Abstract
T-cell therapy has emerged from the bench for the treatment of patients with lymphoma. Responses to T-cell therapeutics are regulated by multiple factors, including the patient’s immune system status and disease stage. Outside of engineering of chimeric antigen receptors and artificial T-cell receptors, T-cell therapy can be mediated by ex vivo expansion of antigen-specific T cells targeting viral and/or nonviral tumor-associated antigens. These approaches are contributing to enhanced clinical responses and overall survival. In this review, we summarize the available T-cell therapeutics beyond receptor engineering for the treatment of patients with lymphoma.
Learning Objectives
To understand how antigen-specific T cells are different from CAR T cells
To understand the broad applicability of the antigen-specific T-cell platform
Introduction
The field of immunotherapy for lymphoma is a prodigious one. Areas under active clinical investigation include vaccines that enhance antigen processing and presentation efficacy, costimulation agonists, adoptive transfer of antigen-specific and specificity-heightened genetically modified T cells, and suppression of T-cell regulatory pathways. Preliminary data from early-phase clinical trials utilizing T-cell therapeutics are promising. Specifically, the development of CD19-directed chimeric antigen receptor (CAR) T cells has revolutionized the treatment of CD19+ B-cell malignancies, including lymphomas, and has elicited some profound clinical regressions. However severe on-target, off-tumor toxicities (healthy B-cell depletion, cytokine release syndrome, and neurotoxicity) mean that these studies can currently only be conducted at institutions that can support patients in an intensive care setting. This, combined with limited suitable antigenic targets, currently restricts the broader applicability of this approach to all lymphomas. However, numerous studies are utilizing non–cell-engineering methods. This review focuses on T-cell targeting using non–gene-modified approaches for patients with lymphoma.
Role of the immune system in lymphoma and immunogenic features of current treatments
Lymphomas arise from cells of the immune system (B cells and T cells), and the tumor microenvironment is a dynamic interplay between tumor and immune cells (Figure 1A). Most lymphomas arise in the secondary lymphoid organs. There are appreciable immune-related differences between the lymphoma tumor microenvironment and the solid tumor microenvironment. The spleen and lymph nodes are immune cell–dense hubs, unlike solid tumors, where immune cell infiltration of cancerous tissue is limited. While discussion of the impact of the microenvironment is outside the scope of this review, it is critical to consider when developing any T-cell therapy approach that immune cell function, frequency, and distribution vary greatly among patients with the same cancer type, and this can impact patient outcome.1
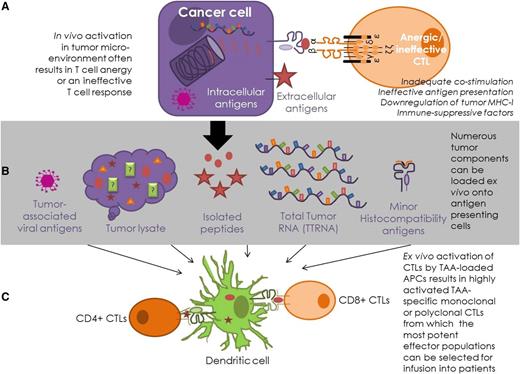
Antigen-specific T-cell strategies for lymphomas. (A) In vivo, intracellular antigens are presented on MHC-I molecules, where CTLs can engage directly with the MHC-I–peptide complex on the surface of the cancer cell. Surface antigens can be targeted indirectly via presentation by antigen-presenting cells or directly by antibodies. This process is often ineffective in cancer patients. (B) Antigen presentation is enhanced in T-cell–mediated therapies, as tumor-derived material is presented by appropriately activated antigen-presenting cells, most commonly DCs. Antigenic DC loading of tumor-associated viral peptides, lysed tumor cells, known antigenic tumor peptides, total tumor RNA (TTRNA), and minor histocompatibility proteins have all been attempted in hematological T-cell–based immunotherapy. (C) T-cell–based therapies enhance the T-cell response by ensuring appropriate costimulation and optimal environmental conditions for T-cell activation. This process allows TAA-specific T-cell clones, or polyclonal multiantigen-specific T cells, to be expanded ex vivo from patients or healthy donors for infusion into patients.
Antigen-specific T-cell strategies for lymphomas. (A) In vivo, intracellular antigens are presented on MHC-I molecules, where CTLs can engage directly with the MHC-I–peptide complex on the surface of the cancer cell. Surface antigens can be targeted indirectly via presentation by antigen-presenting cells or directly by antibodies. This process is often ineffective in cancer patients. (B) Antigen presentation is enhanced in T-cell–mediated therapies, as tumor-derived material is presented by appropriately activated antigen-presenting cells, most commonly DCs. Antigenic DC loading of tumor-associated viral peptides, lysed tumor cells, known antigenic tumor peptides, total tumor RNA (TTRNA), and minor histocompatibility proteins have all been attempted in hematological T-cell–based immunotherapy. (C) T-cell–based therapies enhance the T-cell response by ensuring appropriate costimulation and optimal environmental conditions for T-cell activation. This process allows TAA-specific T-cell clones, or polyclonal multiantigen-specific T cells, to be expanded ex vivo from patients or healthy donors for infusion into patients.
