial cells Question role of PEX |
| Gemcitabine | Dose-dependent endothelial damage, affecting primarily the glomerular arterioles and capillaries Response recorded to eculizumab |
| Mitomycin C | Delayed onset, dose-dependent toxicity, and cumulative Microthrombi affect glomerular capillaries and arterioles Cumulative dose results in irreversible renal damage and no response to PEX |
| VEGF inhibitors, eg, bevacizumab and aflibercept | TMA features limited to the glomerular structures differentiate anti-VEGF-induced thrombotic microangiopathy from other drug causes Supportive care and drug discontinuation |
| Proteosome inhibitors, eg, carfilzomib and bortezomib | Occurs at a median of 3 weeks following medication initiation Favorable response to stopping therapy |
| Thyrosine kinase inhibitors, eg, carfilzomib and bortezomib | Small molecule inhibition of the VEGF receptor Supportive care and drug discontinuation |
| Interferon-β | Direct dose-dependent effect causing endothelial hyperplasia, luminal occlusion, and microaneurysm formation Very delayed (years after initiation) Variable recovery |
| Calcineurin inhibitors, eg, ciclosporin and tacrolimus | Primarily affect glomerular arterioles Reducing the dose/stopping the drug can improve/reverse the TMA |
| Platinum-based drugs, eg, oxaliplatin | Anemia and thrombocytopenia could result from oxaliplatin-dependent antibodies against erythrocytes and platelets |
| Emicizumab (in conjunction with bypassing agents) | Unknown, question excess thrombin resulting in endothelial damage Stop drug, symptomatic symptom control PEX used Drug has been reintroduced on resolution |
Included are some of the more common drugs documented in association with causing TMA. Most are associated with acute kidney injury and usually present with severe hypertension.
Other than stopping the offending medication, therapy remains difficult. PEX is often undertaken but of uncertain utility, as only a small proportion are associated with antibodies to ADAMTS13.28 The pathophysiology of DA-TMAs is varied and can be due to a direct effect on endothelial cells that may be seen with an accumulation of drug dosing, such as with gemcitabine. Alternative mechanisms include antibodies, for example, to red cells or platelets, such as with oxaliplatin.29 Drugs targeting specific proteins, such as vascular endothelial growth factor (VEGF) inhibitors, cause hypertension and microthrombi formation exclusive to glomerular capillaries.25,30
Connective tissue diseases (CTDs) may present with a TMA. Acute scleroderma is differentiated by clinical features and may present with or without severe renal involvement, mimicking TTP or HUS.31
Other conditions include systemic lupus erythematosus (SLE), antiphospholipid syndrome, or vasculitides, such as lupus nephritis or Goodpasture’s syndrome; exclusion of an underlying connective tissue disease requires confirmation by relevant autoantibodies. Caution is required as there are a number of patients with TMAs who have a positive autoantibody screen, especially antinuclear antibody, but do not have SLE.32 Underlying autoimmune disease needs to be excluded for both TTP and HUS presentations.
Specific infections may be associated with a TMA presentation. HIV may present with TTP, severe ADAMTS13 deficiency, and an immune-mediated pathogenesis.33 With the advent of highly active antiretroviral therapies (HAARTs), the incidence of TMAs with a primary renal presentation is significantly decreased, and such presentations are more likely in patients with untreated or resistant HIV disease.34 Many other infections may present with a TMA picture, but they require a careful assessment of history, microbiology, and virology. Examples include Dengue fever,35 cytomegalovirus, and tuberculosis. Others like influenza may be associated with the precipitation of TTP or CM-HUS.36
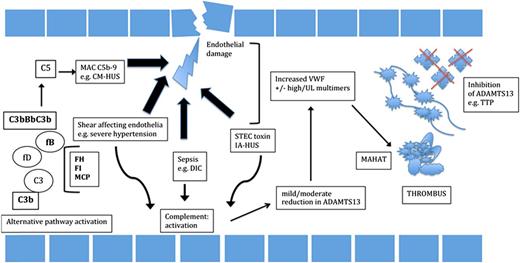
Malignant hypertension may mimic presentation of CM-HUS; indeed, it may be difficult to differentiate it from CM-HUS. Endothelial dysfunction may result in hypertension associated with CM-HUS. However, hypertension causing TMA is thought to result from high intra-arterial pressures causing endothelial damage and then secondary TMA, rather than complement overactivation causing endothelial injury and hypertension (Figure 2).37
Summary of the pathogenesis of TMAs. TTP is the result of a severe deficiency of ADAMTS13, due to an inherited genetic effect in congenital TTP or due to antibodies against ADAMTS13. The result is excess platelet binding to ultra large von Willebrand factor (UL VWF) and thrombi formation, made of these constituents. Triggering of complement (CM-HUS), due to abnormalities in CFH, CFI, MCP, C3, or Factor B, results in overactivation of the terminal complement pathway, the membrane attack complex (MAC), resulting in endothelial damage and thrombi composed of platelets. IA-HUS and resulting toxin, precipitants resulting in DIC and TMAs not associated with severe ADAMTS13 deficiency, cause direct endothelial damage. Resultant activation of complement, release of VWF (including high +/− some UL VWF multimers), causes a reduction in ADAMTS13 levels because of consumption of the enzyme. Thrombi formation is distinct from TTP and CM-HUS, containing a variable proportion of platelets, inflammatory cells, or fibrin. Resulting thrombi cause MAHAT seen clinically.
