Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative option for many disease states. Despite significant improvements in strategies used to prevent and treat acute and chronic graft-versus-host disease (a/cGVHD), they continue to negatively affect outcomes of HSCT significantly. Standard, first-line treatment consists of corticosteroids; beyond this, there is little consistency in therapeutic regimens. Current options include the addition of various immunosuppressive agents, the use of which puts patients at even higher risks for infection and other morbidities. Extracorporeal photopheresis (ECP) is a widely used cellular therapy currently approved by the US Food and Drug Administration for use in patients with cutaneous T-cell lymphoma; it involves the removal of peripherally circulating white blood cells, addition of a light sensitizer, exposure to UV light, and return of the cells to the patient. This results in a series of events ultimately culminating in transition from an inflammatory state to that of tolerance, without global immunosuppression or known long-term adverse effects. Large-scale, prospective studies of the use of ECP in patients with a/cGVHD are necessary in order to develop the optimal treatment regimens.
Learning Objectives
Understand the proposed mechanism of ECP in GVHD
Understand the challenges in establishing optimal treatment regimens
Introduction
Graft-versus-host disease (GVHD) remains one of the most significant causes of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT). Unfortunately, despite many advances in the field of HSCT, the prevention and treatment of GVHD remain suboptimal in that treatment will be refractory to standard first-line therapy in a significant number of patients. First-line treatment with steroids will be refractory in approximately half of patients with acute GVHD (aGVHD); mortality rates in these patients are high.1 In addition, next-line therapies, although numerous, are not standardized and are accompanied by several complications including profound immunosuppression, end-organ damage, risk for secondary malignancies, and metabolic derangements. In addition, completion of rigorous randomized controlled studies in GVHD poses significant challenges including diversity of patient populations, inconsistent grading (especially in chronic GVHD [cGVHD]), and variable definitions of end points and response.2 The development of treatments that modulate the immune system rather than directly suppressing its function, although not dampening a potential graft-versus-malignancy effect, is appealing for use. One such treatment, extracorporeal photopheresis (ECP) is widely available and used. ECP continues to be the only US Food and Drug Administration–approved photocellular therapy for cancer, and its use has expanded to include other T-cell–mediated disease states including GVHD and rejection after solid-organ transplantation. Despite its frequent usage, the optimal role of ECP in the setting of GVHD needs to be better defined.
The incidence of aGVHD after allogeneic HSCT varies widely depending on the degree of HLA disparity, advanced age of either donor or recipient, and other factors. aGVHD is manifested by skin rash; gastrointestinal tract signs and symptoms including nausea, vomiting, abdominal pain, and diarrhea; and elevation of bilirubin levels. Staging systems have been validated and are predictive of mortality.3 The underlying pathophysiology of aGVHD consists of a complex cascade of immune events, which can be described in several phases. Initially, remaining host cells that have the capacity to present antigen become activated and interact with donor T cells, which leads to the secretion of inflammatory cytokines. This mechanism leads to further recruitment and stimulation of donor immune cells, which leads to direct cytotoxicity to host tissue, which is even further amplified by mediators including IL-1, lipopolysaccharide, and tumor necrosis factor-α; this ultimately results in tissue damage and clinical presentation of aGVHD. It generally occurs within the first 100 days after transplantation, although patients who undergo HSCT with nonmyeloablative conditioning or who have mixed-donor chimerism for some time may experience later development of aGVHD.4-8