T-cell receptors (TCRs) on CD8+ T cells can recognize tumor cells expressing peptides in their major histocompatibility complex class I (MHC-I; HLA A, B, C) molecules and become activated against the malignant cell. Alternatively CD4+ T cells can engage with antigen-presenting cells displaying tumor peptides in their MHC class II (MHC-II; HLA DR, DP, DM, DOA, DOB, and DQ) molecules. Antigen-presenting cells with cross-presentation ability, such as dendritic cells (DCs),2-4 B cells,5-8 and macrophages3,4,9-11 can also display tumor-associated peptides on MHC-I. If sufficient costimulation is concurrently provided, a robust activation of the T cell against the tumor peptide ensues. While the term cytotoxic T lymphocyte (CTL) has historically been used to refer to CD8+ T cells, the data are clear that CD4+ T cells are more than just “helper” cells; in addition to providing “help” for B cells and CD8+ T cells, they can act as CTLs in their own right.12,13 These activated antigen-specific T cells form an immunological synapse with the target cell. Subsequent release of the cytokines interferon-γ and tumor necrosis factor–related apoptosis-inducing ligand, as well as upregulation of cytotoxic perforin and granzyme molecules and the transmembrane protein FAS ligand, contributes to the ultimate lysis and apoptosis of the tumor cell. This T-cell–mediated tumor cell killing is believed to occur during the elimination phase of immune surveillance.14
More recent advances in our understanding have identified that, rather than simple elimination of rapidly dividing cells, the success of chemotherapy and radiotherapy is due, in part, to their capacity to induce immunogenic tumor cell death. Immunogenic cell death releases immune-stimulating compounds such as adenosine triphosphate, calreticulin, receptor-interacting protein kinase, heat shock proteins, and uric acid that generate nontargeted innate and adaptive immune activation, which can disrupt immune suppression and break tolerance (reviewed in Emens15 and Zitvogel et al16 ). Immunoadjuvant pathways can be triggered by chemotherapeutic tumor cell death,17-19 while cyclophosphamide can lift immune suppression by selectively depleting regulatory T cells.20 Radiotherapy’s success in cancer eradication can also be attributed to its induction of immunogenic forms of cell death and elimination of immune barriers, which can result in increased immune-cell infiltration, tumor-associated antigen (TAA) presentation, and T-cell activation.21-25 Thus, in addition to their roles as debulking and cytotoxic agents, certain chemotherapies, as well as radiotherapy, are increasingly viewed as potentially useful primers or adjuncts for immunotherapy for cancer.15,21,26-30 However, chemotherapy and radiotherapy are nonspecific treatments, resulting in side effects that bring about appreciable bystander organ toxicities, including late effects and ongoing sequelae. Immunotherapies have the potential to fulfill the vision for efficacious targeted approaches that minimize collateral damage to healthy tissue. The critical role of the immune system in lymphomas is exemplified by the graft-versus-tumor (GVT; graft-versus-lymphoma) effect observed in a subset of patients after allogeneic hematopoietic stem cell transplant (allo-HSCT).31-34 However, this immune-cell–mediated effect is limited, and disease relapse remains the largest cause of HSCT failure.
T-cell therapies for lymphomas
Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) and T/natural killer (NK)–cell lymphoproliferative disorder have all been the focus of cell-mediated therapeutic approaches (Tables 1 and 2).35-60
Non–gene-modified T-cell therapies available for patients with lymphoma
| Treatment approach . | Description . |
|---|---|
| Unmodified DLI35-44 | Infusion of donor lymphocytes to mount GVT response |
| Modified DLI45-49,71 | Depletion of DLI subpopulations, TREG, naive T cells, to select for most potent CTLs |
| Tumor antigen–targeted CTLs50-55,88 | Infusion of ex vivo expanded tumor-specific allogeneic or autologous CTLs for elimination of tumor targets |
| Viral antigen–targeted T cells56,72,84 | Infusion of ex vivo–expanded virus-specific allogeneic or autologous T-cells to minimize viral reactivation during the immunosuppressive period posttransplant |
| Tumor peptide–loaded DC vaccination57-60 | Infusion of peptide-loaded DCs for in vivo presentation of antigen to endogenous T cells |
| Treatment approach . | Description . |
|---|---|
| Unmodified DLI35-44 | Infusion of donor lymphocytes to mount GVT response |
| Modified DLI45-49,71 | Depletion of DLI subpopulations, TREG, naive T cells, to select for most potent CTLs |
| Tumor antigen–targeted CTLs50-55,88 | Infusion of ex vivo expanded tumor-specific allogeneic or autologous CTLs for elimination of tumor targets |
| Viral antigen–targeted T cells56,72,84 | Infusion of ex vivo–expanded virus-specific allogeneic or autologous T-cells to minimize viral reactivation during the immunosuppressive period posttransplant |
| Tumor peptide–loaded DC vaccination57-60 | Infusion of peptide-loaded DCs for in vivo presentation of antigen to endogenous T cells |
DLI, donor lymphocyte infusion; TREG, regulatory T cell.