Summary of the pathogenesis of TMAs. TTP is the result of a severe deficiency of ADAMTS13, due to an inherited genetic effect in congenital TTP or due to antibodies against ADAMTS13. The result is excess platelet binding to ultra large von Willebrand factor (UL VWF) and thrombi formation, made of these constituents. Triggering of complement (CM-HUS), due to abnormalities in CFH, CFI, MCP, C3, or Factor B, results in overactivation of the terminal complement pathway, the membrane attack complex (MAC), resulting in endothelial damage and thrombi composed of platelets. IA-HUS and resulting toxin, precipitants resulting in DIC and TMAs not associated with severe ADAMTS13 deficiency, cause direct endothelial damage. Resultant activation of complement, release of VWF (including high +/− some UL VWF multimers), causes a reduction in ADAMTS13 levels because of consumption of the enzyme. Thrombi formation is distinct from TTP and CM-HUS, containing a variable proportion of platelets, inflammatory cells, or fibrin. Resulting thrombi cause MAHAT seen clinically.
Pregnancy-associated TMAs include specific conditions, eg, TTP or HUS precipitated by pregnancy, or TMAs particular to pregnancy, such as pre-eclampsia (PET), haemolysis, elevated liver enzymes, and low platelets, or acute fatty liver of pregnancy. Differentiating TTP or HUS during pregnancy may be difficult as both conditions can be associated with hypertension, proteinuria, and interuterine growth retardation. TTP can present at any stage during pregnancy, but most commonly it does so in the third trimester. Many cases have been associated with late-onset congenital TTP.38 CM-HUS is thought to be most common in the postpartum period. However, as our understanding of complement-mediated disorders increases, those identified during pregnancy, similar to cTTP, will undoubtedly increase also.39 Consideration for TTP and CM-HUS should be given in patients with thrombocytopenia, particularly with a platelet count <50 × 109/L or a decreasing platelet count. LDH is normal throughout pregnancy, and it is a useful parameter in the consideration of pregnancy-induced TMAs. The incidence of pregnancy-associated TMAs may be greater than originally anticipated. Indeed, 5% of all cases of pregnant women with a platelet count <75 × 109/L during pregnancy have been identified as congenital TTP.40 TMAs should be considered as a possible cause of intrauterine fetal death, particularly in the second trimester, and maternal blood counts should be checked. A role for complement in the pathogenesis of pregnancy-associated TMAs39 is suggested in some cases of severe PET41 and, to a lesser extent, HELLP.42,43
Baseline investigations in a patient with a TMA
There are a collective group of laboratory tests that should be considered in patients with a presentation of TMA. These help not only to elucidate the underlying cause but also to define secondary cases or associated precipitating factors (Table 3).