cGVHD presents later in the clinical course and can manifest in a multitude of organs including the skin, eyes, lungs, liver, and gastrointestinal tract. Patients at highest risk include those who had an HLA-mismatched donor, a history of aGVHD, and had received peripheral blood stem cells as a donor source. cGVHD remains one of the leading causes of late deaths after HSCT.9-11 Recent large-scale studies through the Center for International Blood and Marrow Transplant Research have demonstrated that the incidence of cGVHD is increasing with time, potentially because of the increased use of alternative donors and peripheral blood stem cell products.12 Patients who experience direct progression from acute to chronic forms of GVHD have the most difficult clinical courses, often requiring aggressive immunosuppression for several years. In contrast to the cascade of causative factors in the development of aGVHD, which are well understood, such factors relating to cGVHD are more complex and therefore remain elusive. The clinical presentation of cGVHD resembles many autoimmune disorders and may be related to minor major histocompatibility complex (MHC) antigens. Regardless of the treatment modality in question, it is essential to evaluate patients with cGVHD uniformly in order to be able to accurately compare efficacy; the widely accepted criteria from the National Institutes of Health provide such a platform.13
ECP was developed in the mid-1980s for the treatment of refractory cutaneous T-cell lymphoma, which remains its only Food and Drug Administration–approved application. Since that time, several iterations of the device that is used to treat patients have been developed, and the use of ECP has been expanded to include other T-cell–mediated diseases including GVHD, prevention and treatment of rejection after solid-organ transplantation, and selected autoimmune diseases. Several of these applications are evaluated in the most recently published guidelines from the American Society for Apheresis.14 ECP is an apheresis procedure, which involves the removal of patients’ circulating mononuclear cells with an apheresis apparatus, addition of 8-methoxypsoralen (8-MOP), exposure to UV-A light, and return of the cells to the patient. Approximately 5% to 10% of cells circulating in the peripheral blood are treated in each procedure. Many patient variables need to be considered for such treatments, especially in the context of which device is used for cell collection. Such variables include body weight; permissible extracorporeal volume; clinical status at the time of the procedure; potential need for central venous access; and laboratory values including total white blood cell and platelet counts, hematocrit, and bilirubin levels. ECP may be performed on a closed-system device (predominantly used in the United States) or as an off-line procedure in which cells are collected with a standard cell separator and treated in the laboratory before they are returned to the patient, a procedure more commonly used in European centers.15 Treatment centers that use the off-line method also have frozen collected cells in aliquots to be thawed, treated, and reinfused at a later time, without significant differences noted in cell phenotype. This practice allows the following types of patients to receive treatment: those who travel longer distances, lack appropriate intravenous access, or cannot tolerate multiple apheresis procedures because of the level of illness.16
Although these differences in treatment practices allow practitioners to treat a wide range of patients in multiple clinical settings, they introduce a considerable number of variables that make globalization of the available clinical data difficult to interpret at times. For example, substantial differences in the numbers of cells that are collected, treated, and returned to the patient are significantly different when comparing US devices with the European method of collection. No clinical trials have been performed to determine if a larger number of treated cells are required for maximal clinical effect. In addition, the basic treatment schedule and subsequent tapering of treatments vary considerably from center to center. Some treating centers will refrain from performing ECP depending on certain patient laboratory values resulted on the day of treatment, including absolute lymphocyte or monocyte counts. Finally, although the manufacturer advocates the use of heparin as the principal anticoagulant during the procedure, many centers have adopted the use of citrate either alone or in combination with heparin in patients who have a higher risk of bleeding. As such, ongoing debate exists as to the optimal way to deliver this therapy to patients.15 However, despite these limitations, a favorable safety profile and a nonimmune-suppressing mechanism of action make ECP an attractive therapy for patients with both aGVHD and cGVHD after allogeneic HSCT.