Non–gene-modified T cells for lymphoma
| NCT # (ACRONYM) . | Disease target . | Cell type . | Phase . | Sponsor . |
|---|---|---|---|---|
| NCT00002663 | Lymphoma, leukemia, other adult and pediatric solid tumors | Allogeneic EBV-specific CTLs | 1/2 | Memorial Sloan Kettering Cancer Center and National Cancer Institute |
| NCT01948180 | EBV-positive extranodal NK/T-cell lymphoma | Autologous EBV-specific CTLs (CMD-003) | 2 | Cell Medica Ltd |
| NCT00779337 | Lymphoma | Autologous AdE1- latent membrane–protein-specific CTLs | 1 | Queensland Institute of Medical Research, The Atlantic Philanthropies, Australian Department of Industry, Tourism and Resources, British Society for Haematology, National Health and Medical Research Council, Australia |
| NCT00005606 | Lymphoma, leukemia, multiple myeloma, plasma cell neoplasm | Allogeneic EBV-specific CTLs | 2 | Northwestern University, National Cancer Institute |
| NCT01636388 | HL | Allogeneic LMP-specific CTLs | 2 | New York Medical College, Children's Research Institute, Baylor College of Medicine, MD Anderson Cancer Center, City of Hope Medical Center, Johns Hopkins University, Ohio State University, University of Utah, University of Michigan |
| NCT01956084 | HL,HL, lymphoepithelioma; SCAEBV, leiomyosarcoma | Allogeneic LMP-1/2–specific CTLs | 1 | Children’s Research Institute |
| NCT02057445 | HL, NHL, LPD, | Allogeneic LMP-specific CTLs | 1 | New York Medical College, Children's Research Institute, Baylor College of Medicine, MD Anderson Cancer Center, University of Michigan, University of Utah, City of Hope Medical Center, Ohio University, Johns Hopkins University |
| NCT01498484 | NHL, EBV infection | Allogeneic EBV-specific CTLs | 2 | Atara Biotherapeutics, Memorial Sloan Kettering Cancer Center |
| NCT01447056 | HL, NHL, LPD, nasopharyngeal carcinoma, leiomyosarcoma, SCAEBV | Allogeneic LMP-specific CTLs | 1 | Baylor College of Medicine |
| NCT01555892 (GRALE) | HL, NHL, T/NK LPD, lymphoma | Autologous or syngeneic donor LMP-1/2–, EBNA-1–, and BARF-specific CTLs | 1 | Baylor College of Medicine, National Cancer Institute |
| NCT00062868 | HL, NHL, lymphoepithelioma, leiomyosarcoma | Autologous or allogeneic LMP-1/2–specific CTLs | 1 | Baylor College of Medicine |
| NCT02287311 (MABEL) | HL, NHL, SCAEBV, T/NK LPD | LMP-, BARF-1–, and EBNA-1– specific | 1 | Baylor College of Medicine |
| NCT01956084 | HL, NHL, SCAEBV, lymphoepithelioma, leiomyosarcoma | Allogeneic LMP 1/2 -specific CTLs | 1 | Children’s Research Institute |
| NCT00002663 | Lymphoma, leukemia, unspecified adult solid tumor, protocol specific, unspecified childhood solid tumor, protocol specific | Allogeneic EBV-specific CTLs | 1/2 | Atara Biotherapeutics, Memorial Sloan Kettering Cancer Center, National Cancer Institute |
| NCT02973113 (PREVALE) | HL, NHL, LPDs, EBV-related lymphoma, EBV-related PTLD, EBV-related NHL, EBV-related HL | Autologous EBV-specific CTLs + nivolumab (anti-PD-1 mAb) | 1 | Baylor College of Medicine |
| NCT01333046 (TACTAL) | HL, NHL | 5-azacitidine+ autologous NY-ESO-1, MAGEA4, PRAME, survivin-, and SSX-specific CTLs | 1 | Baylor College of Medicine, National Cancer Institute |
| NCT02203903 (RESOLVE) | Relapsed/refractory hematopoietic malignancies | Autologous or allogeneic WT1, NE, PR3, PRAME, MAGE-A3, MAGE-A4, NY-ESO, and survivin-specific CTLs | 1 | Children’s Research Institute, Johns Hopkins University |
| NCT02822495 | EBV-associated LPD, EBV lymphoma, EBV-related posttransplant LPD, EBV-associated viremia | Allogeneic EBV CTLs | Expanded access | Atara Biotherapeutics |
| NCT # (ACRONYM) . | Disease target . | Cell type . | Phase . | Sponsor . |
|---|---|---|---|---|
| NCT00002663 | Lymphoma, leukemia, other adult and pediatric solid tumors | Allogeneic EBV-specific CTLs | 1/2 | Memorial Sloan Kettering Cancer Center and National Cancer Institute |
| NCT01948180 | EBV-positive extranodal NK/T-cell lymphoma | Autologous EBV-specific CTLs (CMD-003) | 2 | Cell Medica Ltd |
| NCT00779337 | Lymphoma | Autologous AdE1- latent membrane–protein-specific CTLs | 1 | Queensland Institute of Medical Research, The Atlantic Philanthropies, Australian Department of Industry, Tourism and Resources, British Society for Haematology, National Health and Medical Research Council, Australia |
| NCT00005606 | Lymphoma, leukemia, multiple myeloma, plasma cell neoplasm | Allogeneic EBV-specific CTLs | 2 | Northwestern University, National Cancer Institute |
| NCT01636388 | HL | Allogeneic LMP-specific CTLs | 2 | New York Medical College, Children's Research Institute, Baylor College of Medicine, MD Anderson Cancer Center, City of Hope Medical Center, Johns Hopkins University, Ohio State University, University of Utah, University of Michigan |