Summary of investigations to determine the degree of organ involvement and differential diagnosis of an acute TMA
| Investigation . | Tests . | Rationale . |
|---|---|---|
| Hemolytic screen | Hemoglobin, platelet, reticulocytes, LDH, blood film (DCT, haptoglobin) | Diagnosis of MAHAT |
| Renal function | K+, Ur, creatinine urine dipstick: protein/blood/leukocytes protein creatinine ratio | Degree of renal impairment |
| Liver function | Bilirubin | With hemolysis |
| AST/ALT | Question hepatic ischemia | |
| ALP | Liver obstruction | |
| Coagulation screen | PT, APTT, fibrinogen | Normal in TTP/CM-HUS, deranged in DIC |
| Pregnancy test | Urine or serum HCG | In all women of child-bearing age |
| Other biochemistry | Troponin | Degree of cardiac involvement |
| Thyroid function | Useful with pyrexia of TMA to exclude infection | |
| CRP | May be reduced C3 with CM-HUS, C4 in autoimmune disease, eg, SLE | |
| C3/C4 | Consider if possible cancer trigger in men | |
| PSA | ||
| Vitamins | B12/folate | Treat deficiencies, folate may be reduced with haemolysis |
| Virology | Hepatitis A/B/C | Baseline investigations especially pre-PEX |
| HIV +/−viral load | eg, in HSCT | |
| Further virology screen relevant to history, eg, adenovirus, CMV | ||
| Autoimmune screen | ANA/dsDNA | Underlying autoimmune disease |
| RF | ||
| ENA | ||
| ACLA/LA | ||
| Vasculitis screen (if relevant from history) | Anti-GBM antibodies | To exclude Goodpasture’s/underlying vasculitis trigger |
| ANCA | ||
| Microbiology | BCs STEC serology/PCR from stool | With temperature |
| Radiology | USS abdomen | Kidneys’ size, obstruction, lymphadenopathy, organomegaly |
| MRI head | Ischemic changes, infarcts, PRES | |
| CT chest/abdomen/pelvis | If exclude an underlying cancer | |
| Cardiac investigations | ECG echocardiogram | Baseline for cardiac function/conduction defects or ischemia |
| Investigation . | Tests . | Rationale . |
|---|---|---|
| Hemolytic screen | Hemoglobin, platelet, reticulocytes, LDH, blood film (DCT, haptoglobin) | Diagnosis of MAHAT |
| Renal function | K+, Ur, creatinine urine dipstick: protein/blood/leukocytes protein creatinine ratio | Degree of renal impairment |
| Liver function | Bilirubin | With hemolysis |
| AST/ALT | Question hepatic ischemia | |
| ALP | Liver obstruction | |
| Coagulation screen | PT, APTT, fibrinogen | Normal in TTP/CM-HUS, deranged in DIC |
| Pregnancy test | Urine or serum HCG | In all women of child-bearing age |
| Other biochemistry | Troponin | Degree of cardiac involvement |
| Thyroid function | Useful with pyrexia of TMA to exclude infection | |
| CRP | May be reduced C3 with CM-HUS, C4 in autoimmune disease, eg, SLE | |
| C3/C4 | Consider if possible cancer trigger in men | |
| PSA | ||
| Vitamins | B12/folate | Treat deficiencies, folate may be reduced with haemolysis |
| Virology | Hepatitis A/B/C | Baseline investigations especially pre-PEX |
| HIV +/−viral load | eg, in HSCT | |
| Further virology screen relevant to history, eg, adenovirus, CMV | ||
| Autoimmune screen | ANA/dsDNA | Underlying autoimmune disease |
| RF | ||
| ENA | ||
| ACLA/LA | ||
| Vasculitis screen (if relevant from history) | Anti-GBM antibodies | To exclude Goodpasture’s/underlying vasculitis trigger |
| ANCA | ||
| Microbiology | BCs STEC serology/PCR from stool | With temperature |
| Radiology | USS abdomen | Kidneys’ size, obstruction, lymphadenopathy, organomegaly |
| MRI head | Ischemic changes, infarcts, PRES | |
| CT chest/abdomen/pelvis | If exclude an underlying cancer | |
| Cardiac investigations | ECG echocardiogram | Baseline for cardiac function/conduction defects or ischemia |
Samples for ADAMTS13 are taken pre-PEX and for complement mutational analysis, and factor H antibodies will take weeks or months before available.