Mechanism of action in GVHD
ECP exerts multiple effects on the immune system. First is the well-accepted induction of apoptosis in activated lymphocytes within 36 to 48 hours of treatment, which results from intercalation of DNA when 8-MOP is activated by exposure to UV light. This may occur through upregulation of Fas and a resultant increase in Fas-initiated proapoptotic signaling.17 Apoptosis, followed by subsequent engulfment and presentation of antigens, leads to the production of anti-inflammatory cytokines and reduced production of preinflammatory cytokines. The majority of studies in patients with GVHD demonstrates an increase in circulating T-regulatory cells (CD4+/CD25+/FoxP3+); this increase has been associated with positive clinical response to ECP.18-20 In mouse models of contact hypersensitivity, Maeda et al21 found that ECP inhibited the sensitization and effector phases of contact hypersensitivity, and T-regulatory cells generated through the production of IL-10 also inhibited sensitization. Transfer of T-regulatory cells into untreated mice had the same effects.21 However, it is unlikely that the induction of apoptosis of treated lymphocytes represents the main mechanistic drive behind the clinical success of ECP. Rather, the induction of tolerogenic dendritic cells from treated monocytes appears to hold a more pivotal role. Additional mouse experiments by the same group demonstrated that removal of T cells from the treated population of splenocytes by magnetic bead depletion did not alter the inhibitory effects of ECP, whereas depletion of CD11c+ monocytes resulted in a significant decrease in efficacy.22
A series of elegant experiments in both murine and human cells conducted by the Edelson group at Yale University23-26 have clearly demonstrated that treatment of peripheral blood mononuclear cells from both healthy participants and patients with GVHD results in the conversion of monocytes to dendritic cells. In addition, they demonstrated that ECP induced the transcription of several genes involved in the conversion from monocytes to dendritic cells, and that the monocytes, unlike circulating lymphocytes, were not susceptible to apoptosis. As such, monocytes remain viable and are immunologically functional, expressing CD80/86.23 Of particular interest is the finding that the combination of 8-MOP and subsequent exposure of human monocytes to UV light directly stimulates expression of the glucocorticoid-induced leucine zipper; this expression is a known feature of the immunosuppressive dendritic cell phenotype.24 The same group has also demonstrated the importance of interaction of monocytes with activated platelets within the thin plastic plate through which all cells are processed within the treatment device.25,26
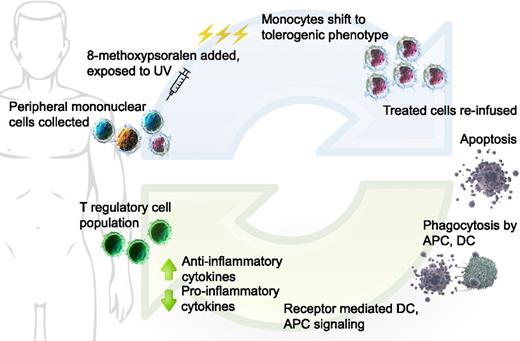
Taken together, the proposed mechanism of action of ECP in GVHD is as follows (Figure 1):
Extracorporeal photopheresis: Buffy coat is collected from patient, 8-MOP added to the collected cells, then treated with UVA. Monocytes shift to DCs with immature, tolerogenic phenotype; all treated cells infused back to patient. Activated lymphocytes undergo apoptosis over 24 to 48 hours. Donor and residual host APCs take up apoptotic bodies, resulting in favorable changes in cytokine milieu. Tolerogenic DCs are unable to stimulate effector T cells; T-regulatory cell population promoted. DC, dendritic cell; APC, antigen presenting cell. (Cell image credits Blausen Medical, US National Library of Medicine; figure design Mia Zierk.)
Extracorporeal photopheresis: Buffy coat is collected from patient, 8-MOP added to the collected cells, then treated with UVA. Monocytes shift to DCs with immature, tolerogenic phenotype; all treated cells infused back to patient. Activated lymphocytes undergo apoptosis over 24 to 48 hours. Donor and residual host APCs take up apoptotic bodies, resulting in favorable changes in cytokine milieu. Tolerogenic DCs are unable to stimulate effector T cells; T-regulatory cell population promoted. DC, dendritic cell; APC, antigen presenting cell. (Cell image credits Blausen Medical, US National Library of Medicine; figure design Mia Zierk.)
Donor T cells recognize minor MHC mismatches existing within the host
Host dendritic cells surviving, despite the conditioning chemotherapy/radiotherapy regimen, display the broad range of self-antigens in the context of MHC class I and play a significant role in the initiation of GVHD
Monocytes exposed to 8-MOP/UV light differentiate into immature, tolerogenic dendritic cells and take up apoptotic material, thereby inducing tolerance to allogeneic antigens
Donor T cells become less able to propagate the GVHD inflammatory response
Clinical use of ECP in HSCT
Publications that describe the use of ECP in HSCT list 3 clinical scenarios in which this therapy can be beneficial to the patient: prevention of GVHD, treatment of aGVHD, and treatment of cGVHD. Only 2 randomized clinical trials that evaluated the efficacy of ECP have been published. Both of these trials involve patients with cGVHD and are discussed in the cGVHD section of this paper. Otherwise, publications have been limited to prospective nonrandomized trials and case reports/series. This limitation, combined with the significant differences among practices mentioned beforehand, makes it difficult to compare the efficacy of ECP with that of other second- and third-line therapies.