| NCT01956084 | HL,HL, lymphoepithelioma; SCAEBV, leiomyosarcoma | Allogeneic LMP-1/2–specific CTLs | 1 | Children’s Research Institute |
| NCT02057445 | HL, NHL, LPD, | Allogeneic LMP-specific CTLs | 1 | New York Medical College, Children's Research Institute, Baylor College of Medicine, MD Anderson Cancer Center, University of Michigan, University of Utah, City of Hope Medical Center, Ohio University, Johns Hopkins University |
| NCT01498484 | NHL, EBV infection | Allogeneic EBV-specific CTLs | 2 | Atara Biotherapeutics, Memorial Sloan Kettering Cancer Center |
| NCT01447056 | HL, NHL, LPD, nasopharyngeal carcinoma, leiomyosarcoma, SCAEBV | Allogeneic LMP-specific CTLs | 1 | Baylor College of Medicine |
| NCT01555892 (GRALE) | HL, NHL, T/NK LPD, lymphoma | Autologous or syngeneic donor LMP-1/2–, EBNA-1–, and BARF-specific CTLs | 1 | Baylor College of Medicine, National Cancer Institute |
| NCT00062868 | HL, NHL, lymphoepithelioma, leiomyosarcoma | Autologous or allogeneic LMP-1/2–specific CTLs | 1 | Baylor College of Medicine |
| NCT02287311 (MABEL) | HL, NHL, SCAEBV, T/NK LPD | LMP-, BARF-1–, and EBNA-1– specific | 1 | Baylor College of Medicine |
| NCT01956084 | HL, NHL, SCAEBV, lymphoepithelioma, leiomyosarcoma | Allogeneic LMP 1/2 -specific CTLs | 1 | Children’s Research Institute |
| NCT00002663 | Lymphoma, leukemia, unspecified adult solid tumor, protocol specific, unspecified childhood solid tumor, protocol specific | Allogeneic EBV-specific CTLs | 1/2 | Atara Biotherapeutics, Memorial Sloan Kettering Cancer Center, National Cancer Institute |
| NCT02973113 (PREVALE) | HL, NHL, LPDs, EBV-related lymphoma, EBV-related PTLD, EBV-related NHL, EBV-related HL | Autologous EBV-specific CTLs + nivolumab (anti-PD-1 mAb) | 1 | Baylor College of Medicine |
| NCT01333046 (TACTAL) | HL, NHL | 5-azacitidine+ autologous NY-ESO-1, MAGEA4, PRAME, survivin-, and SSX-specific CTLs | 1 | Baylor College of Medicine, National Cancer Institute |
| NCT02203903 (RESOLVE) | Relapsed/refractory hematopoietic malignancies | Autologous or allogeneic WT1, NE, PR3, PRAME, MAGE-A3, MAGE-A4, NY-ESO, and survivin-specific CTLs | 1 | Children’s Research Institute, Johns Hopkins University |
| NCT02822495 | EBV-associated LPD, EBV lymphoma, EBV-related posttransplant LPD, EBV-associated viremia | Allogeneic EBV CTLs | Expanded access | Atara Biotherapeutics |
HD, Hodgkin disease; LPD, lymphoproliferative disorder/disease; SCAEBV, severe chronic active EBV infection syndrome.
The CAR era
B-cell lymphomas, including diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and mantle cell lymphoma (MCL) have been shown to be amenable to T-cell therapy with highly promising results in CD19-CAR T-cell studies. Novartis’s CTL019 (tisagenlecleucel) received US Food and Drug Administration (FDA) breakthrough therapy designation and, more recently, FDA approval for pediatric acute lymphoblastic leukemia (ALL). Kite Pharma’s (now Gilead) axicabtagene ciloleucel also received FDA breakthrough therapy designation for DLBCL, transformed FL, and primary mediastinal B-cell lymphoma. However, the excitement of genetic engineering approaches such as CAR T cells and gene-modified TCRs has been tempered by off-target and/or on-target off-tumor toxicities.61 Cytokine release syndrome (CRS), tumor lysis syndrome, neurologic toxicity, and B-cell aplasia are the most common adverse events observed in the current generation of CAR T therapies. Serious side effects necessitating extensive hospitalization are common, but trials continue due to the remarkable results seen in some patients with no other effective options. Patients in Kite Pharma’s ZUMA-1 trial (NCT02348216) all had aggressive refractory B-cell lymphoma. Of the cohort 2 patients (n = 20), 36% had received ≥5 prior therapies, 82% were refractory to second-line therapy, and 18% had relapsed following stem cell transplant. Despite these statistics, this cohort achieved an impressive 73% complete response (CR) rate following treatment with an autologous anti-CD19 CAR T-cell product. Juno’s phase 1 TRANSCEND trial of JCAR017 for NHL and pediatric ALL demonstrated a 60% CR rate in patients with relapsed or refractory CD19-positive NHL. JCAR017 administered at various doses to patients with DLCBL, grade 3b FL, and MCL saw an overall response rate at the lowest dose of 80%. However, development of the Juno Therapeutics’ JCAR015 product (which utilizes the CD28z costimulatory domain as opposed to the 41BB costimulatory moiety used by the JCAR017 product) in adult patients with relapsed/refractory B-cell ALL has been uncertain since November 2016, when the phase 2 ROCKET trial was voluntarily put on hold following the death of 5 participants from cerebral edema. Thus, difficulties with unintended CAR-mediated side effects remain to be resolved.