ALP, alkaline phosphatase; ALT, alanine transaminase; ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; anti-GBM, antiglomerular basement membrane; AST, aspartate aminotransferase; BC, blood culture; CMV, cytomegalovirus; CRP, C-reactive protein; CT, computer tomography; DCT, direct coombes test; dsDNA, double-stranded DNA; ECG, electrocardiogram; ENA, extractable nuclear antibody; HCG, human chorionic gonadotropin; K+, potassium; PSA, prostate-specific antigen; RF, rheumatoid antibody; Ur, urea; USS, ultrasound.
Role of ADAMTS13 analysis in differentiation of TMAs
Acute recognition of TMAs remains a clinical diagnosis. However, the availability of commercial ADAMTS13 assays means confirmation of the diagnosis can be available in real time. ADAMTS13 activity levels <10% is in keeping with TTP.1 ADAMTS13 activity levels between 10% and 20% require exclusion of the presence of anti-ADAMTS13 antibodies. ADAMTS13 activity levels >10% are suggested to be required for the consideration of CM-HUS.44 However, it is very uncommon for HUS cases to have ADAMTS13 activity levels this low, and levels are typically within the middle or normal laboratory range.45 Any reduction in ADAMTS13 levels is the result of consumption of ADAMTS13 due to raised VWF levels. A reduction in ADAMTS13 can be documented in severe sepsis-associated DIC, with an increase in ultra large (UL) VWF multimers and related to the risk of renal failure.46 This may be seen in a number of physiological and disease states.47
Due to the disparity in availability of ADAMTS13 assays, a number of scoring systems have been suggested to help differentiate TTP from CM-HUS and other TMAs. Platelet count >30 × 109/L and serum creatinine level >150 to 200 µmol/L may exclude the likelihood of severely deficient ADAMTS13 activity.44 From the UK TTP registry, which provides ADAMTS13 activity analysis for all cases of TMA, the median platelet and creatinine results confirm these findings. This can be useful; however, basing therapy on these cutoffs can be detrimental. One-third of HUS cases had a platelet count <30 × 109/L and ∼20% had creatinine levels <150 µmol/L, while 17% of TTP cases had a platelet count >30 × 109/L and 15% had a creatinine >150 µmol/L.45 The platelets, lysis, active cancer, stem cell or solid organ transplant, MCV, INR, and creatinine (PLASMIC) score has been suggested as a rapid assessment, in conjunction with clinical assessment, to differentiate TTP due to severe ADAMTS13 deficiency (Table 4).48 Scores of 0 to 4 were associated with low risk of severe ADAMTS13 deficiency (0% to 4% of patients), a score of 5 represented intermediate risk (5% to 24% of patients), and scores of 6 to 7 indicated high risk (62% to 82% of patients). Despite these assessments, definitive confirmation of TTP versus another TMA requires ADAMTS13 activity analysis.
The PLASMIC score to predict TTP associated with severe ADAMTS13 deficiency
| Investigation . | Point . |
|---|---|
| Platelet count < 30 × 109/L | 1 |
| Hemolysis variable* | 1 |
| No active cancer | 1 |
| No history of HSCT | 1 |
| MCV < 90 fL | 1 |
| INR < 1.5 | 1 |
| Creatinine < 2.0 mg/dL | 1 |
| Investigation . | Point . |
|---|---|
| Platelet count < 30 × 109/L | 1 |
| Hemolysis variable* | 1 |
| No active cancer | 1 |
| No history of HSCT | 1 |
| MCV < 90 fL | 1 |
| INR < 1.5 | 1 |
| Creatinine < 2.0 mg/dL | 1 |
INR, international normalized ratio; MCV, mean cell volume. Reprinted from Bendapudi et al48 with permission.
Reticulocyte count >2.5% or undetectable haptoglobin or indirect bilirubin >2.0 mg/dL.
Initiation of treatment of patients with a TMA
The mainstay of treatment of TMAs is PEX. This should be undertaken as soon as the diagnosis is considered, as TTP and HUS are life-threatening disorders. The benefit of PEX has been confirmed in a randomized study.49 PEX should be continued to remission in TTP patients (clinical response after cessation of PEX and maintained for greater than 30 days, associated with normalization of ADAMTS13 activity); but, in other TMAs, it would depend on response and results of further laboratory investigations. Typically, steroids are started in conjunction with PEX for iTTP patients; however, they should be used with caution in other situations, such as for HUS or scleroderma, not least because of associated hypertension at presentation.