Prevention of GVHD
The use of ECP within the conditioning regimen for HSCT has been investigated in both the myeloablative and reduced-intensity transplant settings. Miller et al27 published their experience in 2004, in which they hypothesized that the integration of ECP in a reduced-intensity conditioning regimen consisting of ECP on days −7/−6, a continuous infusion of pentostatin for 48 hours, and low-dose total-body irradiation (600 cGy) would modulate the host effector cells, thereby reducing the incidence of GVHD. GVHD prophylaxis also included cyclosporine and 2 doses of methotrexate. Fifty-five patients with hematologic malignancies were enrolled; the majority (80%) had a matched related donor. The remaining patients had matched unrelated donors. Sixty percent of all patients had relapsed or refractory disease. Almost all received unstimulated bone marrow. aGVHD of higher than grade 2 developed in only 9% of patients, and cGVHD developed in 43%; of these 43%, only 12% had extensive disease. The majority of patients engrafted; of those, close to 90% had a disease response. Miller et al27 concluded that in a heavily pretreated group of patients, this conditioning regimen was well tolerated with a low nonrelapse-related mortality rate, rapid establishment of full-donor chimerism, and low rates of aGVHD and cGVHD.
The next published report of a clinical trial in which ECP was used for GVHD prophylaxis introduced ECP after HSCT in conjunction with etanercept.28 In a phase 2 trial, 48 patients underwent reduced-intensity conditioning that consisted of fludarabine, busulfan, and ± low-dose total-body irradiation (200 cGy). Etanercept was administered twice weekly for 8 weeks after HSCT, and ECP was performed weekly from day +28 post-HSCT to approximately day +70, followed by every other week through day +100 and monthly through day +180 (total of 12 treatments). Additional GVHD prophylaxis consisted of tacrolimus and mycophenolate mofetil. The majority of patients had hematologic malignancies and HLA-identical donors; all but 2 received peripheral blood stem cells. There were no graft failures, and full-donor T-cell chimerism was reached by 1 year. Although aGVHD developed in 57% of patients, the majority of cases were of moderate grade and steroid responsive. However, patients did experience a high incidence of steroid-dependent cGVHD once prophylaxis was discontinued.
Based on findings of Shlomchik et al,29,30 in which they demonstrated that alloantigen expression by host antigen presenting cells protects against the development of GVHD by the induction of T-regulatory cells, Shaughnessy et al31 hypothesized that the use of ECP before initiation of a myeloablative-conditioning regimen would reduce the incidence of GVHD by modulating host antigen–presenting cell function. A total of 62 patients with hematologic malignancies underwent 2 consecutive days of ECP within 4 days of starting the conditioning regimen, which consisted of cyclophosphamide and fractionated total-body irradiation (10-13.5 Gy). Approximately half had matched related donors and only 1 patient had a mismatched related donor; the remaining had HLA-identical unrelated donors. Approximately half received bone marrow; there were no cord blood transplants. Twenty-nine patients (47%) were in second or higher remission at the time of transplant; 7 had primary refractory disease. The remaining 26 patients were in first remission entering transplant. Historical control participants who were matched for similar conditioning regimens and GVHD prophylaxis without ECP were identified through the Center for International Blood and Marrow Transplant Research for comparison with the study population. Patients who received ECP had a statistically significantly lower risk for grades 2 to 4 aGVHD and better overall survival time at 1 year.