Clinicians continue to better anticipate and manage CAR T-mediated cytotoxicities, and Juno reported fewer adverse events with JCAR017 than with other CD19-targeted CAR T-cell therapies. Only 2% of patients (1/44) in the TRANSCEND NHL 001 trial experienced severe CRS; 18% (8/44) experienced severe neurotoxicity, whereas 66% (29/44) did not experience any cytokine release syndrome or neurotoxicity in the core analysis group.62
Nevertheless, CAR T cells are not a panacea, as they are not effective in all lymphoma patients. In some patients, they do not proliferate, for reasons that are not yet understood. Their single target renders them vulnerable to loss of tumor control should tumor mutation result in loss of CD19 expression. Furthermore, CAR approaches are limited by the requirement that the antigen to be targeted must be extracellular, and in the B-cell NHL setting, the targeting of such antigens can potentially result in lifelong depletion of the normal B-cell pool.
Non-CAR approaches
In contrast to the CAR T-cell therapy approaches, non–gene-modified antigen-specific T cells have an excellent safety profile. No instances of CRS, graft-versus-host disease (GVHD), autoimmunity, or neurotoxicity have been associated with these trials to date.
DLIs
Infusions of unmanipulated naive and antigen-experienced lymphocytes can mount a GVT effect against the recipient’s tumor, eliminating neoplastic host cells. This treatment is widely used in leukemia, particularly chronic myelogenous leukemia, in which the majority of molecularly characterized remissions after DLI are durable.63 Although the most compelling evidence for DLI efficacy has occurred in leukemia, responses have also been recorded in relapsed NHL and HL after allo-HSCT.64-67 Nevertheless, relapse and GVHD remain as significant challenges after DLI, particularly in NHL.
A retrospective study of 225 patients with any hematological malignancy found 3-year overall survival (OS) after DLI of 38% in lymphoma patients (8/21). While no statistically significant difference in OS between groups treated with ≤1 × 107, >1.0 to <10 × 107, or ≥ 10 × 107 T cells was obtained, further analysis revealed an increased risk of GVHD with no improvement in OS at ≥10 × 107 cells, irrespective of low- or high-risk disease status.68 Encouraging response rates were reported in another study of unmanipulated DLI for indolent NHL (FL and MCL).67 Twenty-eight patients with progressive disease, with or without mixed chimerism, received escalating doses until full donor chimerism or disease response was attained. A cumulative response rate of 76.5% was achieved in PD patients and 91.6% in patients with mixed chimerism. Seven patients (25%; 5 with FL) achieved full donor chimerism responses in the absence of GVHD.
Investigators attempting to improve the GVT effect of DLI in lymphoma have evaluated “selective depletion” DLI strategies to enhance the GVT response while limiting GHVD utilizing approaches such as the use of anti-CD25 immunotoxin69 and photodepletion70 to selectively deplete host-reactive donor T cells from donor grafts. However, thus far, these approaches have been met with very limited success in the NHL population.
Selective depletion of regulatory CD4+CD25+ cells (regulatory T cells) from DLI populations prior to infusion has been evaluated as a strategy to boost the antitumor effect by removing their suppression of the GVT response.71 This trial cohort included 4 patients with HL and 2 patients with NHL. Overall significant GVHD was 19% (n = 4) at 8 weeks and 33% (n = 7) at 1 year. Eight patients achieved a CR (53%) at dose level 2 (3 × 107 CD3+/kg; n = 15) with an overall response rate (CR + partial response [PR]) of 60%. Responses were observed in 2 patients with HL and 1 patient with NHL.
Reports on DLI efficacy can be confounded by coadministration of antilymphoma treatments (chemotherapy, radiation, and/or rituximab) and corticosteroids for GVHD. Nevertheless, these agents are unlikely to account for responses in heavily pretreated and treatment-resistant patients.
Antigen-specific T cells: targeting tumor-associated antigens
An alternative approach to enhance the graft-versus-lymphoma effect while minimizing GVHD is to selectively expand tumor antigen-specific T cells of interest. CD8+, CD4+, and NK cells have all been associated with GVT responses. Thus selection of tumor-specific CTLs and, to a lesser degree, NK cells targeting tumor antigens or minor histocompatibility antigens has been a strong focus of investigations in relapsed patients (NCT02203903, NCT01333046, NCT00002663, NCT01948180, NCT00779337, NCT00005606, NCT01636388, NCT01956084, NCT02057445, NCT01498484, NCT01447056, NCT01555892, NCT00062868, NCT02287311, NCT01956084, NCT00002663, and NCT02973113). The choice of antigen is crucial, requiring a tumor-specific target that elicits a robust immune response and induces a clinical outcome while sparing healthy tissues. For tumor immunotherapy, this usually entails identifying tumor-associated self-antigen(s) to which T and B cells have not been tolerized during development. Alternatively, tumor-associated non–self-antigens, such as viral antigens, have been used successfully as immunotherapy targets.
EBV as a target antigen in lymphoma
Epstein Barr virus (EBV), which lies latent in ∼1% of B cells post-EBV infection, is strongly associated with ∼40% of lymphoma cases in immune-competent patients with HL and NHL72,73 and with >90% of posttransplant lymphoproliferative disorder (PTLD), making EBV an attractive target for T-cell therapy.