The use of PEX is not without its risks, for example, from central line insertion, infection, citrate toxicity, and reactions to plasma.50 However, many of the investigations to identify the cause of the TMA may be available within 24 to 48 hours from admission, and PEX can be stopped if another diagnosis is suggested for which PEX has no benefit.
If iTTP is confirmed, proceeding to further immunosuppressive therapy, such as anti-CD20 monoclonal antibody therapy, should be considered. Its early use is based on reducing time to remission, less time in the hospital, and reduced need for plasma.51 It is also established at reducing the risk of relapse, both in the short and longer terms, based on a reduction of anti-ADAMTS13 immunoglobulin G antibodies and increased ADAMTS13 activity.51,52 Anti-CD20 therapy takes a median of 10 days to achieve remission. Therefore, there is the high-risk acute period that, until recently, remained precarious, while the platelet counts increase. The use of the nanobody, caplacizumab, which prevents platelet binding to VWF, is associated with a faster increment of platelets, following initiation in the acute presentation. It bridges to allow the effect of immunosuppressive therapy on ADAMTS13 autoantibodies.53 Caplacizumab results in acquired von Willebrand disease, and, therefore, it has a potential bleeding phenotype. TTP is a prothrombotic disorder, in which excess bleeding is not usually documented. However, caution is advised for non-TTP cases before initiation, particularly as there is often a severe thrombocytopenia at presentation.
The role of PEX in HUS has, overall, not been confirmed as demonstrating a benefit. However, PEX in the acute presentation is associated with an improvement in hematology parameters and often stabilization or improvement of renal impairment.54 Certainly longer term use in the past was associated with recurrent exacerbations. In the current era, the C5 complement inhibitor eculizumab has revolutionized CM-HUS patient outcomes and care. From the pivotal clinical trials, there was 100% normalization of hematology parameters and more than 80% of patients were able to come off dialysis.55 Further review confirmed that the earlier the therapy was initiated, the better the outcome, particularly with respect to significant improvements in renal function.56
Discussion concentrates on ensuring the precise diagnosis that drives the appropriate and relevant therapy. Given the diagnosis of CM-HUS is clinical, a minimum requirement and assessment are suggested before starting eculizumab therapy. Exclusion of an autoimmune diagnosis, eg, SLE and virology including HIV. Normal-sized kidneys on abdominal ultrasound and no features of chronic changes of hypertension on ophthalmic assessment. ADAMTS13 activity >20% excludes a diagnosis of TTP. This is the minimum investigative set of investigations before contemplating complement inhibitor therapy.
The use of immunosuppressives or complement inhibitor therapy may be relevant in other TMAs, eg, autoimmune disease and TA-TMA, respectively. It may also be impossible to exclude TTP or CM-HUS, for example, in malignant hypertension, and a trial of complement inhibitor therapy may be required. Furthermore, dual treatment may be required for those cases of TTP or CM-HUS that have an obvious trigger; for example, HIV infection requires PEX, immunosuppressive therapy to clear ADAMTS13 autoantibodies, and also HAART to reduce the viral load and aid remission. With intensified investigation of complement dysregulation in disease pathogenesis aside from CM-HUS, the rationale and benefit of complement inhibitor therapy in other TMAs are increasing.14
In conclusion, TTP and HUS are important life-threatening TMAs that must be differentiated from other conditions resulting in a TMA. As response is time critical, PEX should be initiated as soon as the diagnoses are considered. Clinical assessments in conjunction with further investigations, including ADAMTS13 assays, are imperative to ensure the precise treatment pathways to achieve remission. For TTP this includes immunosuppression such as rituximab, and for CM-HUS it includes eculizumab. The aim is to reduce mortality and improve long-term mobility.
Correspondence
Marie Scully, Department of Haematology, University College of London Hospitals, 235 Euston Rd, London NW1 2BE, United Kingdom. e-mail: m.scully@ucl.ac.uk.
References
Competing Interests
Conflict-of-interest disclosure: M.S. has received research funding from Ablynx and Shire, and has received honoraria form Ablynx, Shire, Novartis, and Alexion.
Author notes
Off-label drug use: Rituximab.