ECP for treatment of aGVHD
Standard first-line treatment of aGVHD grades 2 to 4 consists of corticosteroids (eg, prednisone, methylprednisolone), which range from 1 to 2 mg/kg body weight. The decision as to which second-line therapy to initiate after 3 to 7 days of either lack of improvement or disease progression has been derived from uncontrolled trials or retrospective studies and, therefore, has not been standardized across the HSCT community. Published studies in which ECP was given as second-line therapy are small, with patient numbers evaluated typically at less than 50 per study. In general, complete response rates are highest in patients with skin involvement; the reported rates range from ∼70% to 100%. Greinix et al,32,33 whose study had one of the largest numbers of patients reported, presented 59 patients with steroid-refractory or steroid-dependent aGVHD treated with ECP on 2 consecutive days weekly until improvement on either a pilot trial (n = 21) or phase 2 trial (n = 38). In the phase 2 trial, ECP was initiated earlier than the pilot trial (median, 15 vs 21 days), which resulted in better complete response rates in those with grade 4 and gastrointestinal tract disease. These studies also demonstrated better overall survival duration, lower treatment-related mortality rates, and lower overall steroid exposure.32,33
The largest study of ECP in patients with aGVHD was published in 2014, in which 128 patients from 3 European centers were retrospectively evaluated for freedom from treatment failure at 6 months (no deaths, relapse of primary disease, or addition of third-line immunosuppressive treatment).34 Patients who were diagnosed with aGVHD and found to have steroid-refractory or steroid-dependent disease were treated 2 to 3 days weekly for 4 to 6 weeks, with treatment tapering to every other week. Patients with lower-stage GVHD had better outcomes; an overall response rate of 77% was observed, and 71% of the cohort experienced freedom from treatment failure. The incidence of cGVHD was 34% at 2 years.
In an effort to compare ECP with other second-line therapies, Jagasia et al35 retrospectively evaluated 98 patients with steroid-refractory aGVHD who received either ECP (n = 57) or anticytokine therapy (n = 41) as second-line therapy, for response and survival. Anticytokine therapy included either inolimomab or etanercept. Patients receiving ECP had superior response and overall survival duration in both univariate and multivariate analyses. The retrospective nature of this study led to significant differences in how the patients were treated with respect to conditioning and GVHD prophylaxis regimens (including patients who received either T-depleted grafts vs in vivo depletion and a small number of patients who received donor lymphocyte infusions) and approach to steroid taper, which therefore limits additional inference of superiority. However, the results are intriguing, especially when considering the differences in susceptibility to infections that patients face with anticytokine therapies.
Finally, a recently published review of the literature that pooled 9 studies describing a total of 323 patients with steroid-refractory or steroid-dependent aGVHD revealed an overall response rate of 69%.36 When evaluated for affected organ systems independently, those with skin involvement had the highest response (84%), followed by those with gastrointestinal tract involvement (65%).36 In summary, the clinical reports reflect that patients with steroid-refractory or steroid-dependent aGVHD have good response rates with ECP treatment and are able to taper off corticosteroids faster than those who are not receiving ECP. Early initiation of ECP also appears to be beneficial. Given its favorable adverse effect profile and no documented risk for increased rates of infection, ECP should be considered in all patients with aGVHD.37 Moving forward, randomized controlled studies are crucial to determine the optimal timing of initiation and treatment schedule in patients with aGVHD.