EBV-specific T cells for PTLD
EBV-driven lymphomas that arise in T-cell–deficient patients post-HSCT have not been exposed to immune-selection pressure and as such are highly susceptible to immune targeting. The EBV nuclear proteins EBNA-1, EBNA-2, EBNA-LP, EBNA-3A, EBNA-3B, and EBNA-3C comprise the EBV nuclear antigen complex. EBNA-1 binding to the origin of viral DNA replication sequence on EBV DNA allows the virus genome to be maintained as an episome in infected B cells, which are transformed into cells with indefinite proliferative capacity.74 EBNA-2 is required for EBV-mediated B-cell transformation, transactivating expression of the EBV genes LMP-175 and LMP-2,76 along with other cellular genes. EBNA-LP enhances EBNA-2’s ability to transactivate LMP-1 and LMP-2, while EBNA-3A, EBNA-3B, and EBNA-3C all assist in EBV-mediated B-cell transformation. The latent membrane proteins LMP-1 and LMP-2 are expressed by EBV-transformed B cells. LMP-1 is a constitutively active functional homolog of CD40, while LMP-2 prevents lytic reactivation of EBV-infected B cells and the calcium flux that results from surface immunoglobulin G cross-linking of the B-cell receptor complex.74 BARF-1 protein is a soluble receptor for colony-stimulating factor 1 and inhibits interferon-α secretion by monocytes, which may increase EBV-infected cell survival.74 EBNA-1 expression is observed in Burkitt lymphoma, while EBNA-1, LMP-1 and LMP-2 are all associated with HL. EBNA-2, EBNA-3, and LP are expressed in AIDS-associated lymphomas, LMP is seen in DLBCL, and BARF is seen in Burkitt lymphoma and HL.77
The anti-CD20 monoclonal antibody (mAb) rituximab is used to control EBV-mediated B-cell lymphoproliferative disease in immune-compromised stem cell transplant patients. In some patients, this therapeutic alone can control B-lymphocyte proliferation during the immune cell recovery period, after which the patient’s immune cells can resume control of EBV-infected B cells, dampening their proliferation.
T cells targeting EBV antigens have been a successful adjunct for restoring control of EBV-driven proliferation and can be used in PTLD patients for whom rituximab is not successful. This mechanism underlies the success of non-specific DLI, which was first shown to reinstate EBV immunity and eliminate lymphoproliferative disease in the mid-1990s, albeit with the accompanying risk of severe GVHD.78 This method was subsequently refined with the generation of EBV-specific T cells designed to specifically eliminate EBV-positive tumor cells and reduce the risk of GVHD.79-81 The robustness of this approach was demonstrated in a multicenter setting with experience treating over 100 HSCT patients.81 Of the 101 patients who received donor-derived, EBV-targeted T cells prophylactically, none developed EBV-positive LPD, while 11 out of 13 patients who received CTLs for diagnosed or probable LPD achieved CRs. Toxicity was minimal, with no grade 3 or 4 GVHD post-CTL infusion and chronic GVHD in 13 out of 108 patients (12%). This study was important for demonstrating the utility of EBV-positive CTLs for preventing or treating PTLD post-HSCT. However, the incidence of PTLD post-HSCT has since been considerably lessened due to changes in patient conditioning and posttransplant rituximab. PTLD is currently a more pressing clinical problem after solid-organ transplantation than after HSCT owing to the necessity for ongoing immunosuppression in the solid organ transplant setting and the lack of an evacuated compartment for ex vivo–generated autologous EBV CTL.82 In that setting, EBV-specific CTLs can be effective; however, CTL persistence is less than that observed post-HSCT, suggesting that long-term persistence and function may be impaired in patients receiving ongoing immunosuppression.83 To that end, a recent Children’s Oncology Group study (NHL1522) has opened and is evaluating the use of third-party EBV-specific T cells with Rituxan for patients with newly diagnosed PTLD.
EBV-specific T cells for HL and NHL
EBV-specific CTLs have also been administered to EBV-positive HL and NHL patients whose disease has developed in an immune-competent setting. These tumors express a type II latency of EBV antigen expression, since they have been exposed to immune editing and display minimal expression of EBV antigens. As a result, these tumor cells are less immunogenic and evade immune-mediated control by numerous mechanisms, including downregulation of the highly immunogenic EBNA-3 and EBNA-2 antigens. Nevertheless, the subdominant EBV antigens LMP-1 and LMP-2 are potential targets for T-cell therapy.
Despite HL’s weakly immunogenic expression of LMP1/2, in a trial of 14 patients with relapsed EBV-positive HL, Bollard et al84 reported an 18% CR rate, and persistence up to a year, following treatment with autologous EBV CTLs. No toxicities were reported, and clinical responses were apparent within weeks of infusion. Of 11 patients with quantifiable tumor, 2 CRs (18%), 1 PR (9%), 5 stable disease (45%), and 3 nonresponses (27%) were reported. An additional 3 patients without measurable disease were disease free at 10 to ≥40 months post-CTL infusion. To enhance the activity of the EBV-specific T-cell product for patients with type II latency lymphomas, the group went on to develop a T-cell product with specificity for the EBV antigens LMP-1 and/or LMP-2.72 Adenoviral vector–transduced DCs and EBV-transformed B-lymphoblastoid cell lines were used to activate and expand LMP-specific T cells. Fifty patients comprising HL, NK/T-cell NHL, DLBCL, PTLD, peripheral T-cell NHL, and other lymphomas, including chronic active EBV infection and lymphomatoid granulomatosis, received LMP-specific T cells in this phase 1 dose-escalation study.72 Administration was shown to be safe, with only 2 patients experiencing adverse events that could not conclusively be attributed to CTL infusion. Overall 53% of patients (11/21) in the active disease cohort achieved CR; 2 out of 21 achieved PR, with 1 of those achieving CR following further CTL treatment. The 2-year event-free survival was ∼50% in this group of relapsed/refractory patients, with the frequency of circulating LMP-1 and/or LMP-2–specific CTLs correlating with clinical response. Therefore, this is a promising approach for patients with EBV-positive HL and NHL. However, it is important to consider that this approach is limited to EBV-positive lymphoma, which generally excludes FL and MCL.