ECP for treatment of cGVHD
As in aGVHD, first-line therapy for patients with cGVHD is corticosteroids; nonetheless, approximately half will not respond. Adding to the morbidity and mortality risks associated with cGVHD is that patients require a median of 2 to 3 years of immunosuppressive therapy. Again, no consensus has been reached regarding the optimal second-line therapy in these patients. ECP has been used frequently in patients with steroid-refractory and steroid-dependent disease. Much clinical experience in cGVHD is based again on small case series and retrospective reviews, which have been well detailed by Greinix et al,38 who conclude that ECP is an appropriate second-line therapy for cGVHD in patients whose disease does not respond to corticosteroids.38 Skin, gastrointestinal, oral, and liver signs and symptoms tend to have the highest reported response rates, but ECP is used for other affected organ systems as well, including the respiratory and musculoskeletal systems.36
Two reports on 1 cohort of patients, who were enrolled on a multicenter, randomized clinical trial investigating the usefulness of ECP in patients with refractory cGVHD, have been published. To date, these reports are the only publications to our knowledge that describe the results of a randomized trial in this patient population. The first study describes the initial response in those enrolled in the ECP vs the control group at the 12-week point, whereas the second study describes those eligible to cross over onto the ECP group after progression or lack of response to corticosteroid therapy. A total of 95 patients with biopsy-proven steroid-refractory or steroid-dependent cGVHD of the skin were randomly assigned to receive either standard immunosuppressive therapy (corticosteroids, 1 mg/kg total daily dose; n = 48) or standard therapy plus ECP (n = 47). Those in the ECP group underwent the following treatment schedule: 3 times in the first week, then twice weekly (consecutive days) in weeks 2 to 12. Patients who responded favorably at 12 weeks continued twice-weekly treatments through week 24. Control participants who experienced failure were allowed to receive ECP using the same treatment schedule if they had progressively worsening skin findings, or if their skin score did not improve adequately (<15% improvement in skin score or <25% reduction in steroid dose) by week 12. Steroid doses were maintained until 6 weeks into the study, at which time taper could be initiated if the investigator believed that the skin was improved; blinded investigators performed skin scoring.
The initial report by Flowers et al39 showed a superior response in skin score with a 50% reduction of steroid dose and at least a 25% decrease from baseline at week 12 (8.3% in the ECP group vs 0% in the control group). Twenty-nine of the patients randomly assigned to the standard-of-care group subsequently crossed over to the ECP treatment group because of lack of response or progression of skin manifestations from cGVHD. Parameters for crossover included greater than 25% worsening of skin score from baseline at any time, or less than 15% improvement in skin score or less than 25% reduction in steroid dose at week 12. Of the 29 patients, 90% had extensive cGVHD, and the majority of them (86%) completed the 24 weeks of ECP therapy. A total of 9 patients either experienced complete or partial response, and the median decrease in skin score was −8 at week 12 and −25 at week 24. More than 25% reduction in steroid dose was seen in approximately 30% of patients by week 24.40 It is important to note these publications have demonstrated that the use of ECP in patients with skin manifestations of cGVHD enables a significant decrease in corticosteroid dose and that randomized studies in the field of ECP are feasible.
Conclusions
Although the currently published literature supports the use of ECP for prophylaxis of GVHD and in patients who have already experienced development of steroid-refractory or steroid-dependent aGVHD and cGVHD, significant advances need to be made. Significant improvements in understanding the mechanism of action in these disease entities have been achieved, which will help guide future research in this field. For example, if interactions between monocytes and platelets within the apparatus are critical for differentiation into tolerizing dendritic cells, would patients who undergo ECP benefit from a higher platelet count at the time of treatment? What other variables could be personalized to enhance the treatment effect potency of ECP? The substantial heterogeneity to this point in how ECP is performed (online vs off-line), criteria for laboratory parameters with which to perform (total white cell count vs absolute lymphocyte count), time of initiation, treatment schedule, and tapering schedule continues to be a significant barrier to accurately compare groups of patients. In addition, it perpetuates the uncertainty in how best to deliver this therapy to patients. Currently, studies attempting to identify biomarkers that could predict response and strict response criteria are being conducted and will help to advance the field significantly. Multiple reports of treatments in both pediatric and adult patients with GVHD have been published, and the overall favorable safety profile compared with other available immunosuppressive therapies continues to make this therapy appealing despite all of the unknowns. Clinical trials are essential to define the optimal use of ECP in the field of allogeneic HSCT; studies to evaluate its prophylactic use in haploidentical transplants and as combination up-front therapy should be pursued.
Correspondence
Jennifer Schneiderman, Ann & Robert H. Lurie Children's Hospital of Chicago, Division of Pediatric Heme/Onc/Neuro-Onc/Stem Cell Transplantation, 225 E Chicago Ave, Box 30, Chicago, IL 61611; e-mail: jschneiderman@luriechildrens.org.
References
Competing Interests
Conflict-of-interest disclosure: J.S. has no competing financial interests.
Author notes
Off-label drug use: None disclosed.