Alternative targets for lymphoma-specific T-cell therapies
Reviewing clinical trials for T-cell–mediated lymphoma treatment reveals that while strong immune responses can be induced against lymphoma-associated viral antigens, poorly immunogenic tumor antigens represent more difficult targets. This is not only because they are usually self-antigens to which autologous B and T cells are rendered tolerant during fetal development but also because they develop multiple immune-evasion strategies. Nevertheless since ≈40% of lymphomas are EBV associated, this important avenue of inquiry ultimately has the potential for broader lymphoma applicability. More work in the development of antigen-specific T-cell therapies targeting nonviral antigens has been done in leukemia than in lymphoma; however, several trials are currently investigating expanded TAA-specific T cells for lymphoma (NCT01333046 and NCT02203903).
Expression of the tumor-associated antigens WT1, PRAME, and survivin is low in healthy tissues beyond the developmental stage, making them attractive targets in a range of cancers. Some expression of WT1,85 PRAME,86 and survivin87 does occur in normal adult tissues; thus, the risk does exist for cell-mediated damage to other tissues such as kidney, testes, ovaries, uterus, adrenal glands, hematopoietic cells T cells, neutrophils, and endothelial cells. Nevertheless, PRAME, SSX2, MAGE-A4, NY-ESO1, and survivin-specific T cells can be expanded from HL and NHL patients, and their efficacy is under evaluation in 2 clinical trials (Table 2). The TACTAL trial (NCT01333046) uses autologous T cells targeting PRAME, SSX2, MAGE-A4, NY-ESO1, and SURVIVIN. The RESOLVE trial (NCT02203903) uses autologous or allogeneic T cells targeting WT1, PRAME, and SURVIVIN to treat patients who have relapsed hematologic malignancies, including patients who have relapsed after allogeneic HSCT.
One significant advantage of the TAA-T cell therapies is their safety record. To date, the clinical responses (50%-75%), while very encouraging, still involve small numbers of patients compared with the CAR T-cell studies, making comparisons difficult. However, with the cumulative experience of >20 patients,88,89 <1% product-related severe adverse events have been observed compared with approximately 50% in patients receiving CD-19 CAR T cells.90,91
Neoantigens
Currently, limited clinical data exist for the use of neoantigen-specific T cells in lymphoma. Nevertheless, important progress is being made in identifying future lymphoma-specific neoantigens. Khodaoust et al recently reported on their successful integrated proteomic and genomic approach to identify MHC-I– and MHC-II–restricted MCL neoantigens in 17 patients.92 Surprisingly all identified neoantigen peptides were located in the lymphoma immunoglobulin heavy- or light-chain variable regions, with no mutated peptides discovered in nonimmunoglobulin somatically mutated genes. The peptides arising from these somatic mutations were presented almost entirely on MHC-II, and circulating CD4+ T cells specific for these neoantigens specifically killed autologous lymphoma cells. This study, combined with work in other cancer types showing the efficacy of CD4+ CTLs, indicates that expanded endogenous immunoglobulin-neoantigen–specific CD4+ T cells may prove a useful therapeutic in lymphoma.
Strategies for improving nonengineered T-cell responses in lymphoma
Infused donor T cells are not resistant to endogenous immune suppression or the immune-suppressive tumor microenvironment. Tracking the ability of adoptively transferred cells to home in on tumors, engage with in vivo targets, proliferate, and survive are all measures by which clinicians can gauge the effectiveness of these cell-based therapeutics. Unfortunately, the lack of gene “marking” can hinder the ability to definitively determine whether responses are related to the adoptive T cell transfer. However understanding at which point(s) the infused cells are inhibited facilitates rational design of strategies to circumvent immune-suppressive factors in vivo. One strategy to overcome the tumor-induced immune suppressive microenvironment and to promote homeostatic lymphoproliferation is lymphodepletion prior to T-cell infusion. This approach has the advantage of removing autologous cells that would compete with infused cells for cell-supportive endogenous cytokines.93,94 One study compared patients receiving DLI with or without lymphodepletion by cyclophosphamide and fludarabine.95 In support of the hypothesis, greater lymphocyte expansion was observed in the lymphodepletion plus DLI cohort, but this was accompanied by significantly greater rates of acute and fatal GVHD. The study highlighted the need for effective management of lymphodepletion-mediated toxicity in order to allow its prospective effects on tumor control to be measured. While lymphodepletion prior to non-CAR T-cell therapy may indeed enhance the potency of the infused cells, studies in the DLI setting highlight the need for caution and refinements in the lymphodepletion plus DLI approach.96,97
T-cell expansion in vivo is also aided by judicious administration of exogenous cytokines, such as interleukin-2 and interleukin-15, that support T-cell survival and proliferation. Ex vivo expansion of adoptively transferred T cells that are not impaired by the in vivo malignant microenvironment is another method of generating highly potent cytotoxic T cells (Figure 1B-C). However, adoptively transferred T cells are still susceptible to the suppressive, apoptosis-inducing actions of steroids used to control GVHD post-HSCT.
Downregulation by tumors of HLA class I is one strategy by which they lose recognition and therefore evade direct lysis by CD8+ cells. One study of >100 HL immunohistochemistry samples in which patients were followed up over a median of 9 years showed that reduced MHC-I expression, but not reduced MHC-II expression, correlated with poorer outcomes independent of programed death ligand 1 (PD-L1)/PD-L2 amplification and advanced-stage disease.98 Lack of MHC-I expression occurs in ∼80% of HL99 and has been well described in Burkitt lymphoma,100-103 FL, MCL, DLBCL, and lymphoblastic lymphomas.104 Approximately 29% of DLBCLs display genetic abnormalities at the β2m locus.105 Thus, agents such as interferon-γ, radiotherapy, and chemotherapies that induce tumors to upregulate MHC-I can restore tumor antigenicity.106-110 Indeed the efficacy of certain chemotherapeutics lies in their ability to induce upregulation of MHC-I and/or MHC-II on lymphoma cells. The antigen-presenting cell–restricted MHC-II molecule is likewise downregulated in multiple B-cell lymphoma types, with higher levels of HLA-DR correlating with favorable outcomes in DLBCL,111-115 indicating a role for antigen presentation to CD4+ T cells in the response to therapy.
Tumor cells also downregulate co-stimulatory molecules, thereby limiting CTL activation, and secrete immune-modulating factors such as transforming growth factor β, which drives a regulatory phenotype in DCs and macrophages and directly inhibits T cell function.116 Engineering of antigen-specific T cells resistant to transforming growth factor β to restore antitumor immunity is a strategy under active investigation117 (NCT00368082).
Targeting immune checkpoints has also resulted in promising results for the treatment of patients with lymphomas. Activated T cells upregulate CTLA4 as part of homeostatic contraction of the proliferated population and quiescence into memory cells. CTLA4 ligation with CD80/86 on antigen-presenting cells or tumor cells functions as an “off switch”; thus, anti-CTLA4 mAbs have been used to block CTLA4 binding and maintain T-cell activity in patients with hematological malignancies.118,119 To date, lymphoma responses to anti-CTLA4 alone have been modest but encouraging in numerous trials (NCT02254772, NCT01769222, NCT00089076, NCT00060372, NCT00047164, NCT03013491, NCT01729806, NCT01896999, NCT02408861, NCT01919619, NCT02681302, NCT01445379, NCT02304458, NCT01592370, NCT01822509, NCT02879695, and NCT02693535).
The interaction of programed cell death protein 1 (PD-1) on T cells with PD-L1 on tumor cells is another immune checkpoint that inhibits T-cell proliferation. Increased PD-L1 expression is observed in HL and mediastinal large B cell lymphoma120,121 and upregulation may be driven by the EBV protein LMP1 in NK/T-cell lymphoma.122 PD-L1 overexpression in lymphoma has also been ascribed to PD-L1 gene amplification120 or fusion of the PD-L1 gene with CIITA, the MHC-II transactivator.123 Anti–PD-1/PD-L1 mAbs are able to rescue this inhibition, particularly in HL,124 with overall response rates in HL patients receiving PD-1/PD-L1 inhibition reaching between 65% and 87%.124 Overall responses to PD-1 blockade in NHL have been in the more moderate range of 11% to 30%.
The current consensus is that single immune or T-cell–based therapeutics will not work in isolation and that combination approaches, such as checkpoint inhibition with cell-based therapies, represent the most interesting immediate way forward for a potent antitumoral response in vivo. One clinical trial is actively investigating this concept. Baylor College of Medicine’s trial of nivolumab (α-PD-1) plus LMP, EBNA1, and BARF-specific T cells for HL, NHL, or EBV-associated T/NK-cell or B-cell LPD is open and recruiting (NCT02973113 [PREVALE]). One barrier to the broader application of this approach to patients with EBV-negative lymphomas is identification of tumor-specific targets for T cells to attack once their brakes are released by the checkpoint blockade. However, the current rate of tumor-associated antigen and neoantigen discovery and the clinical use of TAA-specific T-cell therapies for patients with HL and NHL give hope for the future combination of these 2 important therapeutic strategies.
Conclusions
T-cell therapeutics are making excellent progress as effective treatments for patients with lymphoma but are also operating under limitations. DLI can be highly effective in chronic myelogenous leukemia but less so in the lymphoma setting, and identifying reliable strategies to uncouple the GVT effect from GVHD remains the “Holy Grail” that is yet to be discovered. Numerous approaches to enhance cell-mediated antilymphoma effects are under investigation, including selective depletion of particular T-cell populations and generation of TAA-specific T cells targeting specific tumor- or viral-associated antigens. It is envisioned that further refinement of T-cell–based immunotherapies, combined with (1) rational immune checkpoint blockade approaches, (2) genetically enhanced TCRs or chimeric antigen receptors, and/or (3) molecular targeting, will result in highly effective and less toxic treatments for patients.
Correspondence
Catherine M. Bollard, Program for Cell Enhancement and Technologies for Immunotherapy, 111 Michigan Ave, NW, 5th Floor Main Suite 5225, Washington, DC 20816; e-mail: cbollard@cnmc.org.
References
Competing Interests
Conflict-of-interest disclosure: C.M.B. is a member on the board of directors or an advisory committee for Cellectis and Neximmune and has consulted for Neximmune. M.G. declares no competing financial interests.
Author notes
This article was selected by the Blood Advances and Hematology 2017 American Society of Hematology Education Program editors for concurrent submission to Blood Advances and Hematology 2017. It is reprinted with permission from Blood Advances 2017, Volume 1.
Off-label drug use: None disclosed.